Resort to medications dates back million years ago with the use of medicinal plants. In the nineteenth century, significant contributions in medicine appeared in different domains, among which the invention of a specific drug delivery device; the syringe. Nowadays, injection therapy of bio-manufactured drugs is routine practice for chronic diseases but remains constraining and painful. New emerging advanced therapies invest in genetic, electronics and cell-based therapy for addressing unmet needs for the caregivers and the patient. As digital process in health (eHealth) gains momentum, connected advanced bio-electronic devices now offer new strategies for personalized injection therapies. In this review, we take a journey along the genesis path of a new drug delivery system: the Optogenerapy, a synergy between optogenetic and gene therapy. Inside a bio-electronic implant, electronics and optogenetics are interfaced by light as a traceless inducer signal. By controlling a synthetic optogenetic pathway in the cell, therapeutics delivery can be fine-tuned with a precise spatiotemporal control. The technology holds promise of a new modern syringe era capable of producing a drug of interest at will directly inside the patient.
The success of modern medicine results from a synergy between the development of drug delivery devices and the ever-growing pharmacopoeias. The pioneering work of Francis Rynd in 1844, on a syringe-based infusion of fluids into human body led not only to the development of the hypodermic syringe but also revealed the systemic mode of action of an analgesic drug through the circulatory system. The discovery of a precise administration route for opiates will be soon associated with patient repeatedly self-injecting drugs. The first prophylactic targeted use of syringes was not limited to chronic pain. Due to the limited action of the early insulin preparations, diabetic patients had to be injected several times per day. Later, intravenous delivery of penicillin G during World War II popularized the use of a life saving injection. From the 18th century hand-made glass model to the plastic disposable insulin syringe introduced in 1970, today's syringes are mass-produced by billions. The increase in the lifestyle associated chronic diseases (obesity, heart disease, stroke, cancer, type 2 diabetes, and arthritis) generates a worldwide growth of self-administered medications. In 2020, the apprehension is that chronic illnesses will be responsible for almost three-quarters of all deaths on earth.1 As an alternative option to injection therapy, cell transplantation and gene therapy promises may hold true but are not yet delivered. The risk associated with new emerging cell therapies often requires lifetime patient survey. The development of modern medical devices is closely related to innovation in the fields of electronics and photonics. The maturation of the medical technology for the pacemaker and the electroencephalogram (EEG) has set the stage for a bionic heart2 and brain-computer interface breakthrough innovations.3 The exploration and mapping of the brain set new frontiers in genetic engineering. To decipher the neuronal maps, synthetic biology researchers develop a portfolio of optogenetic tools. The genetic reprogramming introduces photo-activable molecular actuator4,5 in the genome of a neuron to shed light on neural network structures. This technology has not yet found its translation path to the patient bedside. In 2016, 17% of new molecular entities (NME) approved by the FDA are personalized medicines.6 New drugs serving unmet medical needs will be soon available. Genetically-engineered therapeutic proteins (antibodies, interleukins, peptides) represent a unique class of drug called biologics. In one hand biologics are difficult to manufacture requiring complex good manufacturing practice (GMP) bioprocessing installations, on the other hand, they offer higher target-specificity and reduced incidence of off-target effects that helps to qualify the drug in preclinical stages.
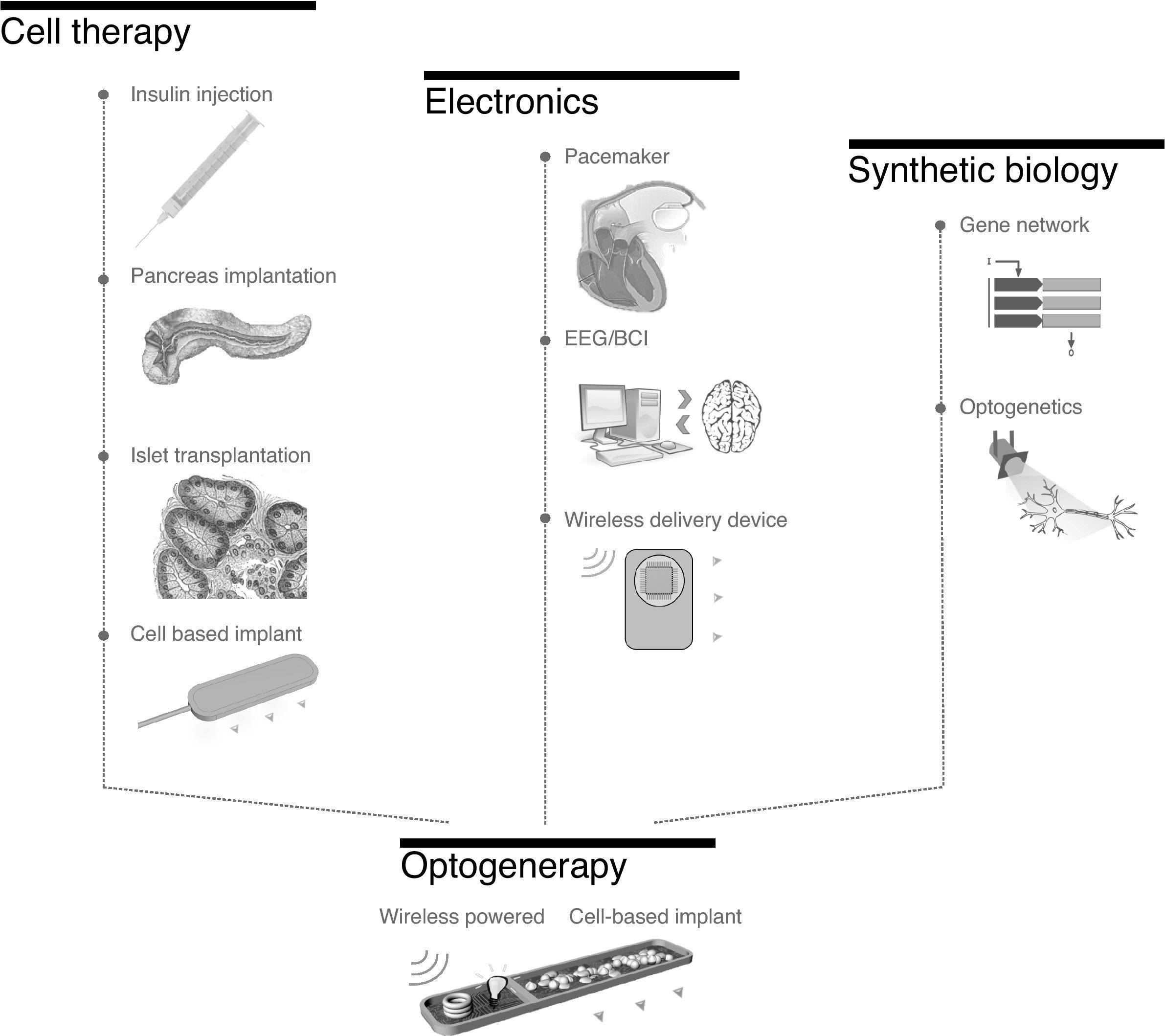
A challenge of tomorrow medicine is to develop a device that would replace the syringe therapy by a new implanted drug delivery device. Optogenerapy system associates the optically controlled bio-manufacturing of the therapeutic protein and its perfusion within the circulatory system. The technology takes its name from the contraction of optogenetics and gene therapy. The optogenerapy multidisciplinary device by combining advances in cell encapsulation, optogenetic and electronic engineering could find its space in a globalized futuristic biocybernetic eHealth scenario. The cybernetic implies a closed loop control of the human by controlling chronic states in concert with a chain of connected biosensors to remote telemedicine. In this review, we will take a journey along the development path of what may be seen as “bio-cybernetic syringe.”
Encapsulated cell technologyA first significant step in the development of the cellular-based drug delivery device of tomorrow is the constant development of cell-transplantation therapy, first applied to diabetes and now translated to other types of disease.
In 1921, the hormone insulin was detected as a treatment for diabetes, but soon the need for long-term therapy appears.7,8 Considering that insulin cannot be taken in a pill form, it is usually injected. Despite considerable research efforts from large medical device manufacturing company like Medtronic with the development of advanced personalized strategy with closed loop system9; alternative options to injection therapy are still today limited.
At first, the pancreas was transplanted as a whole with the constant need for immunosuppression treatment (Fig. 1). Unfortunately full immunosuppression did not offer long-term solution due to the increased risks of incoming infections and the potential causes of cancer. Instead of the whole pancreas the therapy targeted the isolation of islets of Langerhans from the pancreas (Fig. 1). One solution to transplant the islets was to use immune privileged sites, i.e. protected from immune destruction. For example, transplantation of pancreatic pieces in the anterior chamber of the eye was favoured for islets injection.10 These sites were shown to allow the islets engraftments to stay longer before immune-rejection from the patient. In 1933, Bisceglie was the first to propose a treatment of diabetes with encapsulated insulinoma.11,12 Later, by studying immune rejection, Algire et al.13,14 evaluated the possibility to encapsulate the islets in a membrane with different pore sizes. The idea was to prevent immune rejection by circulating cells. As a result of their study creating a chamber with small pores diameters (<0.45μm) offers immune protection to the islets. This semi-permeable membrane confinement strategy offers a double advantage; on one side, the immune cells cannot penetrate the pores of the membrane, on the other side oxygen and nutrients can pass and supply the cells.
Genesis of optogenerapy. The concept of optogenerapy emerged subsequent to the development of the domains of cell therapy, electronics and synthetic biology. Cell therapy, guided by the progress in encapsulated cells for diabetes therapy, opens the path of cell-based implant. Electronic medical devices leaded by the development of pacemakers abled the release of therapeutics wirelessly (Microchip). Finally synthetic biology and optogenetic permit the control of cells protein production capacity simply by light. Optogenerapy, as a multidisciplinary approach, consists of a wireless powered bio-electronic cell based implant to create an embarked optoelectronic circuit. It triggers the bio-manufacture and release of a therapeutic protein by the engineered cells.
A large literature reports the successful transplantation of islets in diverse animal models and patients. The current techniques achieve selective permeability with intra- or extra-vascular macro-devices surrounding islets, micro-devices containing fewer encapsulated islets, coatings with permeability selective material and finally nano-encapsulation to protect each islet8 (Fig. 1).
Micro-devices enclose small number of islets into a hydrogel. Multiple materials for semi-permeable encapsulation are used to isolate the implanted cells from the host. Alginate is one of the most popular due its excellent biocompatibility and facility of utilization.12 Besides alginate beads, agarose, cellulose, chitosan and others materials are reviewed for cell encapsulation by De Vos et al.15
This review will focus on macro encapsulation device. In contrast to the alginate encapsulation and the hydrogels that cannot be explanted, membrane confinement offers the greatest safety for the patient as the genetically modified cells do not circulate in the vascular system and the device could be removed at any time.
Commercialization of macro-devices started at the end of the 1990s by Baxter Healthcare who designed the TheraCyte implant. The device is composed of Teflon membranes and a polyester mesh to allow neovascularization8 after implantation. Studies in rodents were promising but failed to translate into clinical applications in humans. Concomitantly, a small biotechnology company Islet Sheet Device capitalized on alginate sheet to encapsulate islets. A principal difficulty during beta-cell transplantation is ischaemia. As inadequate oxygenation due to a lack of vascularization remains one of the leading cause of implant failure. Beta-O2 devices concentrate their efforts on designing methods to improve the oxygenation of the device by injection or production of oxygen.8
After the success of cell-based therapy from islets transplantation, the field of cell-based implant emerged as an interesting therapeutic option to treat multiple types of diseases. In 2002, Broadhead et al. encapsulated PC-12 cells, in hollow-fibre membrane allowing neurotransmitter release to quantify dopamine level in the culture. While changing the permeability of the membrane, they show that they could fine-tune the neurotransmitter release.16 They further improved the design of their technology by implanting a refillable cell encapsulation device and tested it in rat brains as a treatment of Parkinson's disease.17 As for the first prophylactic target of a syringe, encapsulation was also used to relieve chronic pain. Bovine chromaffin cells were isolated and implanted into the sheep for six weeks. The cells were able to release norepinephrine and met-enkephalin.18
Biologics manufacturing has set up the basis of the empirical selection technology of super producer cell lines. Recent advances in genetic engineering are further refining the technology with specific genome editing tools.19 Synthetic biology applied to encapsulated cell technology emerged as an innovative therapeutic platform to produce biologics directly at their delivery site. This way, the host encounters no risk associated with direct genetic modifications or have genetically modified cell perfused in the body. The genetically modified cells are isolated from the host in a secure manner. As a treatment for Alzheimer's disease, retinal pigment epithelial cells were engineered to produce nerve growth factor and their implantations were evaluated for 12 months in minipig animal model.20 The technology was further improved by the development of particular cell scaffold that supports the growth of tissue like structure in the implant chamber. The cell-based device was successfully tested in Alzheimer's patients. Four patients were implanted for six months with a device containing cells capable of releasing nerve growth factor.21 The acceptance and the safety of the technology were proven after the devices were retrieved from the patient for analysis. The group of prof. Aebischer at the EPFL valued the efficacy of monoclonal antibodies against amyloid to act as a therapy for Alzheimer's disease.22 Their macroencapsulation device successfully secreted anti-amyloid-antibodies in rodent animal model.22 Many therapeutic applications could derive from this technology; non-limiting examples of target disease area include neurological disease: multiple sclerosis, stroke, epilepsy, Huntington's disease, Parkinson's disease.23,24
As cells can be engineered to produce and deliver therapeutic drugs of choice, cell encapsulation offers an excellent method to achieve drug production and delivery directly in the patient itself. Compared to the “traditional syringe”, there is no need for drug manufacturing or galenic formulation before implantation. As the cells may continuously grow, there is assumed unlimited drug availability.
Electronic medical devicesElectronic medical devices such as pacemakers exist for about 50 years (Fig. 1). Before being implantable, pacemakers consisted of large portable devices able to deliver electric pulses. Then the co-founder of Medtronic, Earl Bakken, developed the first wearable pacemaker operated by a battery in 1957.25 Innovations in medical devices are continuously progressing, researchers at Harvard University developed a soft robot surrounding the heart and capable of compressing functions to help the beating.26 The Carmat bioprosthetic heart is now moving forward with phase III clinical trials.2
Most of the implanted electronic devices deliver electricity as therapeutic action (pacemaker, deep brain stimulators, gastric stimulators). Using electronics précision to programme the drug delivery would bring another control on the corrective action and a better patient acceptance. Indeed repeated injections of therapeutics are painful for the patients and result in poor adherence to treatment. Farra et al. achieved the control of a wireless drug delivery implant27 (Fig. 1). The MicroChip consists of multiple doses of drugs divided into reservoirs that can open at will by electro-thermal ablation of the enclosing membrane. Eight women were implanted with the device for a period of four months and demonstrated the efficient release of the human parathyroid hormone as a treatment for osteoporosis.27 Another drug delivery device from Medtronic, integrates a closed loop system where the insulin pump is able to release insulin from a catheter programmed by a wearable glucose biosensor.9 In the future, this machine biology interface could be integrated into a web of connected biosensor that will ultimately be linked to the Internet of Things (IoT) (Fig. 1). It corresponds to the growing network of connected objects able to communicate and to coordinate monitored parameters.
Innovations in electronics are continuing their progresses and overcome the current problems preventing the translation towards effective medical devices. One of the primary challenges in electronic medical devices is the constant need for powering. In the case of miniaturized devices, the utilization of batteries inflicts difficulties due to their short lifetime and the large space required for implementing this component.28 An old engineering principle, discovered by Nicola Tesla is the transmission of energy through air. The solution consists of transferring the power generator to an external source wirelessly. The emitter-coil synchronizes the electricity generation in the implant energy-harvesting antenna. Wireless power transmission relies on an electromagnetic field to transmit energy to a miniaturized implant.29,30 The technology enables not only the electrical powering of the device but also a possible communication route between the emitter and the receiver.
The field of electrical engineering offers new possibilities and tools to engineer implantable miniaturized devices capable of operating as transceivers. For example, advanced Brain Computer Interfaces (BCI) are connected to an articulated prosthesis (Fig. 1). Paralyzed patients by focusing their attention on a brain task can take control of a prosthetic arm.31 The electrical activity of the brain is de-convoluted with the help of sophisticated algorithms and used to programme a computer interface.
Synthetic biology and optogeneticsSynthetic biology engineering approach often employs an electrical engineering vocabulary to describe gene network behavior19 and bio-calculators.32 Designer cells can be a programme to perform simple switch to more complex tasks like logic gate calculation.32 Recent modelling assisted gene networks support the assembly of an ADC converter33; an oscillator;34 a double pole double throw (DPDT) switch35 and boolean logic gates32; essential modules to assemble more complexes calculator networks.19 Genetic engineering offers the possibility to construct robust regulatory pathways for bioprocess36 but also to programme cell fate37 (Fig. 1).
For therapeutic applications, the cells can be programmed to sense a disease marker level and respond accordingly by secreting a therapeutic protein.38,39 Proof of concept of this theranostic gene network was performed for Gout arthritis,39 thyroid disorder,40 diabetes,41 psoriasis.42
Well-characterized light sensor proteins can serve as a genetically-encoded optically-controlled switch43 (Fig. 1). Light irradiation activates a synthetic pathway to trigger a cell potential but also to control the expression of a gene via second messenger signalling pathway.44,45 Light can be use to trigger the expression of one protein but it can also be used to control an organ46,47 or synchronize cellular behaviour's.48–50 Cellular implants responding to blue-light were designed based on hollow fibres containing blue light sensitive designer cells secreting Glucagon peptide GLP-1.51 Blue light cytotoxic properties and the difficulties to accurately dose the gene response with transdermal light-illumination led to the investigation of other synthetic light controlled gene network system. Near infrared (NIR) biolumination source implanted in the brain is showing a promising result for new therapies to prevent neuro-degeneration.52 Using NIR as a traceless inducer of gene network is also an attractive strategy to control a bacteriophytochrome associated nucleotide cyclase domains.4,45 By tapping into second-messenger pathways, prokaryotic phytochrome can control eukaryotic innate immune response pathways or talk to specific chimeric transactivator proteins.45,51,53
An “optogenerapy” concept emerges as an innovative cell-based implant to orchestrate the administration of biologics. It consists of a synergy of mature technology in the domains of macroencapsulation, electronics, and optogenetics (Fig. 1). In contrast to the previous approaches, an electronic module is embarked within the device. It is a first of its kind, as it associates genetically modified cells protected from the immune system thanks to semi-permeable membranes and an optoelectronic interface to control cellular behaviour confined within the implant.53 All implanted therapeutic devices so far were either composed of only electronic or just encapsulated cells, but not a combination of these two areas. The electronic module controlling a light source is used as a trigger for the light sensitive designer cells.
An energy harvesting antenna is powering the wirelessly powered cell-based implant. Its remote-controlled action offers the practician a full control over the infusion therapy. The device can play a fundamental role in many therapeutic applications. Integrating the optogenerapy implanted module to an electronic biosensors network will set the scene for new digital therapeutic processes. Miniaturized wearable biosensors probing patient parameters are already available into clothes and will foster the development of future eHealth platforms.
The engineering field of the wearable biosensor is now developing the algorithms that will enable to integrate patient commands captured with EEG devices. It is now possible with a wearable BCI to control the lightning of a switch or driving a wheelchair simply by thinking.3 As proof of concept for the optogenerapy device, a BCI interface was used to programme the secretion of a reporter protein marker in the bloodstream of a rodent animal model placed on the wireless transmitter.53 A human user wearing an EEG headset was performing a mental task to wirelessly command the illumination time of the implanted cell-based device in mice. The experiment confirmed that it was possible to “mind-controlled” a wirelessly piloted micro-bioprocessor implanted using a biosensor-derived signal.53
The biosensor can directly measure a disease marker, like glucose for type 2 diabetes patients. Ye and co-workers gained advantage from the optogenerapy technology to secrete glucagon like peptide 1 (GLP-1) in a rodent model of diabetes.54 In the experimental set up the glucose monitoring data is integrated via a mobile phone platform in a similar closed loop system as the Medtronic insulin pump except that the wearable biosensor trigger the wireless powered implanted LED.
The translation of the optogenerapy technology from the laboratory to the bedside requires the development of small-scale GMP manufacture of generic designer cells and an ambulatory procedure to implant the device. Each cell type will be designed according to the disease targeted and to the patient needs. The external wearable device controlling the implant would help the patient and the practician to fine-tune the infusion therapy.
ConclusionThe continuous improvements in cellular therapy have set the knowledge to a possible path of cell-based engraftment using macro encapsulation devices. In the case of diabetes, the difficulties in implanting the islets reside in re-creating a particular niche for the cells. Even after implantation the disease is still present and actively target the beta cells. Recent progresses in genetic engineering may help in the development of alternative strategies to provide diabetes with therapeutics.37 The advances in electronic medical device and the development of optogenetic tools open perspectives for engineering an optogenerapy implant. Inside a bio-electronic implant, electronic components wirelessly connected switch on a LED to activate engineered cells rendered capable to respond to light and to trigger the release of therapeutics. Optogenerapy proof of concepts demonstrated how a wireless cell based implant could be controlled by; (i) a brain computer interface to have mind controlled drug delivery; (ii) a connected glucose biosensor for controlling diabetes therapeutic. Further cell engineering research is still required to define the best engraftment and neo-vascularisation parameters that are essential for the success of implanted bio-electronic devices. The ultimate goal is to achieve a closed-loop regulatory system sensing a disease marker and reacting in consequence by releasing the therapeutic drug in a precise and controlled amount. The premises of optogenerapy implants aspire to act as future bio-cybernetic syringes regulating our organisms independently.
Funding sourceThis project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 720694.
Conflicts of interestThe authors declare no conflicts of interest.