The purpose of this work was to characterize the Met34Thr variant in a group of patients with nonsyndromic hearing loss, in order to establish a genotype-phenotype correlation.
Methods13 cases from 4 unrelated Portuguese families were selected, in which one or more hearing-impaired members had Met34Thr variant.
ResultsMet34Thr variant was identified in 11/13 cases. Two cases have an additional mutation – Val153Ile and 35delG. Hearing loss was mild in 2 patients (Met34Thr/Val153Ile; Met34Thr/Met34Thr), moderate in 3(Met34Thr/WT; Met34Thr/35delG; Met34Thr/Met34Thr), severe in 2 (2 Met34Thr/WT) and profound in 1 (Met34Thr/WT). Three individuals with Met34Thr had normal hearing thresholds.
ConclusionThe present data corroborate the hypothesis that the Met34Thr variant is associated with mild-to-severe forms of deafness and that this variant seems to segregate with a dominant hearing loss with incomplete penetrance and a variable expression of the phenotype. However, other factors are likely to also have a pathologic effect.
Hearing impairment affects 1 in 1000 newborns, being the most common congenital sensory impairment.1 Mutations in the GJB2 gene – also known as connexin 26 (Cx26) – are responsible for up to 50% of autosomal recessive nonsyndromic hearing loss (NSHL) in European populations.2–4 The most frequent GJB2 mutation in Caucasian populations is c.35delG, the deletion of one guanine within six-guanine string at the position 35, with an average carrier rate of about 2%.4–7
Another variant found in studies in Portuguese NSHL population was 101 T→C (p.Met34Thr), a methionine-to-threonine substitution at amino acid 34.3,8,9 This substitution will be referred in this work as a variant, as its role in NSHL is still controversial. A recent study from our group estimated the Met34Thr and 35delG carrier rates in a Portuguese sample in about 1 in 50, but studies in other Caucasian popu-lations pointed to a higher carrier rate than of Met34Thr compared with 35delG.6,10–12 Nevertheless, in NSHL patients, Met34Thr is found in a lower frequency compared with 35delG.10
The inheritance of the variant p.Met34Thr, as well as its pathogenicity in NSHL has been questioned over the years and is still controversial.
An autosomal dominant and recessive patterns of inheritance and the possibility of Met34Thr being a benign variant have been previously proposed.13–17 Recent studies combining genetic, clinical, biochemical, electrophysiological properties and structural modeling studies contradicted the hypothesis of this variant being non-pathogenic,18,19 by showing that Cx26 Met34Thr protein is correctly synthesized and targeted to the plasma membrane of HeLA cells, but inefficiently forms intercellular channels that display abnormal electrical activity and retain only 11% of the unitary conductance of Cx26 wild-type (WT).18,19 Moreover, these studies suggested that Met34Thr variant channels resides most of the time in a low conductance state.19
The role of Met34Thr is not clear yet, mainly because of the frequent findings of Met34Thr in individuals without NSHL, but recent studies point to a pathogenic nature of this variant.
The purpose of this work is to characterize the Met34Thr variant in a group of patients with NSHL, in order to establish a genotype-phenotype correlation.
Materials and methodsPatientsAll patients were followed in the Clinic of “Hereditary hearing loss” from 2011 till 2014, presenting mild to profound NSHL, assessed by auditory brainstem response tests (ABR) or pure tone audiometry (PTA) and have been studied for GJB2 mutations. To each patient, a complete medical history has been colected to determine the age of onset and deafness evolution, family and patient's past history; causes of acquired deafness, as infections, surgeries, neonatal diseases and trauma, were excluded. None of them reported a relevant history of otological disease or exposure to ototoxic drugs. Syndromic hearing loss was excluded based on physical examination and complementary diagnostic tests, such as temporal bone computer tomography, and when indicated, ear magnetic resonance imaging, renal ecography and/or intravenous urography. Audiological evaluation of NSHL was performed according to GenDeaf study group recommendations.20 Air conduction thresholds were measured for 250, 500, 1000, 2000, 4000 and 8000 frequencies. The degree of NSHL was classified by the PTA applied to the better ear at 500, 1000, 2000 and 4000Hz. Normal hearing is characterized by a PTA<20dB, mild NSHL 20-40dB, moderate NSHL 41-70dB, severe NSHL 71-95dB and NSHL is classified as profound when PTA>95dB.20
To this study, 13 cases were selected from 4 unrelated Portuguese families, in which one or more hearing-impaired members had Met34Thr variant (homozygous, heterozygous or compound heterozygous).
Genetic analysisAfter obtaining informed consent, DNA was extracted from peripheral blood samples, according to standard protocols. DNA samples were amplified by PCR (Polymerase Chain Reaction), using specific primers for GJB2 gene exon 2. The Taq DNA polymerase used was from Thermo Scientific. After purification with AmpureXP® to remove the contaminants, the PCR products were sequenced using the forward primer and analysed on an automated sequencer (ABI 3500, Applied Biosystems®). The reference sequence used to analyse the results was NG_008358.
Large deletions/duplications screening was done by MLPA (Multiplex Ligation-dependent probe amplification) technique using the SALSA MLPA probemix P163-D1 GJB-WFS1 protocol. The analysis of the amplified products was also performed on ABI 3500® and the results were analysed with GeneMapper® software. Met34Thr variant is referred in this kit as 101 T>C. Other mutations, like IVS1+1G>A mutation on intron 1, GJB2 exon 1 at position 118-119 and a region in upstream GJB2, 18 nucleotides before exon 1, among others, were also analysed with MLPA.
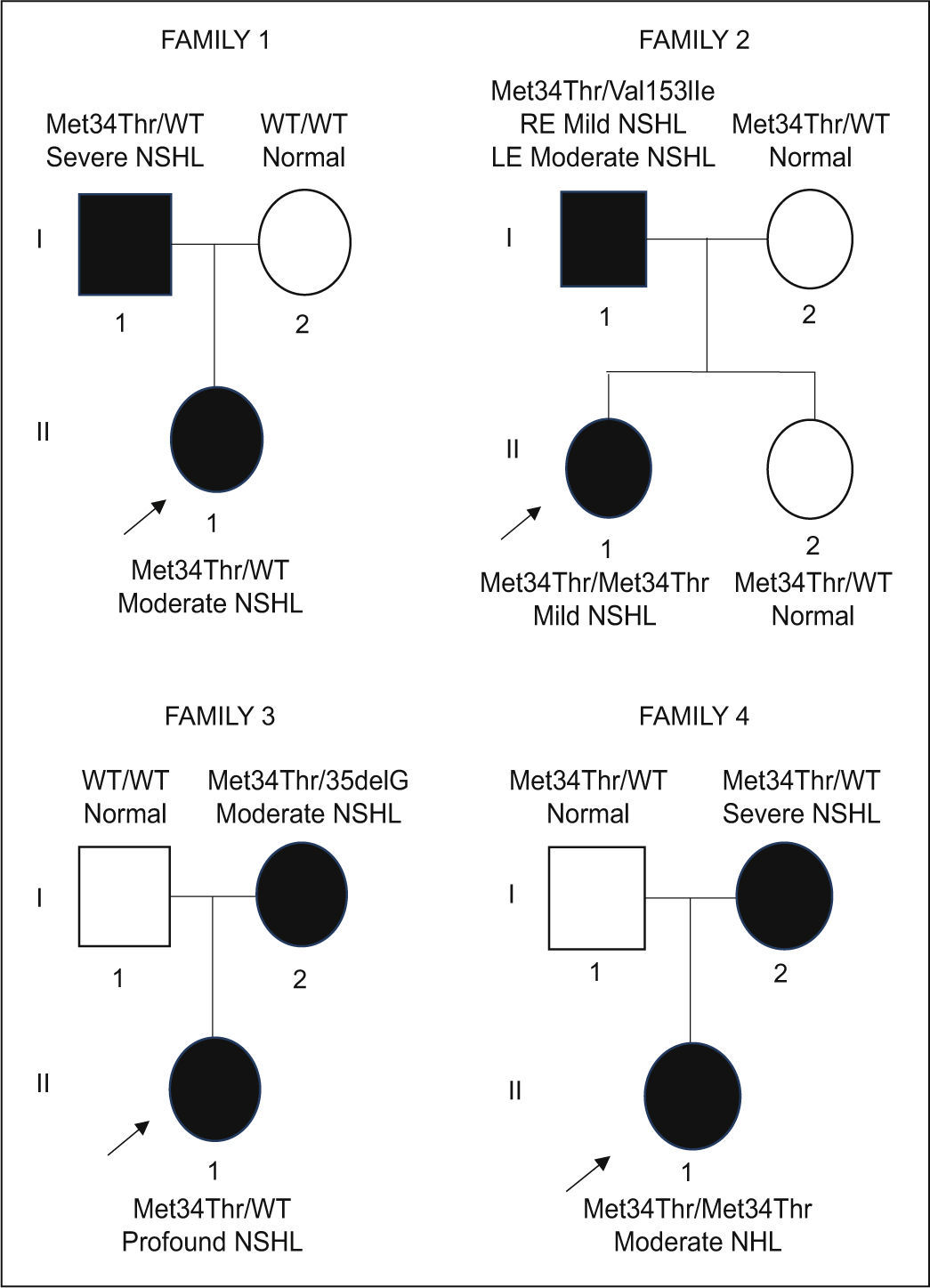
ResultsIn this study, 13 cases from 4 unrelated families were selected, in which one or more hearing-impaired members had Met34Thr variant. The families’ genograms with the individuals’ genotype and the phenotype are presented in Figure 1.
Genogram of the 4 families. PTA (500 to 4000Hz) hearing-impaired symptomatic individuals are indicated by black symbols, unaffected patients with white symbols. The severity of the hearing impairment is indicated. The index case is identified by an arrow. WT- wild type; NSHL- nonsyndromic hearing loss. RE- right ear; LE- left ear.
Syndromic hearing loss was evaluated for all individuals. The case I-1 from family 1 (Fig. 1) presented a pre-auricular pit but other organs lesions (middle ear, kidney or heart) were excluded using complementary diagnostic tests. None of the individuals complained of dizziness, tinnitus or had barotrauma or relevant history of otological disease. History of consanguinity among the parents was also excluded.
The Met34Thr variant was identified in 11/13 individuals, either as heterozygous or homozygous. Two cases have an additional mutation – Val153Ile and 35delG – (compound heterozygosity) presenting bilateral mild to moderate NSHL.
The audiometric features of the four families reported here revealed that NSHL was found in 8 subjects (Fig. 2). The most compromised frequencies were in the range of 2000-8000Hz, which resulted in a sloping type audiogram configuration. Only one individual (family 2: I-1) reported noise exposure in his workplace, which could explain, at least in part, the worsening found in high frequencies.
Mild NSHL was diagnosed in 2 patients (Met34Thr/Val153Ile; Met34Thr/Met34Thr), 3 moderate NSHL (Met34Thr/WT; Met34Thr/35delG; Met34Thr/Met34Thr), 2 severe NSHL (2 Met34Thr/WT) and 1 profound NSHL (Met34Thr/WT), as it is shown in Table 1. Three individuals with Met34Thr variant had normal hearing thresholds (Fig. 1 -3 cases: family 2: I-2 and II-2 and family 4: I-1).
Clinical characteristics of patients from the 4 families. Nr corresponds to the number of family presented in Figure 1. Age of diagnosis in years old (yo). PTA – pure tone average (500-4000Hz); WT – wild type; NSHL – nonsyndromic hearing loss; UNHS - universal neonatal hearing screening.
| Nr. | Case | Age (yo) | Age of diagnosis (yo) | NSHL | Mutation | Observation |
|---|---|---|---|---|---|---|
| 1 | I-1 | 53 | 7 | Bilateral severe; Profound to >8 KHz | Met34Thr/WT | Bilateral Prosthesis |
| I-2 | 53 | – | Normal audition | WT/WT | – | |
| II-1 | 25 | 16 | Bilateral moderate | Met34Thr/WT | Bilateral Prosthesis | |
| 2 | I-1 | 50 | 4 | RE mild and LE moderate | Met34Thr/Val153Ile | – |
| I-2 | 48 | – | Normal audition | Met34Thr/WT | – | |
| II-1 | 17 | 10 | Bilateral mild | Met34Thr/Met34Thr | – | |
| II-2 | 14 | – | Normal audition | Met34Thr/WT | – | |
| 3 | I-1 | 39 | – | Normal audition | WT/WT | – |
| I-2 | 38 | 10 | Bilateral moderate | Met34Thr/ 35delG | Bilateral Prosthesis | |
| II-1 | 2 | UNHS | Bilateral profound | Met34Thr/WT | Cochlear implant | |
| 4 | I-1 | 33 | – | Normal audition | Met34Thr/WT | – |
| I-2 | 33 | 7 | Bilateral severe | Met34Thr/WT | Bilateral Prosthesis | |
| II-1 | 9 | 8 | Bilateral moderate | Met34Thr/Met34Thr | Bilateral Prosthesis |
The presented data corroborate that Met34Thr variant is associated with mild-to-severe forms of deafness. Furthermore, the genogram analysis indicates that Met34Thr might segregate with an autosomal dominant inheritance with incomplete penetrance and variable expression of the phenotype, as described previously in the literature.4,18,21 Recent ideas suggested that this variability can be explained by other factors, as regulatory functions or post transcriptional modifications.22
In this study, 73% (8/11) of patients with Met34Thr present NSHL, which point to a pathogenic nature. All individuals have at least one parent with the Met34Thr variant and even those who inherited only one abnormal allele (heterozygous to Met34Thr) presented NSHL, suggesting an autosomal dominant pattern of inheritance. In fact, domi-nant inheritance has already been described for Met34Thr in 1997, when Kelsell et al. reported a case of two sisters with profound NSHL and Met34Thr allele heterozygosity.8 Nevertheless, later on, a second mutation in GJB2 gene was identified, which segregated with hearing loss, causing doubts on this autosomal dominant trait.13,18 Subsequently, the association of Met34Thr in homozygosity or Met34Thr in trans with 35delG, Val95Met and Arg184Trp with mild to moderate NSHL pointed to a recessive pattern of inheritance.15,16 This interpretation was later questioned due to the frequent findings of normal hearing in compound heterozygous with 35delG. Cases of individuals with this variant and normal audition led to the assumption that this variant could be a normal variant15,17,23,24 or has an incomplete penetrance.18,22
More recently, investigations studying multiple variables of gene function supported the hypothesis that Met34Thr is a pathogenic variant associated with NSHL by showing that, despite Cx26-Met34Thr protein being correctly synthesized and targeted to the plasma membrane, it is unable to form functional efficient intercellular channels.18,19 These studies corroborate the hypothesis here presented that Met34Thr is a pathogenic variant associated with NSHL.
In the present study, we found 3 cases presenting Met34Thr variant without audiometric evidence of NSHL (Fig. 1 - family 2: I-2 and II-2 and family 4: I-1), which corroborates the hypothesis of incomplete penetrance described by Snoeckx et al.4 and Pollak et al.,22 as the genotype is present but the phenotype is not observable. According to estimates from Pollak et al., the penetrance of Met34Thr/35delG genotypes relative to the 35delG/35delG genotypes is approximately 1/10.22 The absence of the phenotype in individual II-2 (14 y.o.) from family 2 might also be explained by later onset of the NSHL. The follow up of this case for a long period could be helpful to determine whether she develops NSHL or not. Reports of a ‘window’ of normal hearing before the diagnosis have been previously described in the literature.22 Indeed, in this work, the age of diagnosis was different among all the individuals (see Table 1) and only one case reported neonatal diagnosis.
Our data also support a phenotype variable expression, because despite these 8 cases showed segregation with NSHL, they all presented with different degrees of severity of the phenotype, even within the same family. For instance, individuals from family 1 (Fig. 1- I-1 and II-1), both Met34Thr heterozygous (Met34Thr/WT), presented different degrees of phenotype severity (I-1 with bilateral severe NSHL and II-1 with bilateral moderate NSHL). This hypothesis has already been described in other studies, suggesting that the effect of GJB2 mutations (including Met34Thr) can be substantially modified by still unknown genetic or environmental factors4,21,25 and can often be masked by these modifiers.22 Bicego et al. suggested that a 10bp deletion in the 5’-untranslated regions of the GJB2 gene could alter the expression levels of the Met34Thr allele in vivo, accounting for the phenotype variability in Met34Thr heterozygous.12,18
It has been suggested that Met34Thr is associated with less severe degrees of NSHL compared with other GJB2 mutations,4,18,26 specially if presented in homozygosity.4,12,17 Our data show that homozygous usually present NSHL ranging from mild to moderate (Fig. 1 family 2: II-1 and family 4: II-1). The only case with profound NSHL (Fig. 1 family 3: II-1) was heterozygous. The case with the genotype Met34Thr/35delG presented moderate NSHL, corroborating previous studies.4,22
The fact that Met34Thr segregates with milder levels of NSHL could explain the lower frequency of Met34Thr found in patients with NSHL compared with 35delG, as persons with milder NSHL, unless involved in family studies, are less likely to undergo genetic testing.4
Another confounding factor is the range of frequencies used to assess NSHL. Previous studies only analysed the 500, 1000 and 2000 Hz frequencies, while recent ones suggest that the assessment of a wider range of frequencies may help uncover mild cases of NSHL associated with Met34Thr.18,20 In the present work, we also considered the frequency of 4000Hz to help overcoming this problem.
A report in general population from UK demonstrated that Met34Thr carriers presented worse hearing thresholds at extra-high frequencies – 16 KHz.11 These frequencies are not usually assessed in standard audiological evaluations, which may underestimated losses. It is important to study these extra-high frequencies for routine, as it provides evidence of early changes of hearing in carrier groups and may be a predictor of later changes in adulthood.
Our study is only based on clinical data, which can be considered a limitation. A recent study from our group compared the carrier rates of Met34Thr found in NSHL patients with the ones from a Portuguese sample in order to better understand the role of Met34Thr variant in hearing loss. No statistically significant differences were found between these two groups, probably due to an ascertainment bias towards more severe NSHL or a low statistical power to find association. However, Met34Thr variant seemed to be conform to an additive model, indicating that the Met34Thr variant may be a risk factor for NSHL.10
In conclusion, our clinical data indicate that Met34Thr variation might be pathogenic and may segregate with a dominant transmission with incomplete penetrance and variable phenotype expression. Other modifying factors are likely to also have a role in the variability of phenotype severity. High frequencies loss is frequently associated with this variant. Therefore, the possible progressive late onset hearing impairment requires a long follow-up of these individuals, with the assessment of 8-20 KHz.
FundingThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.