The sciences are often divided into the ‘basic’ and the ‘applied’. The usual view is that applied sciences depend on basic sciences for essential knowledge, but that the converse is not the case: this might explain why, at least in the author's country, applied scientists seem often to be viewed as ‘below’, in some social sense, their basic-science colleagues. As a hybrid scientist/technologist who has worked for many years in both the basic science side of developmental biology (embryology) and its applied side (tissue engineering), I would argue that each depends very much on the other. This short article will illustrate the point with the story of kidney development–how scientists came to understand how natural kidneys develop and how tissue engineers have learned to engineer realistic kidney organoids in culture.
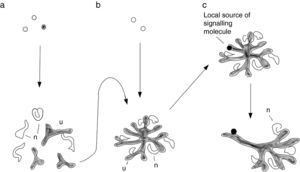
FoundationsAttempts to engineer renal tissue would be futile without some understanding of how natural kidneys form in the embryo. The foundations for this basic knowledge were laid by a small number of anatomists working in the 19th and early 20th centuries.1–6 Between them, they established that the metanephric (permanent) kidneys are the third pair of kidneys formed in development, the first two pairs (pronephros and mesonephros) regressing in females or being adapted for reproductive functions in males. The drainage ducts of the temporary kidneys, the Wolffian ducts, send a branch out from their caudal ends: this branch is called the ureteric bud (UB) (Fig. 1a). The UB crosses the peri-Wolffian mesenchyme to invade a specific area of the caudal intermediate mesoderm, called the metanephrogenic mesenchyme (MM: also called by some authors the ‘metanephric mesenchyme’ although this term is ambiguous, applying equally to the adult, so should be avoided). Once in the MM, the UB begins to branch giving rise, eventually, to the tree-like urine collecting duct system of the kidney (Fig. 1b). As the UB branches, the tips of its branches become capped with a population of mesenchymal stem cells, the ‘cap mesenchyme’. Each cap mesenchyme maintains itself throughout kidney development and it divides with the underlying ureteric bud tip so that new branches are capped. As well as maintaining itself, the cap mesenchyme sheds cells from its distal zone (i.e. the part furthest from the tip), and these cells aggregate, epithelialize and become excretory nephrons, which connect at their distal ends to their nearby UB branches (Fig. 1c). Blood vessel progenitors invade the proximal pole of the new nephron to make a glomerulus, and the glomeruli later connect to the systemic circulation (the timing being revealed by the observation that injecting tracer into systemic circulation fails to dye all glomeruli of the early kidney2). It should be noted that, although contemporary authors tend to cite 21st century research papers for almost all of the above, it was all known before 1930 and even terms like ‘cap mesenchyme’ and ‘stem cell’ were in use by then.4,5,7
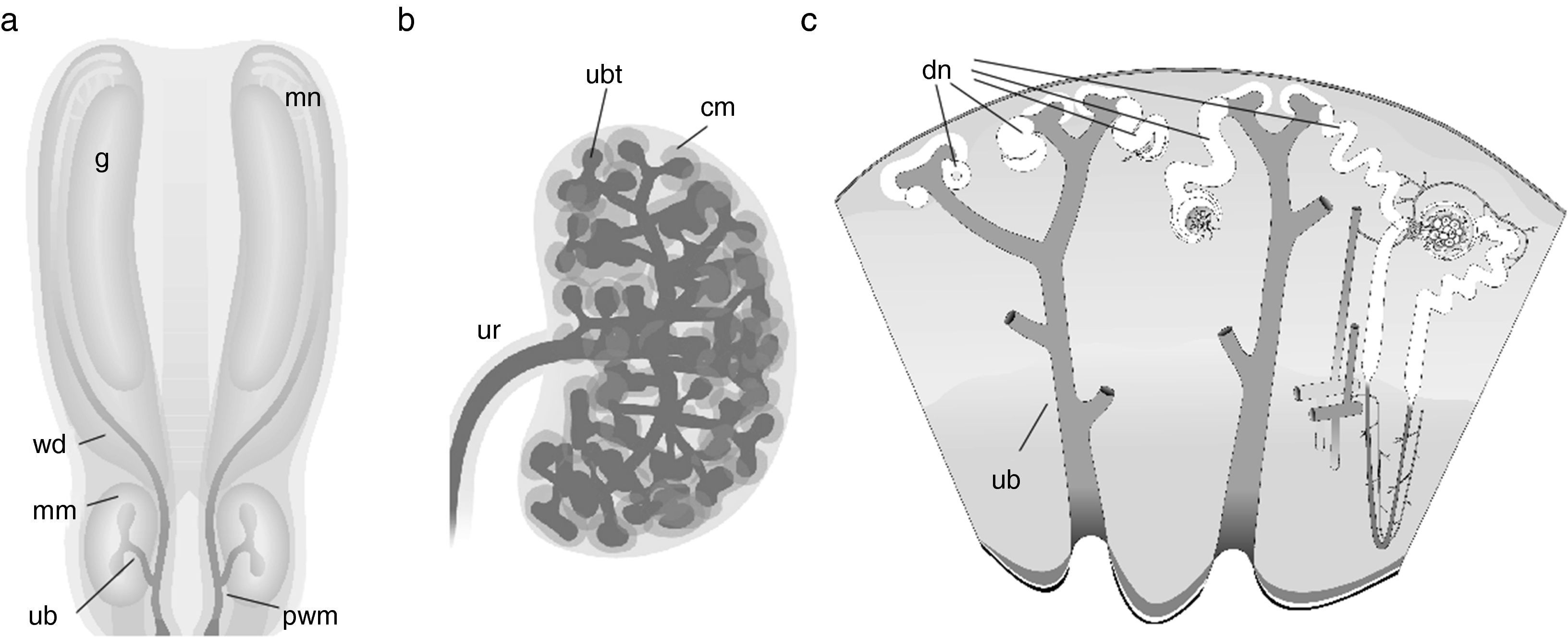
Normal development of the metanephric kidney. (a) Depicts the early embryo, in which the Wolffian ducts (wd) run caudally from the mesonephros (mn) and associated gonad (g) towards the cloaca: a ureteric bud (ub) crosses the peri-Wolffian mesenchyme (pwm) to enter the metanephrogenic mesenchyme (mm). (b) Shows the unbranched portion of the utereric bud becoming a ureter (ur) and he branching portion becoming a collecting duct tree: cap mesenchyme (cm) forms around the ureteric bud tips (ubt). (c) Depicts a time series of stages of nephron development: developing nephrons (dn) forr from cells left behind by the cap mesenchyme and undergo a stereotyped sequence of morphogenetic events to become a complicated, elgongated tube: the excretory nephron. Images have been obtained with permission from the GUDMAP database (http://www.gudmap.org/Schematics).
The first steps towards a mechanistic understanding of kidney development, in terms of establishing how particular events depend on other events, came from early attempts to build embryonic kidney components in isolation from the rest of the kidney. The work was made possible by the development of techniques for culturing kidneys in the lab, at first in clotted serum or hanging drops and, later, on filters in relatively simple media.5,8,9 An attempt to produce nephrons outside the body by culturing MM without its UB failed: the mesenchyme produced no nephrons and just rounded up and died.8 Isolation of MM and recombining it with UB, however, resulted in the formation of a kidney rudiment with nephrons, indicating that nephrons depend for their formation on an inductive signal from the ureteric bud. The observation that other tissues such as dorsal spinal cord could substitute for ureteric bud in inducing nephrons10 indicated, in the language of the era, that the induction was permissive (telling cells they could go ahead and follow a pre-determined path) rather than instructive (specifying to naive cells exactly what they should do: as we have understood more about the nature of signalling, the concept of instructive induction has largely disappeared from embryological thinking).
Attempts to construct renal tissues and, in particular, failure to do so, therefore had the effect of exposing very important features of normal kidney development. In the molecular era, the pathway mediating the induction of nephron formation has been identified by a combination of pharmacological,11 gene expression, and knockout studies. A reciprocal induction of UB branching by MM was also identified, led by the implications of a knockout phenotype that abolished branching.12,13 In recent years, many more signalling interactions have been identified (see McMahon14 for a recent review), leading to the view that the developing kidney organizes its anatomy through the exchange of signals mediating rich feedback (feeding back information about what has been built to the processes that will build the next stage). This feedback makes the system highly adaptive and tolerant of error.
Making renal organoids by reaggregation of stem cellsThe discovery that kidney development is regulated by a web of cell interactions that give it self-organizing character, capable of adapting to create realistic anatomy even under unusual constraints such as developing on a flat surface, raised an interesting question: could a random mix of renogenic stem cells (UB and MM types) organize itself into realistic kidney tissue? An early attempt to do this, by disaggregating the UB and MM of a young kidney rudiment into a cell suspension and then reaggregating it met with failure: too many cells died by apoptosis.15 This was not surprising, perhaps, for it is known that mammalian cells depend on normal cell–cell and cell–matrix contacts for their survival and in the absence of these contacts they die: the process is called anoikis.16
In the course of a quite different project, centred on the basic-science question of the role of actin reorganizion in the morphogenesis of renal tubules,17 it had been observed that inhibition of Rho-dependent kinase (ROCK), the focus of attention for the study because it is a regulator of actin organization, greatly reduced apoptosis in developing kidneys. The finding was incidental, not considered in the paper that came out of the work, but it was mentioned in an internal lab meeting. Remembering it, Unbekandt and Davies15 used ROCK inhibition to give re-aggregating renogenic stem cells temporary ‘life support’ as they re-established connections with one another. This prevented excessive apoptosis and allowed the cells to undergo significant self-organization. The result was an oragnoid in which UB cells formed distinct cysts that matured into collecting duct treelets. Around these, the MM formed nephrons which divided into segments (proximal tubule, distal tubule etc.) and connected to nearby collecting ducts (Fig. 2a).
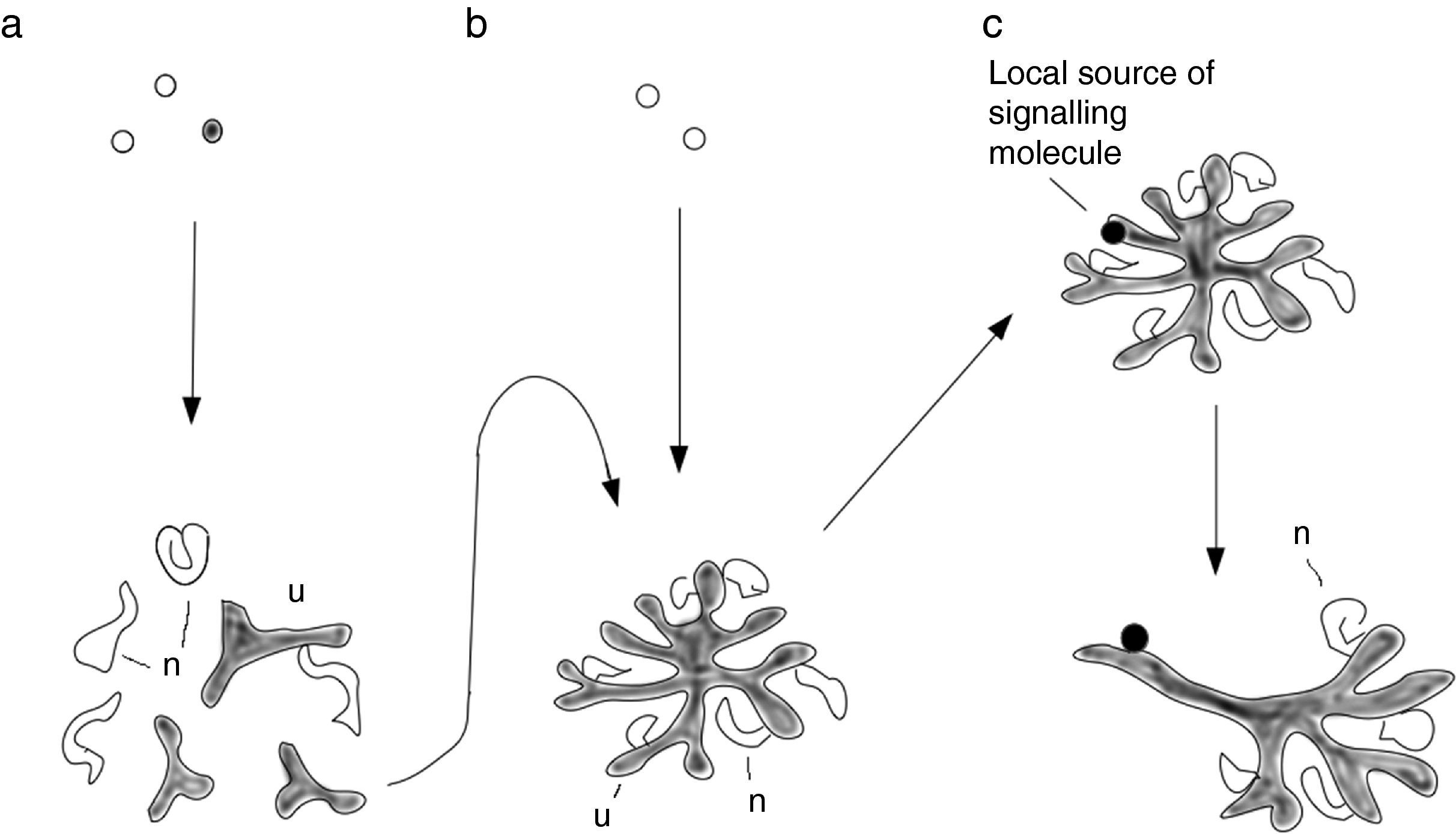
Methods of renal tissue engineering. (a) Shows a basic reaggregation method (Unbekandt & Davies, 2010), in which mixed renogenic stem cells (UB and MM cells) are reaggregated and form small UB treelets and young nephrons near and connected to them. (b) Shows a serial-aggregation method, in which just one UB treelet is isolated from a primary aggregate and aggregated with fresh aggregated MM cells: now the tissue organizes itself around a single, large UB/collecting duct tree (Ganeva et al., 2011). (c) Shows the effect of applying local growth factors, characteristic of peri-Wolffian mesenchyme, near one developing collecting duct in the Ganeva system. This duct ceases to branch and nephrons do not develop around it, polarizing the whole organ so that this branch is ureter-like, and even expresses uroplakin.
Organoids formed by self-organization, as described above, have realistic micro-scale anatomy but completely lack organ-scale features, such as being organized around a single collecting duct tree, with a distinct cortex and medulla. This apparent boundary between fine and large-scale features is a typical feature of self-organizing organoid systems. It suggests that critical information, missing from self-organizing organoids, must be present in the embryo. Searching for it, to improve organoids, raises new questions about natural embryogenesis. The presence of multiple collecting duct treelets in simple renal organoids formed by re-aggregation, for example, draws attention to the critical importance of a natural feature of normal kidney development: the collecting duct progenitor (UB) enters the MM as one single tubule that never loses its integrity. This creates an asymmetry in the system that ensures that everything is arranged around the single tree. Extracting one ureteric bud treelet from a re-aggregate and combining it, intact, with reaggregated MM cells, so that there is now only one source of UB cells in the system, results in a more realistic renal anatomy with everything arranged around a single collecting duct tree (Fig. 2b).18 This improved organoid even develops a distinct cortex and medulla in a suitable culture system.19 This observation, made in the tissue engineering arena, informs embryological knowledge by confirming the critical role of the collecting duct tree in organizing large-scale anatomy.
The collecting duct system of an organoid made as above does not, however, have an exit tube: all branches of the tree are collecting ducts, with no ureter. Gene expression studies conducted for basic embryology have shown that the peri-Wolffian mesenchyme, through which the ureter passes before entering the MM, expresses signalling molecules distinct from those of the MM itself. Cut-and-paste experiments that result in the (immature) ureter stalk being surrounded by MM, or the branching part of the UB being surrounded by peri-Wolffian mesenchyme, suggest that UB cells’ decision about whether to make a branching collecting duct tree or a non-branching, uroplakin-expressing ureter, is governed by signals from the mesenchyme that surrounds it. This knowledge can be used to add further realism to the organoid system: surrounding just one young collecting duct of an organoid based on a single collecting duct tree with signals characteristic of peri-Wolffian mesenchyme causes that duct to branch no more, to induce no nephrons to form near it, but instead to thicken and express uroplakin (Fig. 2c). This manipulation, effectively a further symmetry-breaking step, increases the realism of the organoid considerably. It also enriches basic embryological knowledge by positively identifying the cause of the UB stalk becoming ureter not collecting duct.
Kidneys from pluripotent stem cellsThe tissue-engineering experiments described above all used ex fetu cells that were already committed to a renogenic fate and came from the area about to form a kidney. Clearly the routine construction of human kidneys from human ex fetu sources would be neither practical nor, to many people, ethical. Producing them from pluripotent human cells, for example hiPS cells,20 is the obvious alternative, but this depends on the development of a method to ‘program’ pluripotent stem cells to the renogenic fate. Designing such a method is usually done by ‘walking’ cell through the sequence of signalling environments that would be experienced in the life of a cell that reached a renogenic fate in a real embryo (reviewed by Little and Takasato21). Establishing this sequence depends on two types of basic embryological information: lineage and signalling. The lineage information, from epiblast to intermediate mesoderm to nephrogenic zone, was established long ago, mainly by careful observation with no need for genetic cell marking (reviewed by Saxen7). The sequence of signalling events was worked out by a combination of observation of expression patterns of signalling molecules, and assessing the effects of defined signalling factors on cells at various points of the lineage.22,23 The first people to apply this information to the problem of engineering renal tissue from pluripotent stem cells (in this case, mouse ES cells) were Kim and Dressler,24 who treated ES cells with retinoic acid, activin and BMP7 to make them into intermediate mesoderm cells that would differentiate into kidney tubules when placed into an embryonic kidney or combined with embryonic spinal cord. The cells did not, however, make kidneys on their own, suggesting that they may have represented MM but not UB.
Several subsequent attempts to make kidneys from ES or iPS cells met with similar frustrations in generating either MM or UB but not making both in a form that would generate a renal organoid.25–28 This led Taguchi and colleagues29 to return to basic developmental biology, to examine more carefully the origins of, and the environments experienced by, the cells that make the UB and the MM. The conclusion from this careful analysis is that cells that give rise to the rostral end of the intermediate mesoderm, and thus eventually the UB, experience retinoic acid earlier and for longer than those in the caudal end of the intermediate mesoderm that make the MM. Using this new basic embryological information, discovery of which was prompted by frustrations on the applied side, Takasato and colleagues30,31 returned to the problem of programming iPS cells to make self-organizing kidneys on their own. They showed that they could, by varying the length of exposure to a particular growth factor during programming, choose whether the cells become almost all MM, almost all UB, or a mixture. The mixture, after a short induction by a Wnt agonist, produced organoids very similar to those made by reaggregation of ex fetu murine renogenic stem cells described above.15 The important difference was that Takasato's organoids31 were made from human cells taken all the way from the pluripotent state. It will no doubt only be a matter of time before the realism of these organoids is improved using the symmetry-breaking tricks described above for the murine system.
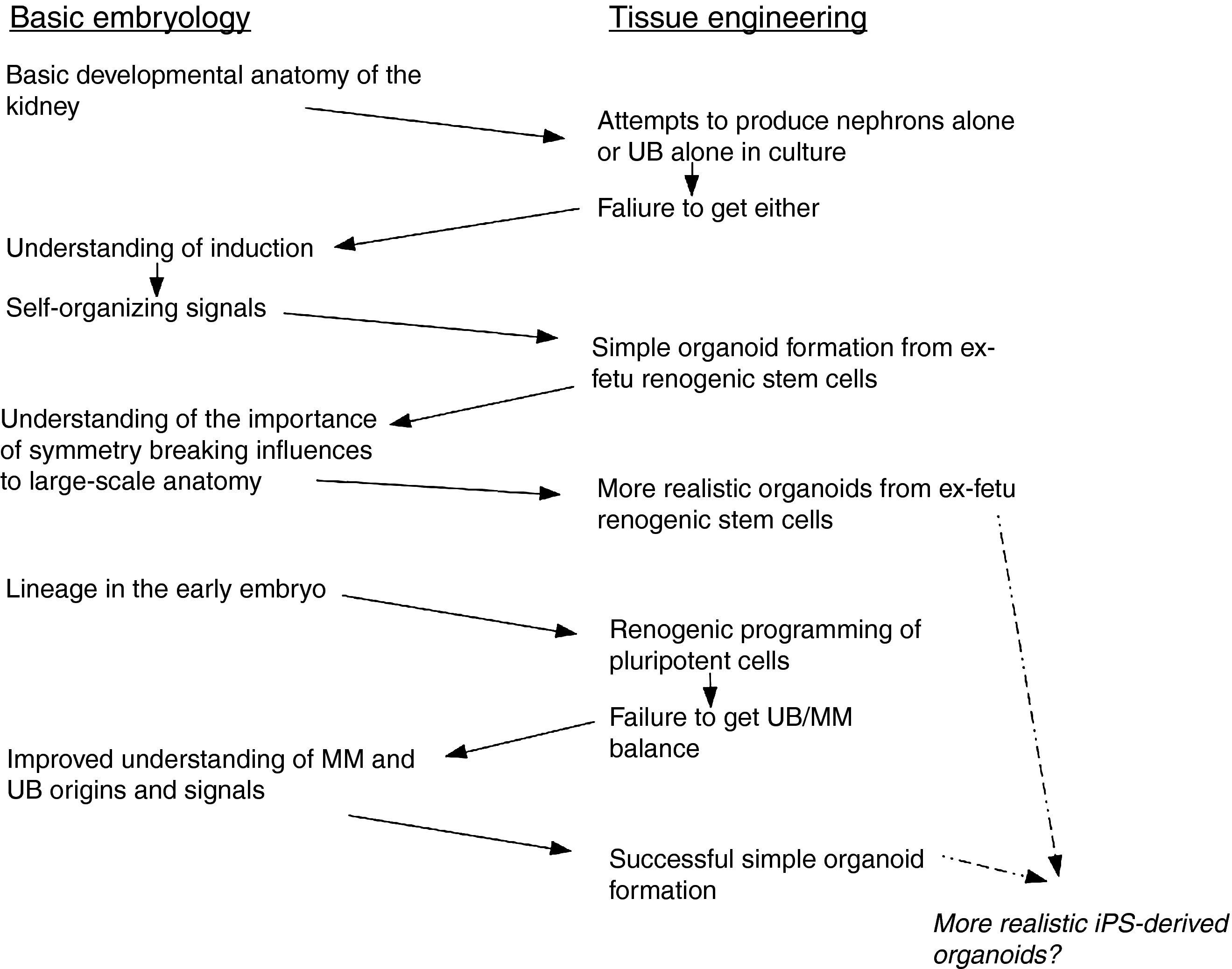
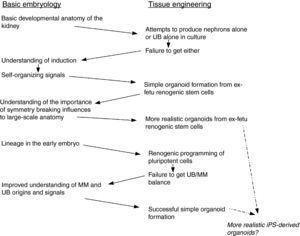
Concluding remarksAlong the path to iPS-derived renal organoids, described above, applied science has both drawn on and informed basic science, and basic science has in its turn drawn on the results of tissue engineering work. The interchanges of information, some of which are summarized in Fig. 3, emphasizes the falseness of the dichotomy between basic embryology and tissue engineering. It also suggests the foolishness of the way we tend to organize research communities. Embryologists and tissue engineers tend to publish in different journals (though with some overlap, for example in Organogenesis and Development) and go to different conferences. How much faster might both fields progress if the communities mixed more, and how much faster might other fields of biomedical science and technology advance if they did the same?