In addition to the known lipid-lowering effects, statins are now widely accepted to have anti-inflammatory and immunomodulatory effects. Adjunctive use of statins has proven beneficial in the context of a wide range of inflammatory diseases, including rheumatoid arthritis. Evidence also suggests that statins may also have utility in the management of uveitis, a form of sight threatening inflammation which occurs in the eye. In this article, we outline our rationale behind a clinical trial of simvastatin as a steroid-sparing agent in uveitis, to which patient recruitment started last year. Potential risks associated with the clinical use of statins, including putative effects on the eyes, are discussed.
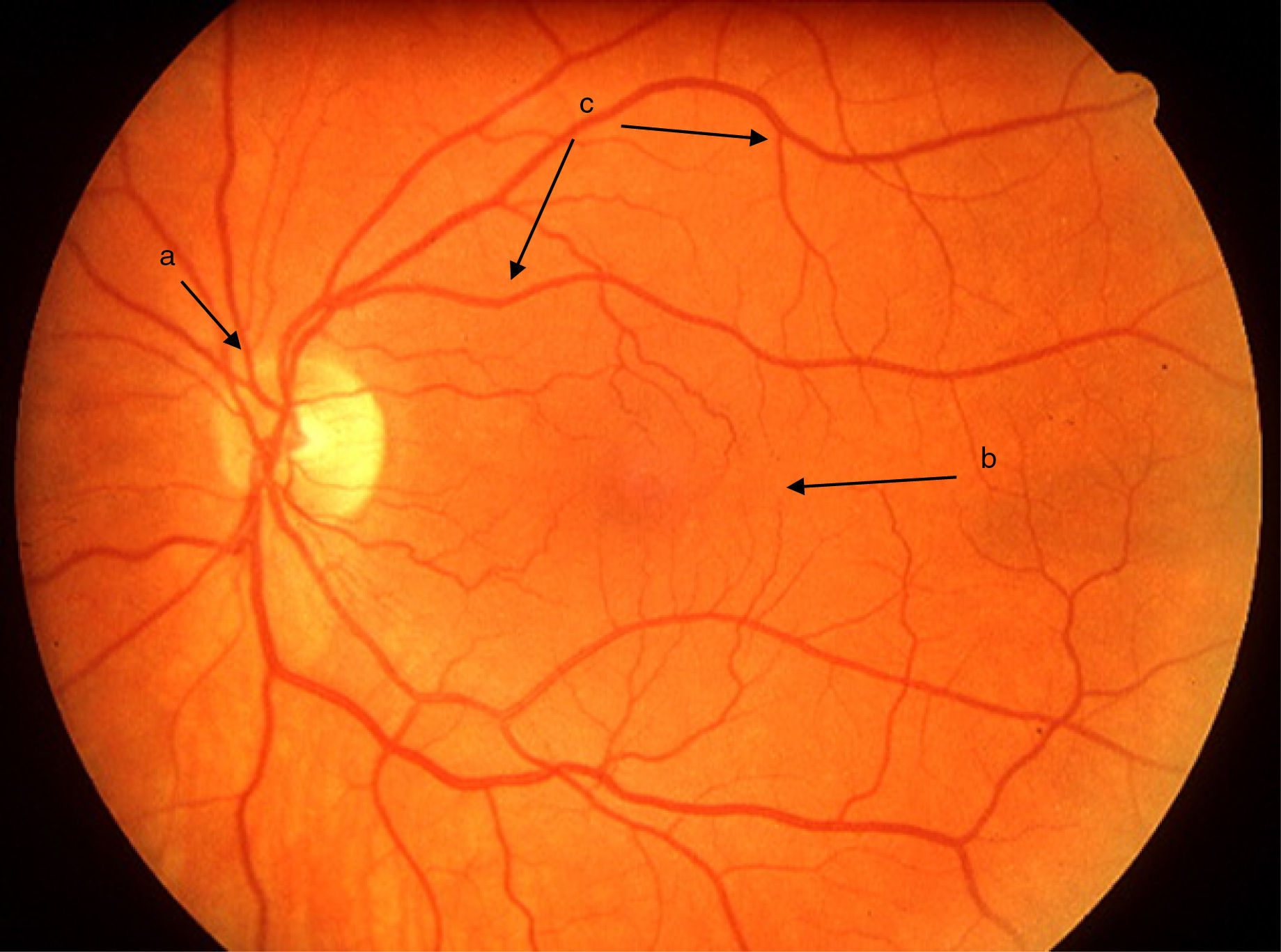
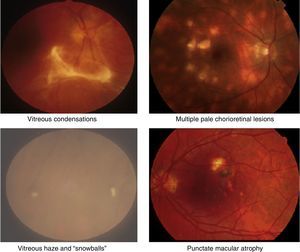
Uveitis could be “the most important eye disease you’ve never heard of”. It is a significant but largely unrecognised cause of permanent visual impairment worldwide. Uveitis denotes inflammation of the uvea, which is the highly vascular pigmented middle layer of the eye, between the sclera and the retina. The uveal tract is composed of the iris and ciliary body, anteriorly, and the choroid, posteriorly.1 However, the term “uveitis” has now become almost synonymous in ophthalmic clinical practice with any inflammation involving structures inside the eye, including vitreous gel and the retina (Figs. 1 and 2).
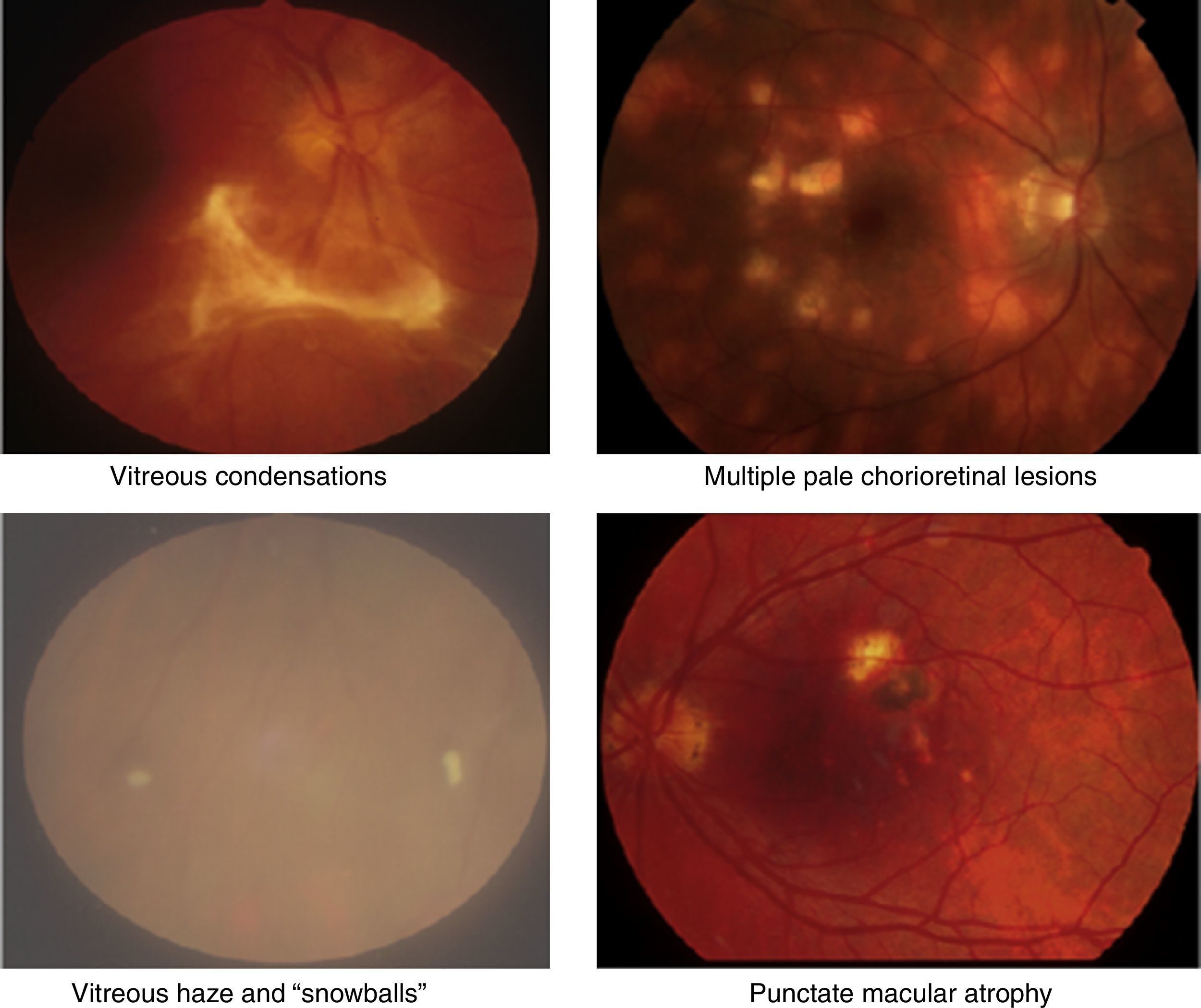
Clinical retinal photography images illustrating how sight-threatening noninfectious uveitis might manifest in the eye. Inflammation appears as pale white/yellow chorio-retinal spots and/or haziness with “floaters” in the vitreous gel, which fills the posterior chamber of the eye. Inflammation may lead to visual loss and blindness by causing pale, atrophic scarring of the retina, with exposure of the underlying black retinal pigment epithelium.
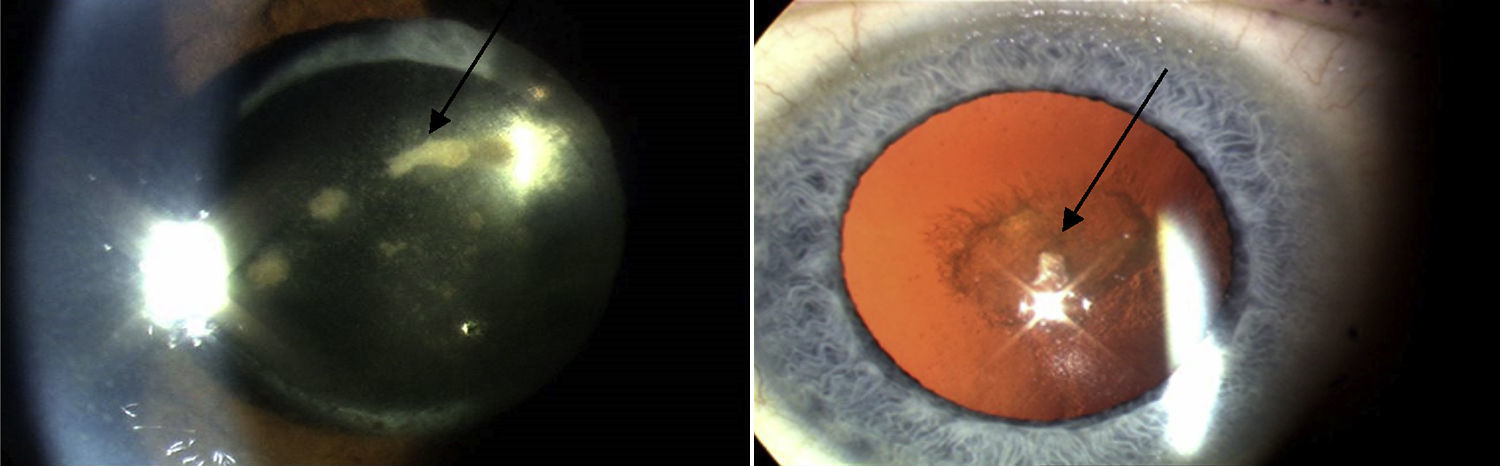
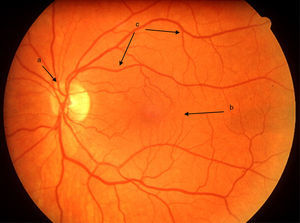
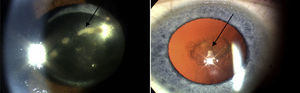
In the Western world, the current incidences of uveitis vary between 38 and 200 per 100,000.2 Many cases are chronic, and lead to numerous sight-threatening complications, including decompensation/degeneration of the cornea (the clear anterior window of the eye); cataract (opacity of the crystalline lens of the eye); raised intraocular pressure; glaucoma (optic neuropathy with a characteristic peripheral visual defect); and retinal pathology (such as macular oedema and retinal detachment), which affects retinal function (Fig. 3).
Around 10–15% of all causes of vision loss and 20% of cases legally recognised as “blindness” are attributed to uveitis.3 It can affect people of all ages but occurs most frequently in the working age population (20–50 years), where the socio-economic impact of visual impairment is thought to be comparable to diabetic retinopathy.4 Visual loss in uveitis has been historically underestimated due of the lack of data concerning the incidence of sight threatening complications. A recent study conducted by our group on the long-term clinical outcomes of patients attending a tertiary centre uveitis clinic indicates that the incidence of visual impairment in these patients is 19%.5
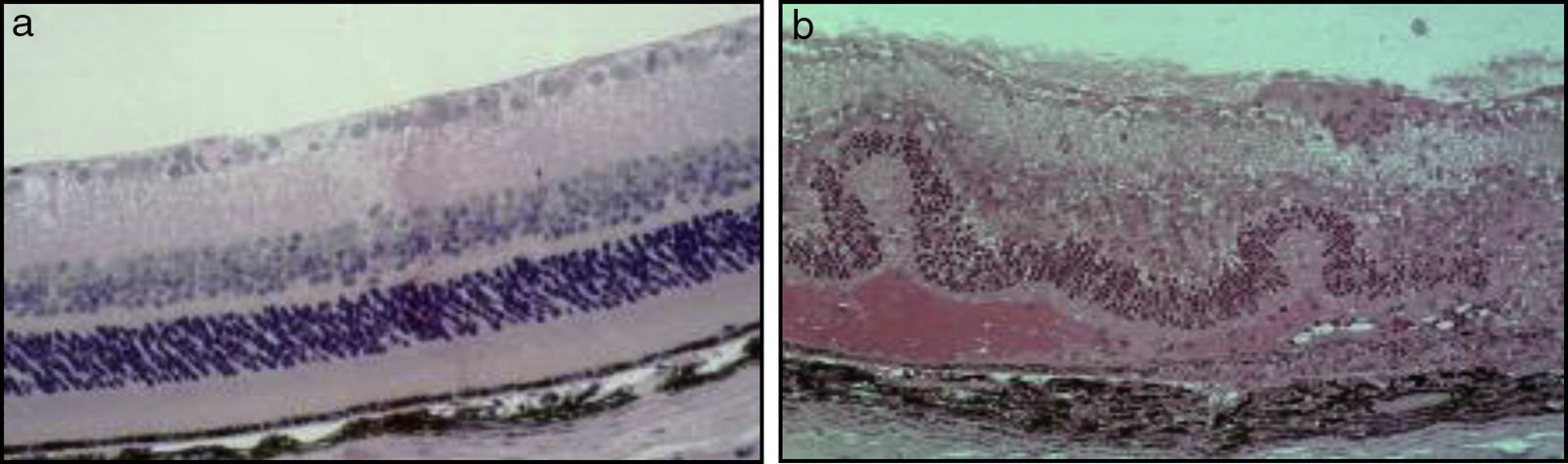
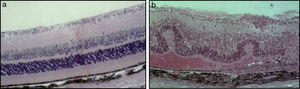
In the majority of cases, the aetiology of inflammation in uveitis is non-infectious and idiopathic. Non-infectious uveitis has been classically described as an autoimmune disease, mediated by Th1 and Th17 subsets of self-reactive CD4 T-lymphocytes, which secrete “signature” pro-inflammatory cytokines, specifically, interferon (IFN)-¿ and interleukin (IL)-17. Our understanding of the pathogenesis of non-infectious uveitis has been facilitated by a mouse model of experimental autoimmune uveitis (EAU), in which a soluble retinal antigen and interphotoreceptor retinoid-binding protein (IRBP) are injected to create an immune response (Fig. 4).6
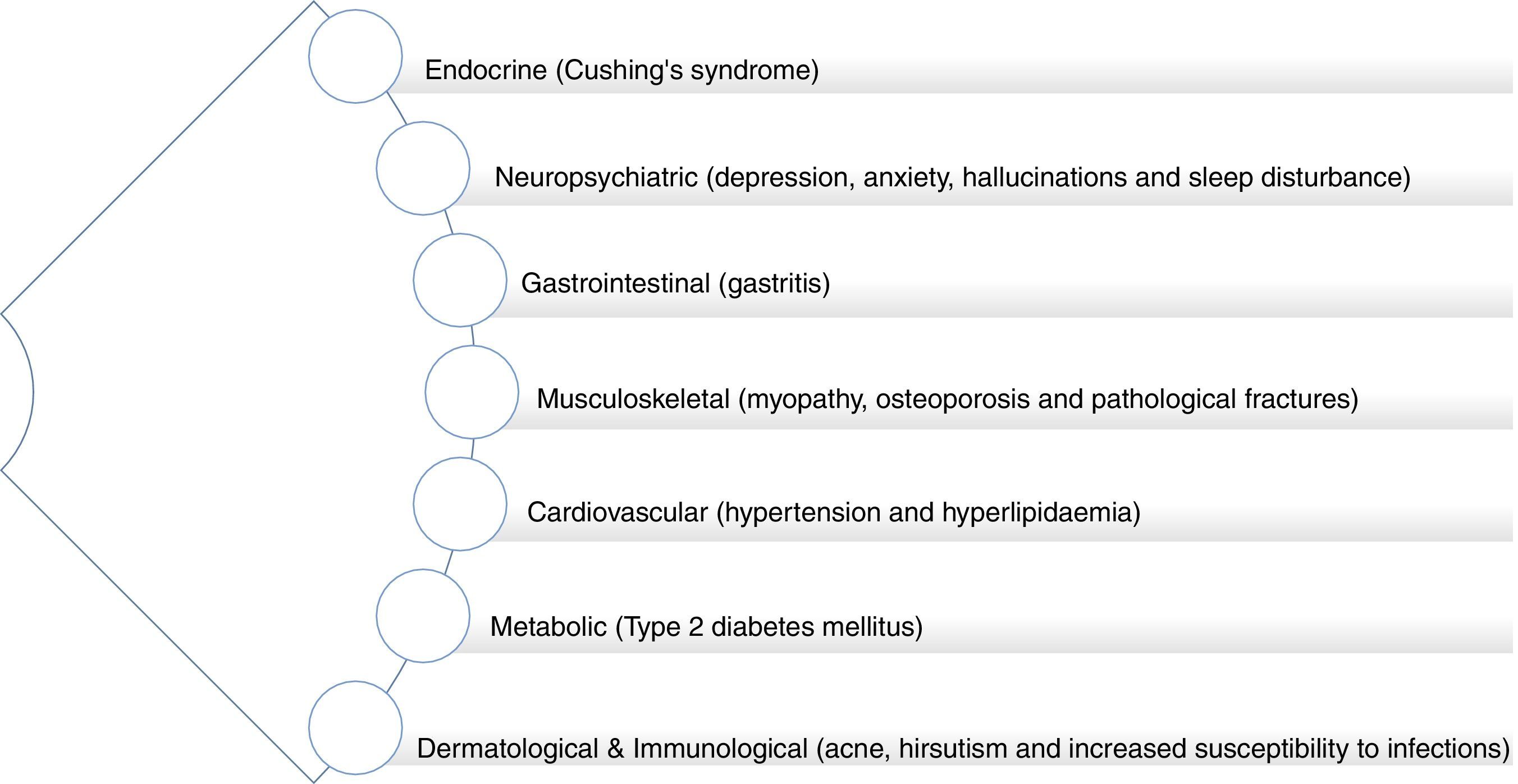
As with other forms of autoimmune inflammatory disease, the mainstay treatment of non-infectious uveitis is high dose corticosteroids with additional second-line immunosuppressive agents (such as mycophenolate, methotrexate, azathioprine or cyclosporine A), as required to reduce the corticosteroid doses and associated side effects. Corticosteroids and other immune-modulating treatments are not curative but rather suppress the immune system, thereby reducing the ocular tissue damage and detrimental consequences on vision, which result from immune system hyperactivity. The ocular side effects of corticosteroids include raised intraocular pressure, glaucoma and cataracts. In addition to the risk of infection associated with immunosuppression, given systemically, these drugs together have significant side effects which include hypertension, diabetes, liver dysfunction, osteoporosis and potential malignancy. In other chronic immune-related inflammatory conditions, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), it is known that there is an associated increased risk of premature atherogenesis and cardiovascular disease (CVD).7,8 Furthermore, prescription corticosteroid use, itself, is associated with increased long-term CVD risk (Fig. 5).9
The burden of the current immunosuppressive drugs (costs, side effects and monitoring), plus the often relapsing and remitting course of inflammatory disease, are a major problem for patients, clinicians and ophthalmic services in the Western world. As we continue to learn more about the different mechanisms and biological pathways leading to ocular inflammation, newer biological anti-inflammatory therapeutic approaches are being developed and tested.10 However, these therapies are expensive and the long-term safety and efficacy are not known. An alternative approach is drug re-positioning, that is, application of known drugs and compounds to treat new indications.
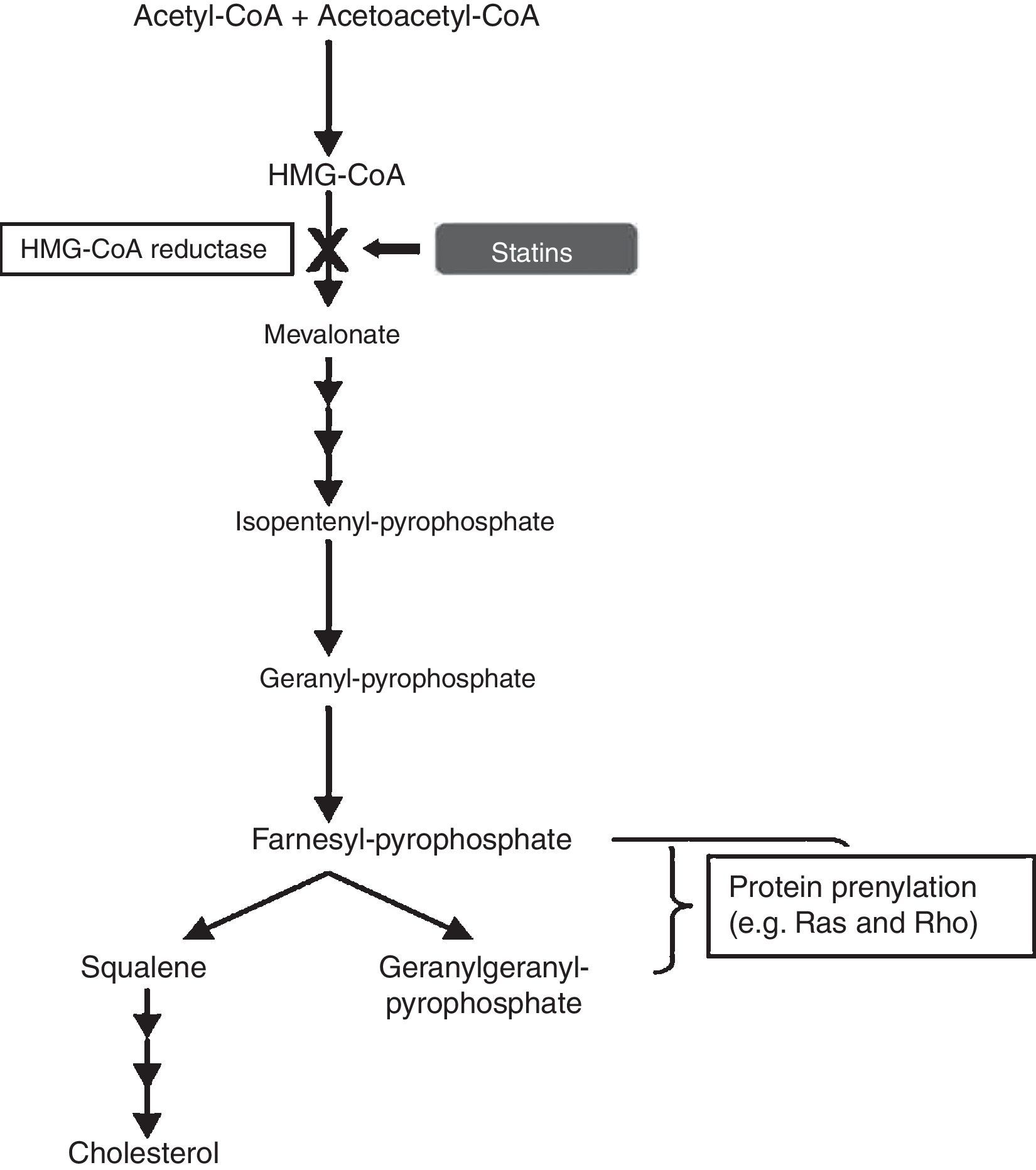
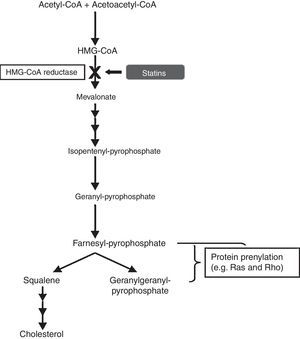
Statins as anti-inflammatory agentsStatins have been used for decades as a therapy for hypercholesterolemia, in order to reduce the risk of developing atherosclerosis and cardiovascular disease. They are pharmacological inhibitors of the conversion of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), into L-mevalonate, the rate-limiting step in cholesterol synthesis, by competitive blocking of the active site of the enzyme HMG-CoA reductase. Blockade of the mevalonate pathway thereby affects cholesterol production and, ultimately, reduces serum low-density lipoprotein (LDL) cholesterol levels, which are associated with increased risk of cardiovascular disease (Fig. 6).
In the cholesterol synthesis pathway, mevalonate is the precursor not only for cholesterol, but also for many non-steroidal isoprenoid compounds. The mevalonate pathway plays a vital role in various cellular functions, including cell signalling, cell differentiation and proliferation, myelination, cytoskeleton dynamics, and endocytotic/exocytotic transport.11 There is compelling evidence that statins have pleiotropic effects, independent of cholesterol lowering, and this is due to their role in induction of post-transcriptional modifications of important isoprenoid intermediates downstream of mevalonate.12 This process is known as “prenylation” and affects numerous signal transduction molecules in inflammatory, as well as vascular and myocardial pathways. The small guanine-triphosphate (GTP)-binding proteins, which include Rho, Rac and Ras, are an important group of proteins involved in the intracellular signalling pathways modulated by statins. These small GTP proteins regulate pro-atherogenic, pro-inflammatory pathways and are activated by isoprenylation.13 Rho proteins, in particular, are involved in the expression of pro-inflammatory cytokines. A study proposed that simvastatin reduced the activity of RhoA, which is involved in tumour necrosis factor (TNF)-¿-induction and the activation of nuclear factor (NF)-¿B and cytokine secretion.14
Early evidence of the anti-inflammatory effects of statins derives from studies on patients with CVD. An important study that initially addressed this was the PRINCE (pravastatin inflammation/CRP evaluation) randomised controlled trial (RCT), where statin use resulted in reduction of C-reactive protein (CRP) levels in these patients.15 CRP is a systemic inflammatory marker, produced by the liver under the direct influence of IL-6. This effect on CRP by statins was shown to be independent of the cholesterol lowering effect.16,17 Clinical studies have also demonstrated the anti-inflammatory effects of statins in RA. In the randomised controlled Trial of Atorvastatin in Rheumatoid Arthritis (TARA), statins had significant effect on reducing disease activity. The authors noted that the effect of atorvastatin could prove beneficial in the context of disease-modifying therapy combination design, in which statins offer both vascular protective and adjunctive immunomodulatory potential.18 Nagashima et al. showed that statins induced potent inhibition via protein geranylgeranylation (another form of prenylation) of pro-inflammatory cytokine production (TNF-¿, IL-1¿, IL-6 and IL-8) by rheumatoid synovial cells.19 Further studies have demonstrated the anti-inflammatory effect of statins in other clinical contexts. Statins decrease the recruitment of monocytes and T-cells into the arterial wall and inhibit T-cell activation and proliferation in vitro, leading to the belief that the immunomodulatory effects of statins may be of help in recipients of organ transplantation.20 A landmark study then showed that statins decreased rejection rate and prolonged survival of cardiac transplant patients.21 Simvastatin was also shown to have a positive effect on brain atrophy rate in secondary progressive multiple sclerosis. An RCT in which patients had 80mg simvastatin in addition to disease modifying agents, showed 43% reduction in the annual rate of brain atrophy.22 However, other studies conducted on early stage multiple sclerosis using simvastatin or atorvastatin showed no effect on relapse rate or MRI appearance.23,24
A study from our ocular immunology group has shown that statins can synergise with conventional immunosuppressive agents to modulate T-cell proliferation and pro-inflammatory cytokine production.25 Other specific effects of statins on the immune system include reduction in antigen presentation via inhibition of IFN-¿, which is necessary for the induction of major histocompatibility complex (MHC) class II expression, and inhibition of T-cell differentiation into the pro-inflammatory Th1 sub-type (again via protein geranylgeranylation).26–28 One of the key issues underlying the pathogenesis of autoimmune disease is the process by which systemic immune cells “home” to target organ sites of inflammation. The mechanism for this involves leucocyte migration, adhesion to vascular endothelial cells and the expression of self-adhesion molecules. The integrin lymphocyte function associated antigen-1, which binds to intercellular adhesion molecule-1 (ICAM-1) is altered by statins, leading to inhibition of lymphocytes adhesion and activation.29 Reduction in leucocyte infiltration of the eye is a consistent finding in autoimmune uveitis treated with statins.12
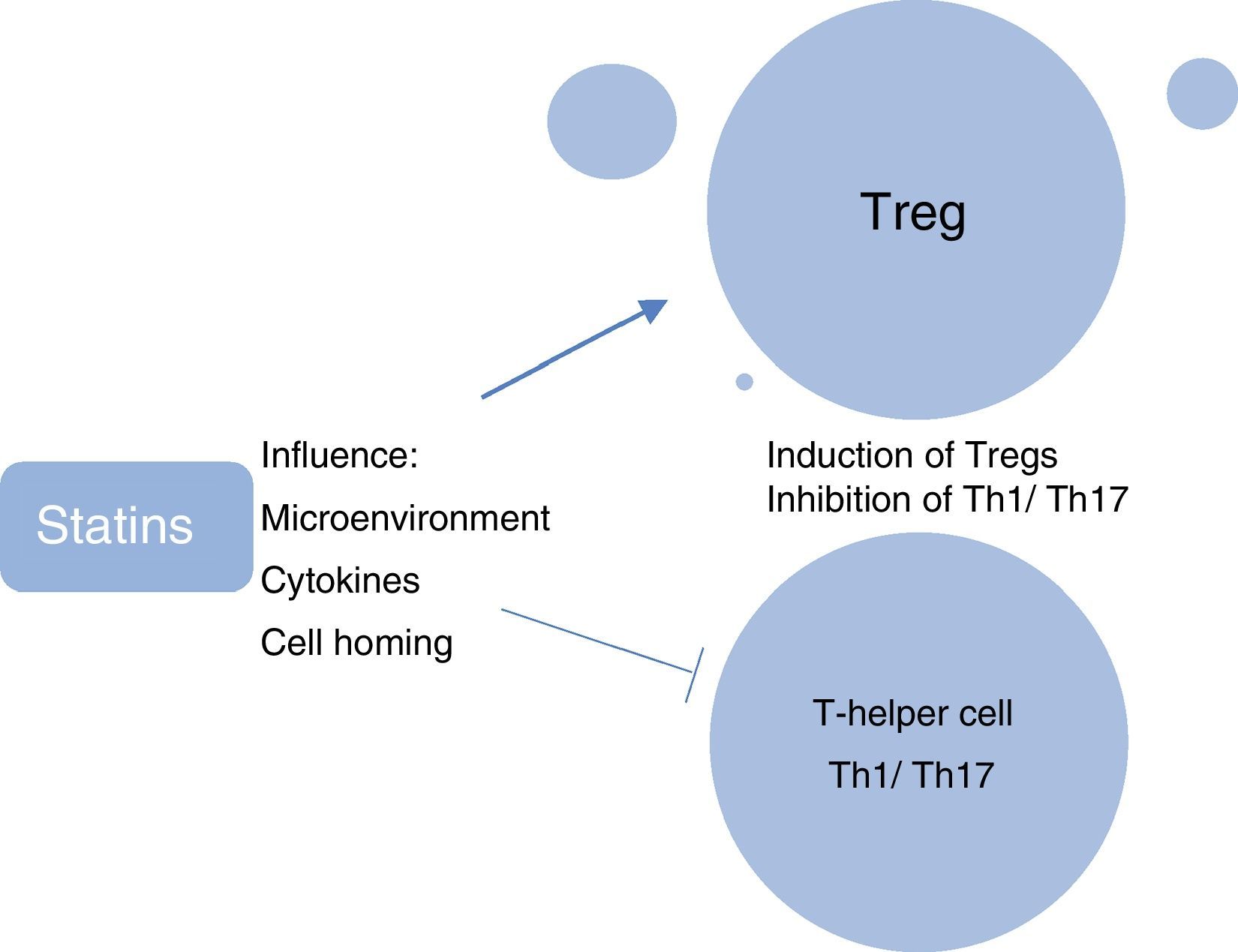
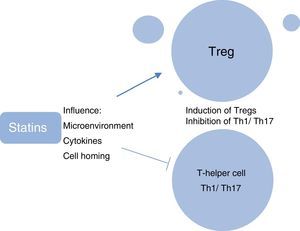
A further research focus in ocular immunology relates to the investigation of the role of systemic phenotypically categorised CD4+CD25+FoxP3+ T-regulatory cells (Tregs) in the reduction in ocular inflammation and induction of clinical remission of uveitis. Tregs are considered to be the “guardians of peripheral tolerance” and their role in the prevention of autoimmune diseases such as SLE and RA is well recognised. In these diseases it appears that there has been a failure of Tregs to keep pace with the activation of effector cells, resulting in an inability to resist ensuing inflammation.30 In patients with active uveitis compared with healthy controls, decreased peripheral blood levels of Tregs have been found, with levels being significantly upregulated during disease remission.31 We have shown recently that in ocular Beçhets, a systemic inflammatory disease, Treg levels are increased by treatment with the immunomodulating therapy, pegylated interferon-¿ (pegIFN-¿), and the increased levels of Tregs are still detectable at 12 months, 6 months after cessation of therapy.32 In animal models, the immunosuppressive effect of simvastatin has been attributed partially to its ability to promote the generation of T-regs.33 Studies have elucidated the myriad ways in which statins modulate Treg biology, which include influencing the microenvironment required for the induction and maintenance of Tregs; directly enhancing the induction of Tregs; influencing the migration of Tregs and homing of Tregs to inflamed tissues; and inhibiting the induction of pro-inflammatory Th1 and Th17 T-cell subtypes (Fig. 7).34
Rationale for a clinical trial of simvastatin in uveitis patientsOur group and others have reported on the pleiotropic effect of simvastatin, highlighting its anti-inflammatory effect and potential use as a steroid-sparing agent. Patients with uveitis are often required to adhere to steroid therapy for a long periods of time. It is, of course, preferable for these corticosteroids to at the lowest effective dose necessary to keep the inflammatory drive under control. However, high initial loading doses of corticosteroids are often the best option for rapid control of inflammation in the case of a uveitic relapse. Inflammatory flare-ups are inevitable in most forms of non-infectious autoimmune uveitis, which tend to follow a relapsing and remitting disease course.
As previously mentioned, corticosteroids are associated with a wide array of adverse effects including hypercholesterolemia and atherosclerosis. As a result, patients are at risk of having ischaemic heart disease or stroke in the long-term, even if they tolerate systemic steroid therapy in the short-term. Statins provide benefit to those patients at risk by reducing high cholesterol levels and may confer additional anti-inflammatory effects. A retrospective population based case control-study showed that the likelihood (odds) of developing uveitis among statin users were half than that of patients not taking statins.35 Not all statins have the same activity in modulating the inflammation, for example atorvastatin appears less efficacious in retinal inflammatory disease while lovastatin demonstrated a better effect.36 Our group has shown that simvastatin, in particular, has a unique ability to suppress the activity of inflammatory cells.25
The blood-brain barrier and blood-ocular barrier demonstrate similar transport and permeation characteristics.37 A study compared the ability of three statins (simvastatin, lovastatin and pravastatin) in their ability to cross the blood brain barrier and to alter gene expression in mice.38 Simvastatin, which is a lipophilic compound was able to cross the blood brain barrier, and was found in cerebral cortex of mice in vivo. Furthermore, simvastatin showed greatest effect on gene expression when compared to lovastatin and pravastatin. Simvastatin was able to alter 23 additional genes involved in cell growth, signalling and trafficking. Of particular importance was the expression of genes involved in apoptotic pathways, which could be a fundamental contributor to immunosuppression.38 In the EAU mouse model, statins were shown to suppress leucocytes infiltration to the retina and decrease vascular leakage.36 It is thought simvastatin might be able to exert a similar local immunomodulatory effect on ocular tissue in humans.
With this in mind, we are currently conducting the first RCT to assess such effects in patients with uveitis: “Can Simvastatin Significantly Reduce the Amount of Immunosuppressive Medication Required by Patients With Sight Threatening Uveitis? A Phase 2b, Single Site, Randomised, Placebo Controlled, Double Blinded Trial” (ID NCT02252328, http://www.clinicaltrials.gov). The primary outcome for this RCT is the change in dose (mg) of prednisolone after 12 months of treatment. The reduction in the corticosteroid (prednisolone) dose will be a surrogate measure for the control of inflammation. The efficacy of statins as anti-inflammatory agents will be assessed by measuring the reduction of oral steroid dose required by uveitis patients, with concomitant use of simvastatin. Patient recruitment to this trial is currently ongoing.
Potential risks associated with clinical use of statinsStatins are generally well-tolerated and safe and, moreover, are considered among the safest drugs used in medical practice.39 This is supported by long-term follow-up studies of randomised clinical trials in the management of hypercholesterolaemia, ranging from 10 to 15 years.40,41 The risk for mortality or permanent organ damage associated with statins is almost entirely related to rhabdomyolysis, that is, drug-induced damage to muscle tissue that leads elevation of creatine kinase (CK), myoglobin protein release into the bloodstream and potential renal failure. RCT-reported case fatality rate from hospitalized rhabdomyolysis was about 1 in 10.5.42 Thus mortality caused by statin treatment is rare. Statins cause myopathy, as well as rhabdomyolysis. Myopathy is defined as diffuse muscle symptoms (pain, tenderness, weakness) with elevated CK, sufficient to consult a physician but insufficient to warrant hospital admission. It is probable that myopathy and rhabdomyolysis constitute a spectrum of the same muscle disorder, rhabdomyolysis being the more severe, characterised by a higher CK level and myoglobinuria.43 It is common for patients who are started on statins to develop symptoms of muscle soreness, however, in many cases this resolves spontaneously. Due to the aforementioned risk of serious statin-induced muscle damage and mortality, it is imperative to monitor CK levels in these patients. Apart from muscle disease, toxicity attributable to statins, if it occurs at all, is rare. Although statin treatment may result in substantial increases in serum liver enzyme levels (transaminase levels, alanine aminotransferase and/or aspartate aminotransferase, in 0.3–3% of patients in a dose-dependent manner), for this to result in actual liver disease is very rare.39,44 There is currently no conclusive evidence that statins cause renal disease or cognitive decline.44 On the other hand, statins may cause peripheral neuropathy, but the attributable risk is small (12 per 100,000 person-years).42 Of course, the effects of statins as modulators of the immune system may, in theory, have detrimental systemic effects influencing the appearance of neurodegenerative disorders, such as motor neurone disease, infections and cancer development and progression.34 However, in a Danish cohort it was shown that statins significantly reduced mortality related to cancer when compared to individuals who had never used statins.45
Increased incidence of type 2 diabetes mellitus (Type 2 DM) is a relatively newly identified statin adverse effect. The results of a recent network meta-analysis suggested that, statins, as a class, increased the risk of diabetes, particularly with more intensive statin therapy.46 The frequency of Type 2 DM is mainly increased in those with other elements of the metabolic syndrome or prediabetes such as obesity, glucose intolerance, and hypertension. However, it is thought that the benefits from statins, as measured by the number needed to treat for a reduction in myocardial infarction and stroke are much more favourable than the number needed to harm from incident diabetes.39 Statin-induced interstitial lung disease is another recognised side effect of statin therapy.47 The mechanism of lung injury is not defined and it is thought that discontinuing the statin improves, and may completely resolve, symptoms. Furthermore, in a recent study, amongst patients with interstitial lung disease, statin use was associated with reduced all-cause mortality.48
Of particular relevance to the use of statins in patients with uveitis are the potential ocular effects. A previous study has shown a possible relationship between statin therapy and eye muscle/movement disorders, specifically: diplopia, ptosis and ophthalmoplegia.49 A plausible mechanism by which these may occur is myositis of the extraocular muscles, the levator palpebrae superioris (upper eyelid) muscles, or both. The frequency of ocular adverse events among the reported adverse drug reactions from the Food and Drug Administration (FDA) and Adverse Drug Reactions Advisory Committee (ADRAC) data has been recently published.50 Among 131,755 cases of patients taking statins, there were 2325 cases reported ocular adverse events after using statins (1.8%). The most highly reported ocular adverse events associated with statins were blurred vision (48.4%) and visual impairment (25.7%), however results indicated that the ocular problems formed a greater proportion of the adverse events for subjects taking atorvastatin (2.1%).50 The use of statins in Age-Related Eye Diseases Study 2 (AREDS2) trial participants was associated with cataract surgery and progression of both cortical and posterior subscapular type cataracts.51 The risk of these outcomes seemed to be greater for women and patients under 75 years who used statins. The relation between serum lipids, statin use and the macular pigments in the serum and retina, was examined in another study.52 The xanthophyll carotenoids lutein and zeaxanthin are found in and around the macula of the retina, where they are termed macular pigment. Dietary lutein and zeaxanthin are absorbed with fat in the gut and transported on lipoproteins to the retina. Both macular pigment and serum lipoproteins have been related to risk for neurodegenerative diseases, such as age-related macular degeneration (AMD). Macular pigment optical density (MPOD) was not found to be lower in statin users when compared to matched non-statin users, but MPOD decreased significantly with increased duration of statin use.52 However, statin use was not statistically significantly associated with progression to late AMD in the AREDS2 participants, and these findings are consistent with findings in the majority of previous studies.53
ConclusionsThe anti-inflammatory effects and immunomodulatory of statins are now widely accepted. This is supported by RCTs, which have demonstrated that further to their lipid-lowering ability, statins also exert anti-inflammatory effects in a wide variety of disease states. Statins are well tolerated and have a favourable side effect profile compared to other medications. However, further research is required to clarify the impact of statins on ocular disease. An RCT is currently underway to investigate the role of simvastatin as steroid-sparing agents in sight-threatening non-infectious uveitis. This data, in conjunction with experimental studies, should contribute to our understanding of the utility of statins in the management of sight-threatening ocular inflammatory disease.
Conflicts of interestThe authors declare no conflicts of interest.