The introduction of levodopa in clinical practice represents a hallmark in the treatment of the neurodegenerative disease, Parkinson's Disease. However, levodopa induced motor complications, namely dyskinesias and motor fluctuations, develop in the majority of Parkinson's Disease patients.
Objectiveto identify which Parkinson's Disease's, patient's and therapeutics’ initial features are more associated with dyskinesias or motor fluctuations development.
MethodsPatients with diagnosed Parkinson's Disease attending neurology outpatient clinic at Centro Hospitalar São João were selected. For this observational study, data was retrospectively collected from patient's clinical records. A survival analysis model with univariate and multivariate regression analysis was used.
Results87 patients with a mean of 72±9.7 years were included. After a median follow-up of 6 (range 1–17) years, 35.6% patients developed dyskinesias; and with a median of 5 (range 1–16) years, 32.2% developed motor fluctuations. After multivariate analysis, the akinesia/rigidity subtype was found to have a higher risk of dyskinesias and motor fluctuations development. Age of onset ≤50 years was associated with motor fluctuations development.
ConclusionIn conclusion, our results suggest that Parkinson's Disease patients’ initial characteristics, such as subtype or age of onset, are independently associated with the development of motor complications.
Parkinson's Disease (PD) is a neurodegenerative disorder described in 1817 by James Parkinson.1 Since his publication, “An Essay on Shaking Palsy”,1 there has been a crescent scientific interest on this subject. According to the most accepted theory, it was suggested that PD has a spreading character correlated with deposition of abnormal aggregates which create a significant loss of dopaminergic enervation in Substantia Nigra pars compacta.2 Motor symptoms begin when loss in dopamine uptake becomes ≥50%.3
In clinical practice, main symptoms are muscular rigidity, rest tremor and/or postural instability.4 One of the most common and simplest used scales to assess motor progression and severity of PD is the Modified Hoehn and Yahr Scale (H&Y).5
As a definitive solution is yet to be found, treatment can be the most challenging phase. General measures can reduce the impact of motor symptoms, but not completely, and surgical treatments are indicated in advanced disease6,7; differently, pharmacological therapy is widely used.8 Many drug classes are available – such as MAO-B inhibitors, dopaminergic agonists (DA) and anticholinergic drugs – but the best drug in relieving motor symptoms is levodopa.9,10 Associations with aromatic l-amino acid decarboxylase inhibitor and catechol-O-methyltransferase inhibitors are used to optimize drug action and reduce side effects like nausea and vomiting.11
With the risk of undertreatment, levodopa introduction is sometimes delayed because of the most feared levodopa-induced motor complications (MC): dyskinesia (DK) and motor fluctuations (MF).12
MF include “wearing-off” phenomena - symptoms re-appearance just before the next levodopa dose – and generally it is the first MC. MF also include “on-off” phenomena – sudden changes between normal and parkinsonic motor state; and “delayed on” phenomena – when symptoms relief takes longer.12,13
DK are defined as involuntary movements, the most common are chorea and dystonia, that can affect any body region, usually an extremity.12 This region can coincide with the first one affected by motor symptoms of PD.13 Initially they are peak-dose related, but can also be dysphasic – according to levodopa blood levels rise and fall – or “off dyskinesias” – when these levels are low.13,14
Although less and later than in the past, nowadays a significant part of PD patients develop these MC: 9 or more years after beginning levodopa, close to 90% of patients will develop DK and 70% MF.15 In Portugal, it was reported that patients with MF have 2 times more socio-economic costs than MF-free patients.16 Also, quality of life is greatly impaired in PD patients with MC. Their mobility, daily living activities or communication skills can be compromised, and stigmatization is frequent.17
MC are important obstacles to the everyday life of PD patients. It is vital not only to understand their etiology and pathophysiology, but also to understand what can be made to prevent them right since the first medical approach.
Our objective was to identify which Parkinson's Disease's, patient's and therapeutics’ initial features are more associated with dyskinesias or motor fluctuations development.
MethodsStudy designThis is an observational, analytical, non-interventional, non-comparative, retrospective, longitudinal study. It took place in a central university hospital, Hospital São João, Porto, Portugal.
Source of information were clinical records of patients followed in the hospital's outpatient clinic by two movement disorders neurologists (C.G. and M.J.R.) in periodic appointments.
The research protocol was certified by the ethical committee of Faculty of Medicine of Porto University and by the ethical committee of Hospital São João, respecting the Declaration of Helsinki principles.
Patient selectionPatients were selected according to the following inclusion criteria: appointments in Hospital São João outpatient's clinic, diagnosis of PD, and attendance of at least two appointments of movement disorder since 2012. In these appointments, the diagnosis of PD is based on UK Parkinson's Disease Society Brain Bank.4
Patients who had secondary causes of parkinsonism, uncertain diagnosis, missing data about first pharmacological therapy, levodopa not included in therapy, follow-up time less than 3 years or previous treatment with neurosurgical treatment, were excluded.
Data collectionEach patient's entry date was the year of first appointment and, for all, follow-up ended in August 2014. No blinding procedures were needed.
To gather all the information, a database was created using Microsoft Office Excel 2007. The studied variables were: gender, age at symptoms onset, time between symptoms onset and diagnosis, PD subtype at onset (tremor dominant (TD), Akinesia/rigidity (AR) or mixed), H&Y class at levodopa onset, time between DA onset and levodopa onset, initial drug (levodopa, DA or other), evolution of first year levodopa dose, presence of MF and/or DK and time since levodopa onset until the occurrence of MF and/or DK. The last one was used as our dependent variable.
Statistical analysisStatistical analysis was performed with IBM SPSS Statistics 22 according to a survival model. Dyskinesias-free survival (DFS) and Motor fluctuations-free survival (MFFS) were defined as the time from levodopa onset until the occurrence of DK or MF, respectively, or the most recent follow-up. Patients without DK or MF were censored in the last follow-up date. Continuous variables were expressed as the median, percentile 25 (p25) and percentile 75 (p75). For further data analyses, these variables were categorized.
A Kaplan–Meier estimate was used to calculate the median survival time and survival rate free of each MC. Outcome predictors were evaluated using univariate and multivariate Cox proportional regression analysis. Multivariate analysis was used to account for confounding variables; the ones included were those with a significance of p<0.20 in univariate analysis. Results were expressed as hazard ratio (HR) with 95% confidence interval (CI). A p-value <0.05 was used as criteria for statistical significance in the multivariate model.
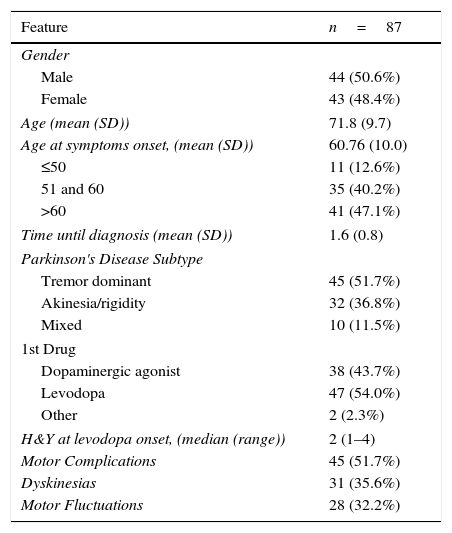
ResultsBaseline featuresAfter applying inclusion and exclusion criteria, 87 patients were included in this study. Patients excluded for not having levodopa included in their therapy, did not develop MC, until follow-up ended.
General features from study population are described in Table 1.
General features.
| Feature | n=87 |
|---|---|
| Gender | |
| Male | 44 (50.6%) |
| Female | 43 (48.4%) |
| Age (mean (SD)) | 71.8 (9.7) |
| Age at symptoms onset, (mean (SD)) | 60.76 (10.0) |
| ≤50 | 11 (12.6%) |
| 51 and 60 | 35 (40.2%) |
| >60 | 41 (47.1%) |
| Time until diagnosis (mean (SD)) | 1.6 (0.8) |
| Parkinson's Disease Subtype | |
| Tremor dominant | 45 (51.7%) |
| Akinesia/rigidity | 32 (36.8%) |
| Mixed | 10 (11.5%) |
| 1st Drug | |
| Dopaminergic agonist | 38 (43.7%) |
| Levodopa | 47 (54.0%) |
| Other | 2 (2.3%) |
| H&Y at levodopa onset, (median (range)) | 2 (1–4) |
| Motor Complications | 45 (51.7%) |
| Dyskinesias | 31 (35.6%) |
| Motor Fluctuations | 28 (32.2%) |
SD, standard deviation; H&Y, Hoehn and Yahr class.
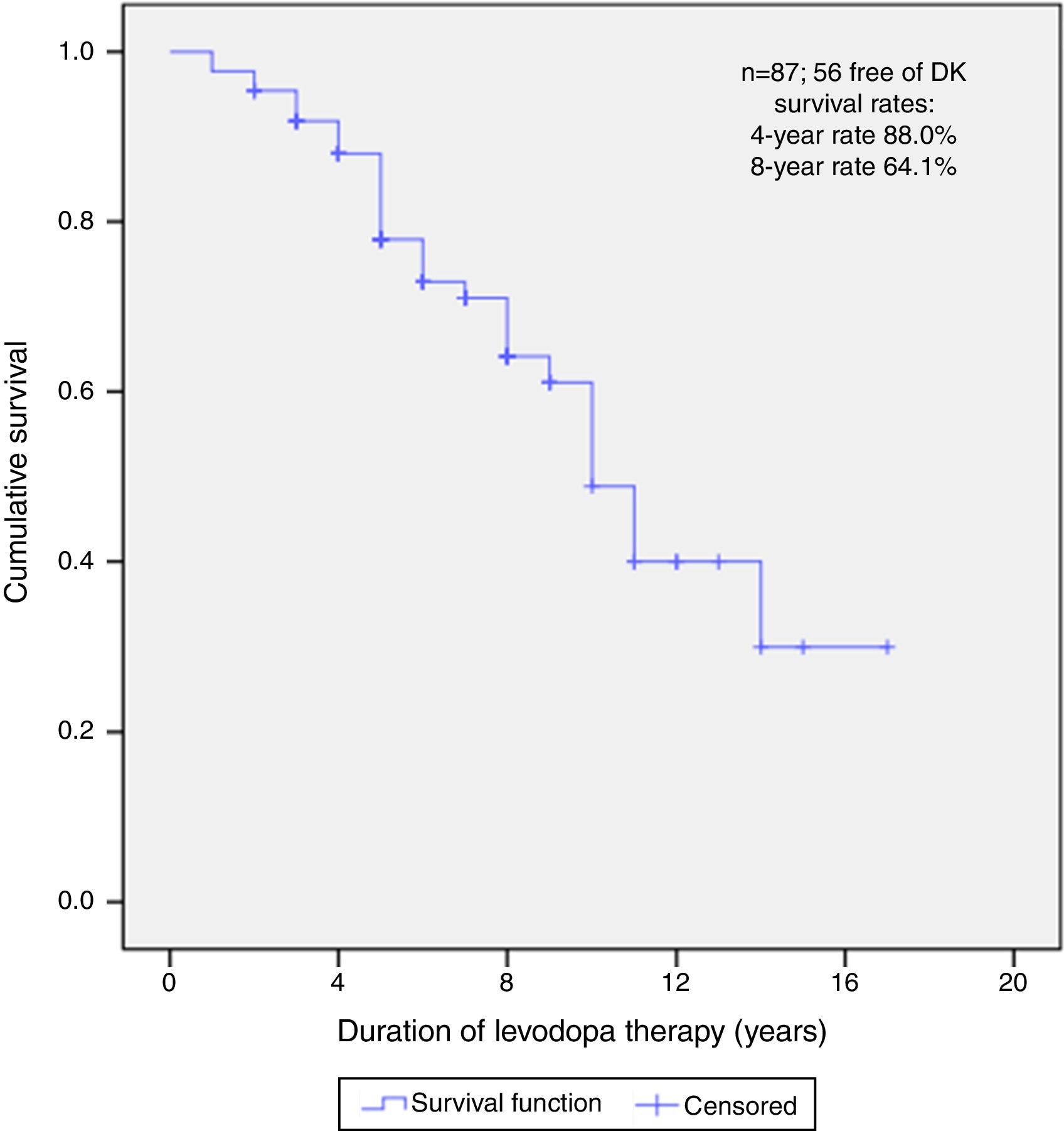
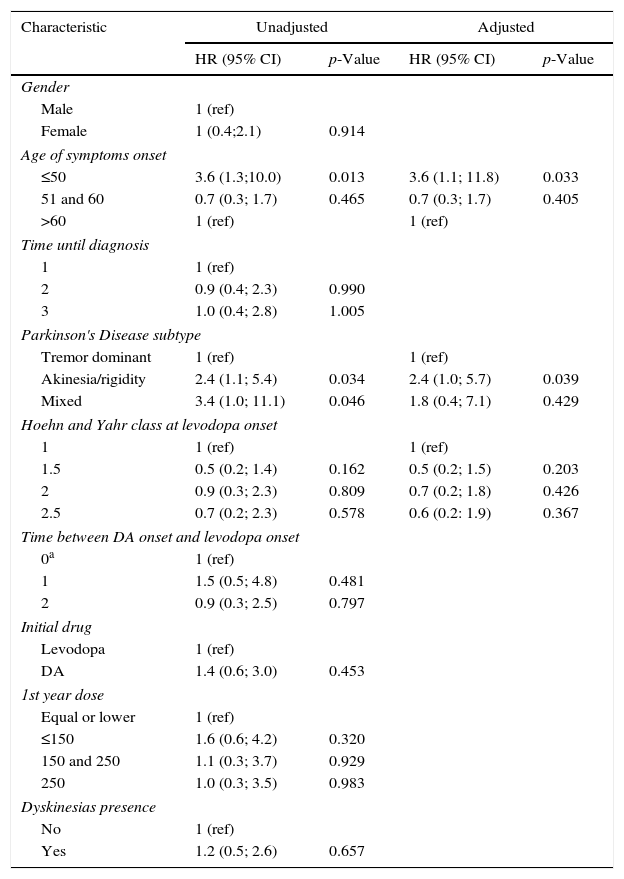
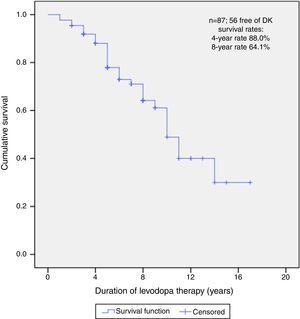
After a median follow-up of 6 years (range 1–17), 56 (64.4%) patients did not present DK at the time of this report. The median free-survival rate for DK was 10 years.
Kaplan–Meier curve about DFS estimate shows that, of all patients, 88.0% did not present DK on the 4th year and neither did 64.1% on the 8th year (Fig. 1).
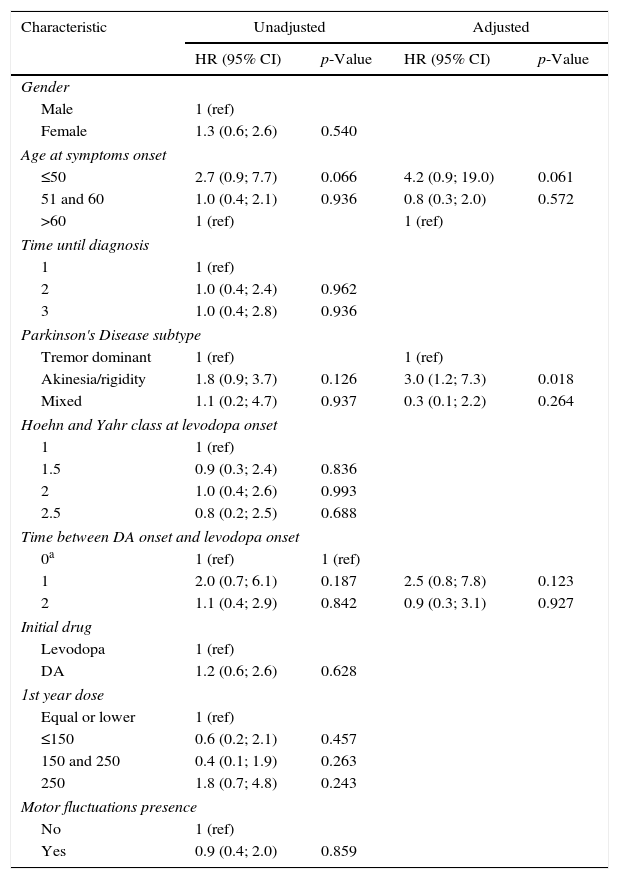
As presented in Table 2, multivariate Cox regression analysis was performed on the age group, subtype of PD and time between DA onset and levodopa onset, as they were statistically significant (p<0.20) in the univariate analysis.
Factors associated with DFS: unadjusted and adjusted HR.
| Characteristic | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | ||||
| Male | 1 (ref) | |||
| Female | 1.3 (0.6; 2.6) | 0.540 | ||
| Age at symptoms onset | ||||
| ≤50 | 2.7 (0.9; 7.7) | 0.066 | 4.2 (0.9; 19.0) | 0.061 |
| 51 and 60 | 1.0 (0.4; 2.1) | 0.936 | 0.8 (0.3; 2.0) | 0.572 |
| >60 | 1 (ref) | 1 (ref) | ||
| Time until diagnosis | ||||
| 1 | 1 (ref) | |||
| 2 | 1.0 (0.4; 2.4) | 0.962 | ||
| 3 | 1.0 (0.4; 2.8) | 0.936 | ||
| Parkinson's Disease subtype | ||||
| Tremor dominant | 1 (ref) | 1 (ref) | ||
| Akinesia/rigidity | 1.8 (0.9; 3.7) | 0.126 | 3.0 (1.2; 7.3) | 0.018 |
| Mixed | 1.1 (0.2; 4.7) | 0.937 | 0.3 (0.1; 2.2) | 0.264 |
| Hoehn and Yahr class at levodopa onset | ||||
| 1 | 1 (ref) | |||
| 1.5 | 0.9 (0.3; 2.4) | 0.836 | ||
| 2 | 1.0 (0.4; 2.6) | 0.993 | ||
| 2.5 | 0.8 (0.2; 2.5) | 0.688 | ||
| Time between DA onset and levodopa onset | ||||
| 0a | 1 (ref) | 1 (ref) | ||
| 1 | 2.0 (0.7; 6.1) | 0.187 | 2.5 (0.8; 7.8) | 0.123 |
| 2 | 1.1 (0.4; 2.9) | 0.842 | 0.9 (0.3; 3.1) | 0.927 |
| Initial drug | ||||
| Levodopa | 1 (ref) | |||
| DA | 1.2 (0.6; 2.6) | 0.628 | ||
| 1st year dose | ||||
| Equal or lower | 1 (ref) | |||
| ≤150 | 0.6 (0.2; 2.1) | 0.457 | ||
| 150 and 250 | 0.4 (0.1; 1.9) | 0.263 | ||
| 250 | 1.8 (0.7; 4.8) | 0.243 | ||
| Motor fluctuations presence | ||||
| No | 1 (ref) | |||
| Yes | 0.9 (0.4; 2.0) | 0.859 | ||
DKS, Dyskinesias-free survival; HR, hazard ratio; CI, confidence interval; DA, dopaminergic agonists.
Subtype was an independent predictor for onset of DK in PD patients. AR subtype had a statistically significant higher risk for DK onset when compared to TD subtype, after adjusting for all other factors (HR=3.0; 95% CI: 1.2; 7.3). On the other hand, age of onset, time between DA and levodopa onset, did not remained statistically significant.
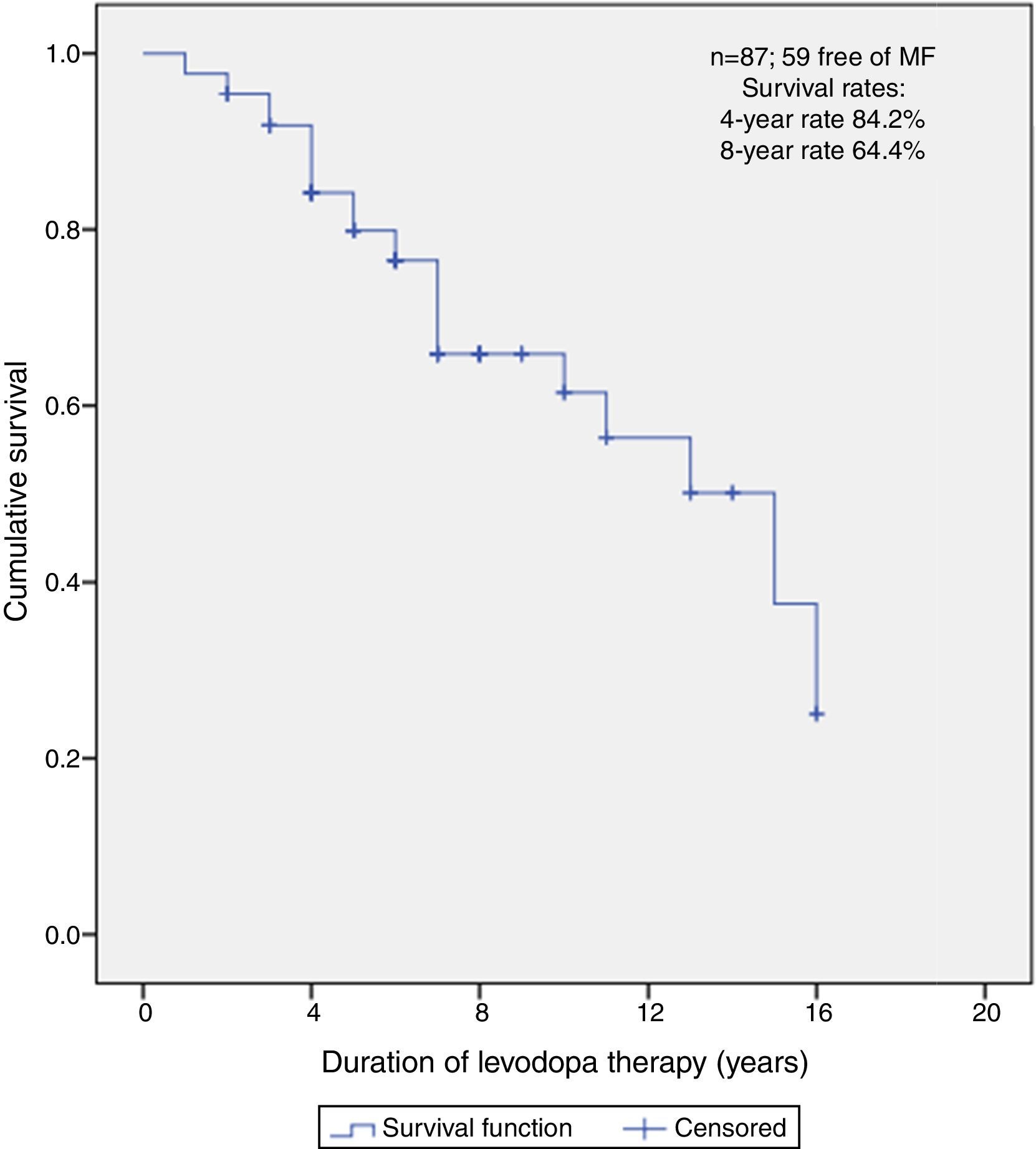
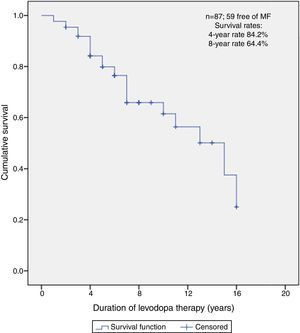
Predictors of MFFSAfter a median follow-up of 5 years (range 1–16), 59 (67.8%) patients did not present MF at the time of this report. The median free-survival rate for MF was 15 years.
Kaplan–Meier curve about MFFS estimate shows that, of all patients, 84.2% did not present MF on the 4th year and neither did 64.4% on the 8th year (Fig. 2).
As presented in Table 3, multivariate Cox regression was performed on the age group, subtype of PD and H&Y class at levodopa onset, as they were found to be statistically significant (p<0.20) in the univariate analysis.
Factors associated with MFFS: unadjusted and adjusted HR.
| Characteristic | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | ||||
| Male | 1 (ref) | |||
| Female | 1 (0.4;2.1) | 0.914 | ||
| Age of symptoms onset | ||||
| ≤50 | 3.6 (1.3;10.0) | 0.013 | 3.6 (1.1; 11.8) | 0.033 |
| 51 and 60 | 0.7 (0.3; 1.7) | 0.465 | 0.7 (0.3; 1.7) | 0.405 |
| >60 | 1 (ref) | 1 (ref) | ||
| Time until diagnosis | ||||
| 1 | 1 (ref) | |||
| 2 | 0.9 (0.4; 2.3) | 0.990 | ||
| 3 | 1.0 (0.4; 2.8) | 1.005 | ||
| Parkinson's Disease subtype | ||||
| Tremor dominant | 1 (ref) | 1 (ref) | ||
| Akinesia/rigidity | 2.4 (1.1; 5.4) | 0.034 | 2.4 (1.0; 5.7) | 0.039 |
| Mixed | 3.4 (1.0; 11.1) | 0.046 | 1.8 (0.4; 7.1) | 0.429 |
| Hoehn and Yahr class at levodopa onset | ||||
| 1 | 1 (ref) | 1 (ref) | ||
| 1.5 | 0.5 (0.2; 1.4) | 0.162 | 0.5 (0.2; 1.5) | 0.203 |
| 2 | 0.9 (0.3; 2.3) | 0.809 | 0.7 (0.2; 1.8) | 0.426 |
| 2.5 | 0.7 (0.2; 2.3) | 0.578 | 0.6 (0.2: 1.9) | 0.367 |
| Time between DA onset and levodopa onset | ||||
| 0a | 1 (ref) | |||
| 1 | 1.5 (0.5; 4.8) | 0.481 | ||
| 2 | 0.9 (0.3; 2.5) | 0.797 | ||
| Initial drug | ||||
| Levodopa | 1 (ref) | |||
| DA | 1.4 (0.6; 3.0) | 0.453 | ||
| 1st year dose | ||||
| Equal or lower | 1 (ref) | |||
| ≤150 | 1.6 (0.6; 4.2) | 0.320 | ||
| 150 and 250 | 1.1 (0.3; 3.7) | 0.929 | ||
| 250 | 1.0 (0.3; 3.5) | 0.983 | ||
| Dyskinesias presence | ||||
| No | 1 (ref) | |||
| Yes | 1.2 (0.5; 2.6) | 0.657 | ||
MFFS, motor fluctuations-free survival; HR, hazard ratio; CI, confidence interval; DA, dopaminergic agonist.
After multivariate analysis, the age group ≤50 years was associated with worse free survival, presenting an increased HR of MF (HR=3.6; 95% CI: 1.3; 10.0). Also, AR subtype had a statistically significant higher risk of MF development when compared to TD subtype, after adjusting for all other factors (HR=2.4; 95% CI: 1.0; 5.7). H&Y class did not remain statistically significant.
DiscussionSeveral observational retrospective studies were conducted aiming to find risk factors associated with the development of levodopa induced MC.18–20
In the present study, DK development was correlated with AR subtype at the onset of PD symptoms. AR subtype was also related with a higher risk of developing MF. Another factor found to be independently related with MF was age at symptoms onset: patients with ≤50 years old were more prone to develop MF.
Comparing present results to previous retrospective studies, similar rates of MC were reported. In the present sample, 51.7% of the patients developed MC, comparing to 62% in another study.21 Regarding studied MC, 35.6% patients developed DK and 32.2% MF; in previous studies DK rates ranged from 28% to 30.1% and MF rates from 40% to 46.3%.19,20
Concerning Kaplan–Meier analysis, 12% of patients presented DK after 4 years of levodopa therapy and 35.9% after 8 years. MF were present in 15.8% of patients after 4 years and in 35.6% after 8 years. In a cross-sectional study, DK were present in 13% of the patients with 5 or less years of treatment duration, and in 36% after 6 to 9 years. MF were present in 21% of patients with 5 or less years and in 56% after 6–9 years.20 Except for MF at 8-years, similar rates were described, showing that our population's MC characteristics can be comparable to others.
Previous retrospective studies reported that initial TD subtype was associated with lower probability of DK, independently of other risk factors.22–24 A clinical review concluded that recognition of the phenotype is important for PD managing since patients with TD subtype are associated with a more benign disease course and fewer MF.25 A possible explanation for these results was suggested by imaging studies that correlated AR subtype with a higher clinical progression and dopaminergic loss in putamen.26 Although a cross-sectional study reported that AR subtype was more common in patients with MF than with DK,20 our study found AR subtype to be independently correlated in MF and DK development.
Average age at symptoms onset was 60.76 (Standard Deviation (SD) 10.0) years, comparable with mean age in other studies: 63.8 (SD 8.5) and 64.5 (SD 7.3) years.20,27 Younger ages at PD symptoms onset were demonstrated to influence MF development in a previous cross-sectional, retrospective study.27 No relation with DK occurrence was found in the referred studies.20,27 Similarly, in other studies, including a survival community-based cohort, younger age was not found to be an independent risk factor for DK.18,28 Analogous results were obtained in our study: younger age of onset was associated with development of MF, but not DK. Although in some studies DK were influenced by age of symptoms onset,19,24 it has been suggested that MF and DK have a different pathophysiology.29
Some studies reported that DK was more common in females.18,19 Though, in previous studies and in ours, gender does not appear to influence development of MC.20,24,27
Time between symptoms onset and diagnosis did not appear to be associated with development of DK or MF. This variable was not studied in others studies of our knowledge. Given that most patients start treatment at the moment of diagnosis, there are similar results in other studies concerning levodopa onset.19,20
H&Y class at levodopa onset had a median of 2 (range 1–4), and no relationship was found concerning MC development. In a previous study relating H&Y class and MC onset, it was described that earlier MC occurred when levodopa was introduced in H&Y class 3. Yet, in the mentioned study, patients were drug-free before levodopa onset, unlike our study.30
In patients who started DA before levodopa, time between them was related neither with MF nor DK. Concerning first pharmacological choice, other studies support our finding that there is no association between patients who start with DA or with levodopa.20 Although some clinical-trials found it statistically significant,21 a recent clinic-pathological study concluded that delaying levodopa onset in order to avoid levodopa is unnecessary; MC were associated with disease progression, not levodopa.31 Also, a placebo-controlled trial concluded that, in a clinical perspective, levodopa therapy, in early-onset PD patients, does not accelerate PD progression.32 It is interesting to note that some studies, trying to find differences between starting medical therapy with a specific DA or with levodopa, suggest that development of MC is similar in both groups at long term.33,34 Mortality in patients who start levodopa in early stages of PD symptoms onset was found to be similar to those who start it in later stages.35 It was suggested that levodopa therapy would reduce mortality, possibly by improving motor symptoms in later PD stages.36 A major advantage on using levodopa is the fact that it does not cause cognitive impairment as DA does, so it can be used in older patients.12
We tried to evaluate if 1st year dose evolution influenced the development of MC but there were no statistically significant results. A hospital-based study concluded that initial levodopa dose was higher in patients with MC.19 Information in other studies about levodopa dose evolution in the 1st year was not found.
Presence of MF did not change DK appearing and neither the reverse. In other studies, the majority of patients with MF developed DK or there was a relationship between them.19,21 It was suggested that DK would predict MF appearing37 but that is yet to be determined.
Measures can be taken to manage MC. A huge matter of debate is whether the use of DA has a protective effect on MC or not. Studies suggest that the delay on MC when using DA as first medical approach is due to the delay in levodopa introduction and not a neuroprotective process.34,38 However, after longer follow-ups, there are contradictory findings about the existence of a “catch-up” mechanism or a real MC delay.33,34,39 It seems to be a consensus that using the lowest levodopa dose necessary for good clinical control minimizes both DK and MF.40
These comparisons are restricted because of the statistical method used in the present study: we are not aware of other studies with the similar analysis method and objective. A survival analysis model was considered the best option since participants in our study had a different follow-up time and at the end of follow-up not all participants developed MC; it has the advantage of retrieving information about those patients too.41 Different results between studies may also occur because of different treatment regiments or different population characteristics.
Although ideal to chronic diseases like PD, the present study has some limitations related to its retrospective observational nature. A major limitation was data collection: many patients were excluded by lack of data and, although trustable, the only information we had access was the written one. Due to undefined guidelines, confounding/treatment-by-indication bias may be present, for example the tendency to start with DA, not levodopa, in younger patients, which is also a variable related with the outcome. We tried to control confounding factors by using multivariable analysis. Other disadvantage is the absence of a randomized sample which would confer more external validity to the study. As there was no direct control, we also have to consider the possibility of low adherence to medication in these patients.42
Regarding advantages when comparing to other studies, recording-bias is not in question, making data more accurate. Also, patients’ information was written in clinical records by 2 movement disorders neurologists and therapeutic adjustments in drugs were made only by them. Besides, since our study is a non-interventional observational study, a more natural and realistic approach to day-to-day PD and its’ MC was made. Finally, follow-up length (16–17 years) allows for more precise information.
In conclusion, our results suggest that patients’ disease initial characteristics, such as subtype or age of PD onset, are independently associated with the development of MC, rather than the first drug used in therapy.
Conflicts of interestThis study was not funded and conflicts of interest are inexistent.
I thank Carolina Garrett, Joselina Barbosa and Maria José Rosas who provided insight and expertise that greatly assisted the research.