To determine the safety and efficacy of angioplasty with a retrievable stent in treating vasospasm secondary to subarachnoid hemorrhage (SAH) due to an aneurysm.
MethodsWe retrospectively analyzed prospectively collected data from consecutive patients undergoing endovascular angioplasty with a retrievable stent to treat vasospasm related to SAH due to an aneurysm in four neurointerventional radiology departments between January 2018 and July 2019. We included patients aged >18 years with vasospasm >50% of the internal carotid artery (ICA), anterior cerebral artery (ACA), and / or middle cerebral artery (MCA) secondary to SAH due to an aneurysm treated with endovascular angioplasty with a retrievable stent. The variables used to measure safety were complications of the procedure and clinical complications. The variables used to measure radiological efficacy were improvement in the degree of stenosis after endovascular treatment and improvement or normalization of cerebral circulation time CTT).
ResultsWe included 16 angioplasty procedures with retrievable stents in 13 patients, in which 33 arterial segments were treated (10 ICA, 15 MCA, and 8 ACA). We observed no complications of the procedure in any patients and no clinical complications in patients who were not intubated. All but one of the patients who had delayed CTT at the beginning of the procedure showed improvements in CTT. The mean improvement in the degree of stenosis was 18% ± 11.65% in the ICA, 30.67% ± 18.45% in the MCA, and 28.38% ± 15.49% in the ACA. No statistically significant associations were observed between endovascular treatment variables and the degree of improvement in stenosis.
ConclusionAngioplasty with a retrievable stent is a safe and efficacious treatment for vasospasm secondary to SAH due to an aneurysm, improving CTT and stenosis.
El objetivo es informar de nuestro estudio multicéntrico de seguridad y eficacia acerca de la angioplastia con stent recuperable en el vasoespasmo secundario a la hemorragia subaracnoidea por aneurisma (HSAa).
MétodosRealizamos un análisis retrospectivo de una base de datos prospectiva de pacientes consecutivos sometidos a tratamiento endovascular mediante angioplastia con stent recuperable para el vasoespasmo relacionado con la HSAa en cuatro departamentos de neurorradiología intervencionista, entre enero de 2018 y julio de 2019. Incluimos a pacientes consecutivos mayores de 18 años con vasoespasmo mayor del 50% de la arteria carótida interna (ACI), la arteria cerebral anterior (ACA) y/o las arterias cerebrales medias (ACM) secundario a HSAa, tratado endovascularmente mediante angioplastia con stent recuperable. Las complicaciones del procedimiento y las complicaciones clínicas se registraron como variables de seguridad. Las variables de eficacia radiológica del procedimiento se definieron como la mejora en el porcentaje de estenosis después del tratamiento endovascular y la mejora o normalización del tiempo de circulación cerebral (TCC).
ResultadosIncluimos 16 procedimientos de angioplastia con stent recuperable en 13 pacientes, con 33 segmentos arteriales tratados (10 ACI, 15 ACM y 8 ACA). No encontramos complicaciones de procedimiento en ningún paciente, ni complicaciones clínicas en pacientes no intubados. Todos los pacientes con TCC retrasado al principio del procedimiento, excepto uno, mostraron una mejora en el TCC. La mejora en el porcentaje de estenosis en la ACI fue de 18 ± 11,65 (media ± DE), 30,67 ± 18,45 en la ACM y 28,38 ± 15,49 en la ACA. No encontramos ninguna asociación estadísticamente significativa entre las variables de tratamiento endovascular y la mejora en el porcentaje de estenosis.
ConclusiónLa angioplastia con stent recuperable como tratamiento del vasoespasmo secundario a la HSAa es un procedimiento seguro y eficaz que mejora el TCC y la estenosis del vaso después del tratamiento.
Aneurysmal subarachnoid hemorrhage (aSAH) is a severe subtype of stroke with high morbi-mortality consequences after the acute phase of the disease.1 Cerebral vasospasm (CVS) is the most important complication of aSAH in the subacute phase (5–14 days), it is the leading cause of severe delayed cerebral ischemia (DCI) which is the main reason of unfavorable clinical outcome.2 The exact cause for DCI is not completely understood, however the prevention or early treatment of cerebral vasospasm seems to improve patient outcome.3 Endovascular treatment of vasospasm is controversial with different approaches, such as arterial vasodilator infusion or intracranial angioplasty, according to different institutions and personal preferences. The purpose of the study is to report our safety and efficacy multicentric study about stent-retriever angioplasty in vasospasm secondary to aSAH. A secondary objective is to evaluate the endovascular procedure variables that can be associated with the improvement of the percentage of the vessel stenosis.
Materials and methodsStudy design, patients, and data collectionWe conducted a retrospective analysis of a prospective database of consecutive patients that underwent endovascular treatment with stent-retriever angioplasty for aSAH related vasospasm in four high-volume interventional neuroradiology departments, between January 2018 and July 2019. Informed consent for the endovascular procedure was obtained from all patients; we were not required to present additional information for institutional authorization due to the retrospective nature of the study.
We included consecutive patients older than 18 years-old with more than 50% vasospasm of the internal carotid artery (ICA), anterior cerebral artery (ACA) and/or middle cerebral artery (MCA) secondary to aSAH, treated endovascularly with stent-retriever angioplasty. Patients with severe tortuosity of the anatomy or arterial occlusion that prevents the deployment of the device were excluded.
Demographic, clinical and radiological variables at hemorrhagic presentationDemographic variables (age and sex) and cardiovascular risk factors variables (arterial hypertension, diabetes mellitus, smoking, familiar history of brain aneurysm, fibromuscular dysplasia, and polycystic disease) were recorded. The clinical situation of the patients was assessed at admission with two neurological scales: Hunt and Hess Scale (HH) and Glasgow coma scale (GCS).
Non-enhanced Computed Tomographies (CT) were reviewed to assess the severity of aSAH using modified Fischer CT scale and the presence of intraparenchymal hematoma. Digital Subtraction Angiography (DSA) was analyzed by an interventional neuroradiologist to determine the number of aneurysms, the shape (saccular or fusiform), size (maximum size in mm), location (Anterior communicating artery, posterior communicating artery, middle cerebral artery, anterior choroidal artery, bifurcation of internal carotid artery, ophthalmic segment of internal carotid artery, bifurcation of basilar artery, other locations) and side (left or right) of the bleeding aneurysm.
Clinical and radiological variables related to vasospasmDuring the episode of vasospasm, time period from the bleeding (days after the hemorrhage), patient intubated (yes or no) and main neurological deficit were recorded.
DSA was analyzed by an interventional neuroradiologist to determine the location (internal carotid artery, anterior cerebral artery and/or middle cerebral artery) and side (left or right) of vasospasm, percentage of pretreatment stenosis (compared to DSA performed during the endovascular treatment of bleeding aneurysm) and cerebral circulation time (CCT) (normal or delayed defined as more than 8 s from the appearance of contrast in the ICA to venous drainage).
Endovascular treatment variablesAll procedures were carried out with systemic heparinization according to local protocol and with a unsheath-resheath method, unlike the drag-method used in mechanical thrombectomy, in order to avoid the endothelial damage and iatrogenic vasospasm. Regarding endovascular treatment variables, the location (internal carotid artery, anterior cerebral artery segment A1 and/or middle cerebral artery segment M1) and duration of device placement (minutes), side (left or right) of the treatment, type of device (Trevo[Stryker, Mountain View, CA], Solitaire [Covidien, Dublin, Ireland], Vesalio NeVa-VS [Vesalio, Nashville, TN], Catch/Catch-Mini [Balt, Montmorency, France], Aperio device [Acandis, Pforzheim, Germany], Preset device [Phenox, Bochum, Germany]), and size of the device were recorded.
The use of vasodilator drug, type and catheter from where the drug was administered (microcatheter, guiding-catheter, other) were also recorded. At the end of the procedure, the percentage of posttreatment stenosis and improvement of CCT were recorded.
Procedural complications (groin hematoma, perforation, dissection, vasospasm, other) and clinical complications as new neurological deficit or worsening of a previous neurological deficit were recorded as safety variables during the endovascular treatment. Procedural radiological efficacy variables were defined as the improvement in percentage stenosis after the endovascular treatment and improvement or normalization of cerebral circulation time. Clinical efficacy variables were defined as an improvement of a previous neurological deficit.
Statistical analysisDescriptive analysis included frequencies and percentages for categorical variables and mean (Standard Deviation; SD) or median (Interquartile Range; IQR) for continuous variables. Size of the stent-retriever and duration of device placement were dichotomized in 4 mm and 5 min respectively, in order to detect any associations with the improvement of the percentage of the vessel stenosis. Univariate analysis was done using the U Mann-Whitney test for continuous variables and the χ2 test or Fisher exact test for categorical variables. Statistical analyses were performed using the Statistical Pack-age for the Social Sciences software, Version 20.0 (SPSS, Chicago, Illinois). A p value < 0.05 was considered significant.
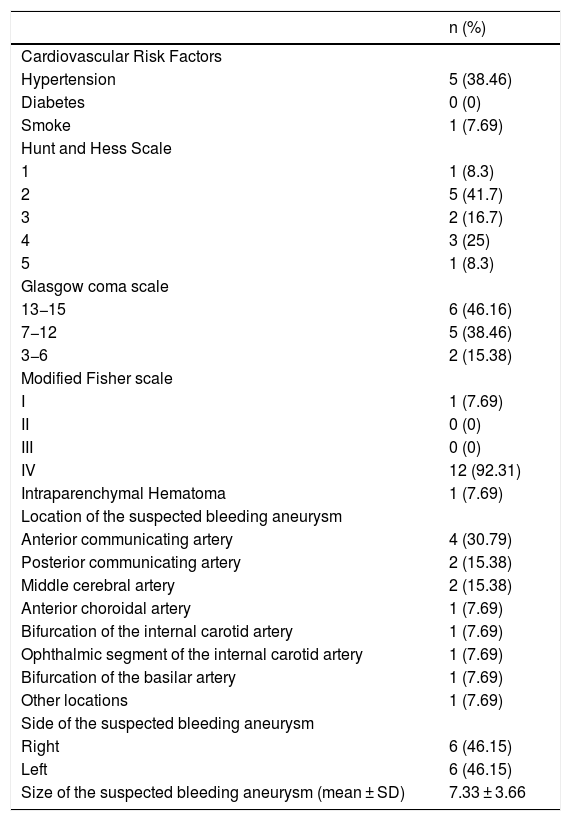
ResultsPatient populationWe included 16 stent-retriever angioplasty procedures in 13 patients, with no patients excluded because of the inability to deploy the device related to tortuosity or arterial occlusion. The mean age was 52.46 ± 6.9 (mean ± SD) years-old and 10 (76.92%) patients were female. Clinical and radiological variables are summarized in Table 1. There were no patients with brain aneurysm familiar history, fibromuscular dysplasia, or polycystic disease. Eight patients (61.5%) had only one aneurysm; five patients (38.5%) had more than one aneurysm (two patients had two aneurysms, one patient had three aneurysms, and two patients had four aneurysms). All suspected bleeding aneurysms were saccular, except one which was a dissecting aneurysm. All other aneurysms characteristics are summarized in Table 1.
Clinical and radiological variables related to aSAH.
| n (%) | |
|---|---|
| Cardiovascular Risk Factors | |
| Hypertension | 5 (38.46) |
| Diabetes | 0 (0) |
| Smoke | 1 (7.69) |
| Hunt and Hess Scale | |
| 1 | 1 (8.3) |
| 2 | 5 (41.7) |
| 3 | 2 (16.7) |
| 4 | 3 (25) |
| 5 | 1 (8.3) |
| Glasgow coma scale | |
| 13−15 | 6 (46.16) |
| 7−12 | 5 (38.46) |
| 3−6 | 2 (15.38) |
| Modified Fisher scale | |
| I | 1 (7.69) |
| II | 0 (0) |
| III | 0 (0) |
| IV | 12 (92.31) |
| Intraparenchymal Hematoma | 1 (7.69) |
| Location of the suspected bleeding aneurysm | |
| Anterior communicating artery | 4 (30.79) |
| Posterior communicating artery | 2 (15.38) |
| Middle cerebral artery | 2 (15.38) |
| Anterior choroidal artery | 1 (7.69) |
| Bifurcation of the internal carotid artery | 1 (7.69) |
| Ophthalmic segment of the internal carotid artery | 1 (7.69) |
| Bifurcation of the basilar artery | 1 (7.69) |
| Other locations | 1 (7.69) |
| Side of the suspected bleeding aneurysm | |
| Right | 6 (46.15) |
| Left | 6 (46.15) |
| Size of the suspected bleeding aneurysm (mean ± SD) | 7.33 ± 3.66 |
Vasospasm episode was presented in the day 9.2 ± 3.6 (mean ± SD) after the aSAH. Six patients (37.5%) were under general anesthesia when the endovascular treatment was made, so it was not possible to evaluate them clinically. In those patients, the indication of endovascular treatment was based on transcranial doppler (TCD) or DSA vasospasm, with more than 50% of stenosis of internal carotid artery (ICA), middle cerebral artery (MCA) and/or anterior cerebral artery (ACA).
In the ten remaining patients without general anesthesia, the neurological examination showed motor deficit in eight patients (80%), bradypsychia or fluctuation of consciousness level in six patients (60%) and aphasia in one patient (10%). Delayed cerebral circulation time was observed in 11 patients (68.75%).
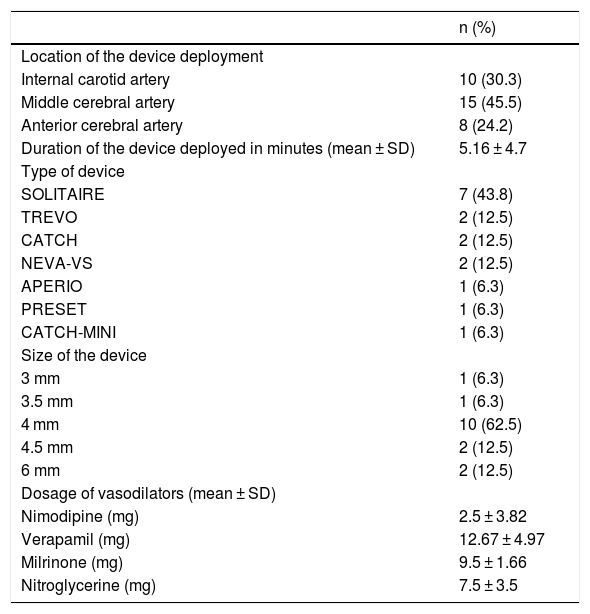
Vasodilator drugs were used in all patients, in nine (56.3%) the vasodilator drug was infused through the guiding-catheter and in seven (43.8%) patients it was infused through the microcatheter before or after the stent-retriever angioplasty. The vasodilator drug used was verapamil in 3 patients (18.8%), nimodipine in 3 patients (18.8%), verapamil plus nimodipine in 4 patients (25%), nimodipine plus milrinone in 4 patients (25%), verapamil plus nitroglycerine in one patient (6.3%) and verapamil plus nitroglycerine plus nimodipine in one patient (6.3%). Dosage of vasodilators are summarized in Table 2.
Endovascular treatment variables.
| n (%) | |
|---|---|
| Location of the device deployment | |
| Internal carotid artery | 10 (30.3) |
| Middle cerebral artery | 15 (45.5) |
| Anterior cerebral artery | 8 (24.2) |
| Duration of the device deployed in minutes (mean ± SD) | 5.16 ± 4.7 |
| Type of device | |
| SOLITAIRE | 7 (43.8) |
| TREVO | 2 (12.5) |
| CATCH | 2 (12.5) |
| NEVA-VS | 2 (12.5) |
| APERIO | 1 (6.3) |
| PRESET | 1 (6.3) |
| CATCH-MINI | 1 (6.3) |
| Size of the device | |
| 3 mm | 1 (6.3) |
| 3.5 mm | 1 (6.3) |
| 4 mm | 10 (62.5) |
| 4.5 mm | 2 (12.5) |
| 6 mm | 2 (12.5) |
| Dosage of vasodilators (mean ± SD) | |
| Nimodipine (mg) | 2.5 ± 3.82 |
| Verapamil (mg) | 12.67 ± 4.97 |
| Milrinone (mg) | 9.5 ± 1.66 |
| Nitroglycerine (mg) | 7.5 ± 3.5 |
We did not find procedural complications in any patient, or clinical complications in non-intubated patients like worsening of a previous neurological deficit or the appearance of a new neurological deficit. Out of all 13 patients, four patients were retreated. Three of them were treated endovascularly twice within 10−12 hours, in the framework of local endovascular vasospasms protocol, without clinical or vasospasm worsening; and the other patient was endovascularly re-treated with vasodilator drugs, because of the appearance of left hemiparesis, the patient was previously treated because of right hemiparesis days before, considering it as a new episode of symptomatic vasospasm. None of the other 9 patients were re-treated in the same side of stent-retriever angioplasty treatment.
All patients with delayed-CCT at the beginning of the procedure except one showed improvement in the CCT (10 out of 11 patients; 90.91%); however, none of them went back to normal. In non-intubated patients, the improvement of a previous neurological deficit was observed in 5 patients out of 8 patients (62.5%) in which the patient could be evaluated.
Regarding the endovascular treatment variables, thirty-three segments were treated with stent-retriever angioplasty (Fig. 1). All the rest of the variables were summarized in Table 2. The percentage of pretreatment and posttreatment stenosis and the improvement of it were summarized in Table 3. The relationship between endovascular treatment variables and the improvement of the percentage of stenosis were summarized in Table 4. Internal carotid artery and anterior cerebral artery segments analysis could not be done because of the small sample size.
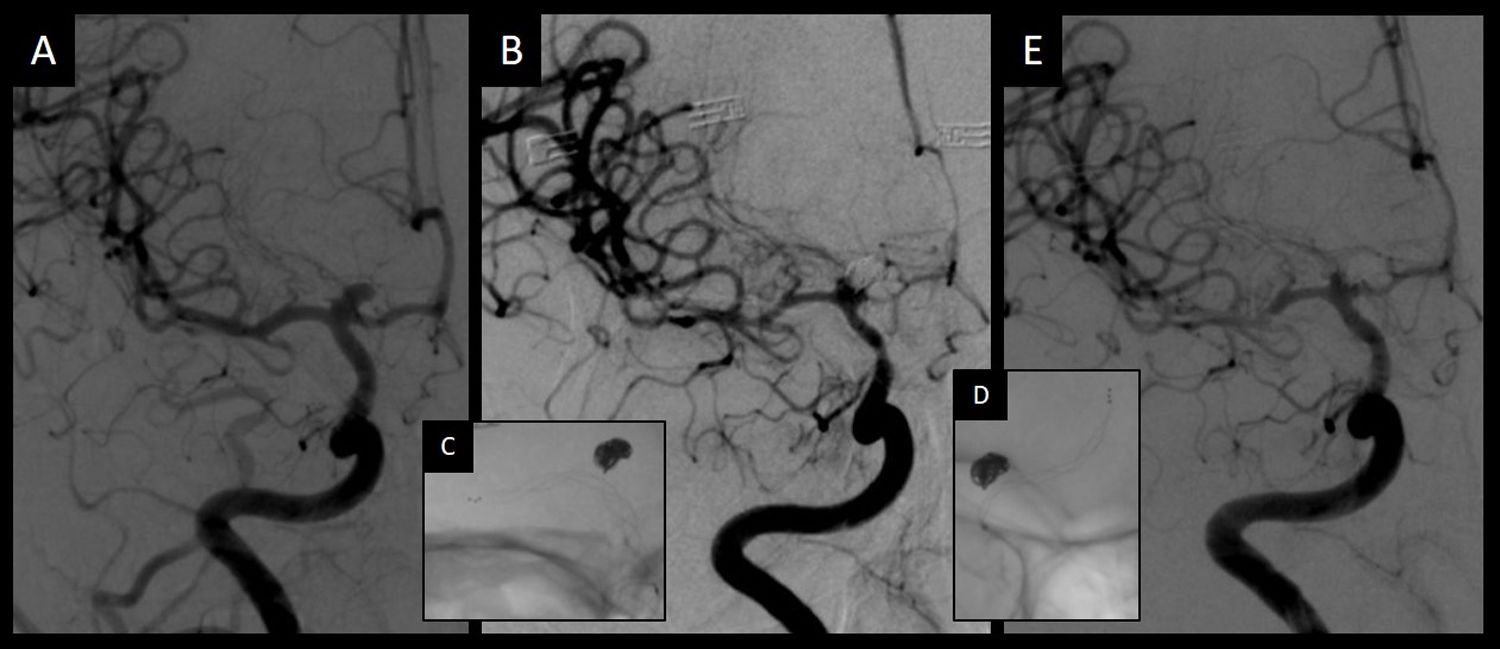
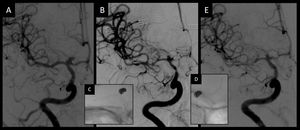
Admission angiography (A) showed a normal caliber of intracranial vessel and a terminal internal carotid aneurysm. Eight days later, pretreatment angiography (B) showed spasm of the intracranial vessel in the internal carotid artery, the anterior cerebral artery and the middle cerebral artery. After stent-retriever angioplasty in the middle cerebral artery and the internal carotid artery (C) and in the anterior cerebral artery (D), posttreatment angiography (E) showed an improvement in the caliber of the three vessels.
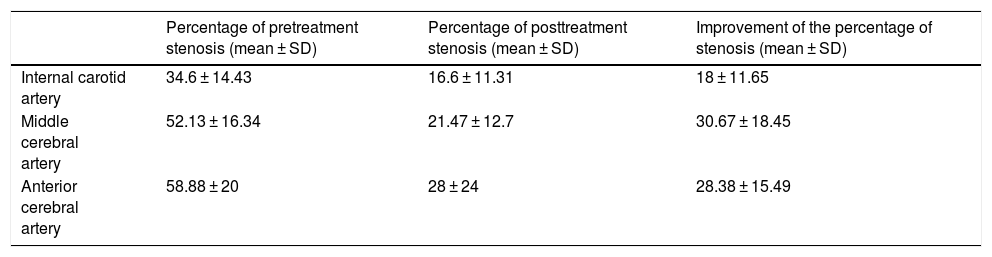
Efficacy endovascular treatment variables related to arterial stenosis.
| Percentage of pretreatment stenosis (mean ± SD) | Percentage of posttreatment stenosis (mean ± SD) | Improvement of the percentage of stenosis (mean ± SD) | |
|---|---|---|---|
| Internal carotid artery | 34.6 ± 14.43 | 16.6 ± 11.31 | 18 ± 11.65 |
| Middle cerebral artery | 52.13 ± 16.34 | 21.47 ± 12.7 | 30.67 ± 18.45 |
| Anterior cerebral artery | 58.88 ± 20 | 28 ± 24 | 28.38 ± 15.49 |
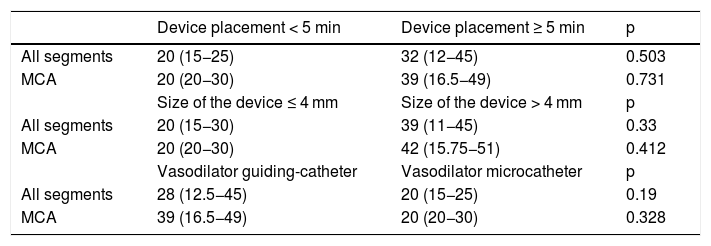
Relationship between endovascular treatment variables and the improvement of the percentage of stenosis. Median (Interquartile range).
| Device placement < 5 min | Device placement ≥ 5 min | p | |
|---|---|---|---|
| All segments | 20 (15−25) | 32 (12−45) | 0.503 |
| MCA | 20 (20−30) | 39 (16.5−49) | 0.731 |
| Size of the device ≤ 4 mm | Size of the device > 4 mm | p | |
| All segments | 20 (15−30) | 39 (11−45) | 0.33 |
| MCA | 20 (20−30) | 42 (15.75−51) | 0.412 |
| Vasodilator guiding-catheter | Vasodilator microcatheter | p | |
| All segments | 28 (12.5−45) | 20 (15−25) | 0.19 |
| MCA | 39 (16.5−49) | 20 (20−30) | 0.328 |
The present multicentric study showed the stent-retriever angioplasty as treatment for vasospasm secondary to aSAH, as a highly effective procedure in terms of improvement of the CCT (90.91%) and the percentage of the vessel stenosis (up to 30% average improvement in the vessel diameter at the middle cerebral artery and close to 20% average improvement in vessel diameter at the internal carotid artery), with a very low procedural and clinical complications rate. We did not find patients in need of an endovascular re-treatment secondary to recurrence of symptoms or vasospasm at the same treatment side. We did not find any statistically significance associations between endovascular treatment variables and the improvement of the percentage of stenosis.
Our data are consistent with the literature already published. A proof of principle paper was published in 2017 by Bhobal et cols4 showing a retrospective series of 4 patients; all patients were treated with stentretriever for an aSAH vasospasm. They had no complications but 2 patients (50%) experienced recurrent vasospasm, without specifying whether they needed secondary endovascular treatment or not.
Kwon et cols5 published in 2018 a retrospective single center experience, twelve patients with vasospasm due to aSAH were treated with stent-retrievers, which was deployed in 53 segments. The deployment of the stent-retriever was technically feasible in all cases. They divided all patients into two groups, 5 patients were treated first with intraarterial vasodilators and stent-retriever deployment afterwards (VD-first) and 7 patients were treated first with the deployment of the stent-retriever and intraarterial vasodilators right after (SR-first). Vasodilation occurred in 71.4% of segments in the VD-first group and 82.1% of segments in the SR-first group. No recurrent vasospasm was detected in the SR-first group; however, recurrent vasospasm was detected in 3 patients (60%) in the VD-first group.
In this recently paper published,5 three procedural complications happened, that accounts for 25% of the patients. Two thromboembolic complications in M2 and M3 segments were observed, both completely resolved with 1 and 0.5 mg of tirofiban respectively. In one case, perforation was caused by the microwire tip during navigation of the microcatheter, vessel rupture was solved with the occlusion of the branch with coils without clinical consequences for the patient. No permanent clinical sequelae or mortality were observed in those three patients because of the procedural complication.
Our results are comparable to those published previously, with a high rate of efficacy and low rate of clinical complications. However, our study showed less rate of procedural complications than the previous report. We speculate that these results might be partially due to the continuous flushing of a vasodilator through the guiding catheter in most of the cases, and the deploy of the stent-retriever only in proximal intracranial segments (ICA, MCA, ACA). These results might also be related to the experience of neurointerventionalists with stent-retrievers, thanks to thrombectomy in acute ischemic stroke, making this procedure increasingly safe.
The main limitations of this study are the small size of the sample and the retrospective design, as well as the variability in the devices and vasodilators used, limiting the external validation of our results. Other possible limitation is that we don´t compare our sample with other types of treatment, such as balloon angioplasty or the use of intraarterial vasodilators only. Future studies should aim to replicate these results in a larger multicentric cohort and compare the two main angioplasty methods (stent-retriever and balloon) targeting large-vessel stenosis, in order to determine the highest safety and efficacy profile. It is important to address the difference in the cost of the stentretriever compared to the angioplasty balloon, more than triple in some countries, making it an important consideration in expanding its use in the clinical setting.
The main strength is the multicentric design across four high-volume tertiary level hospital with similar results, obtaining a low procedure complication rate with a good enough efficiency, being used as a first step in the endovascular treatment of vasospasm secondary to aSAH. The multicentric nature of the study also determines different endovascular treatment protocols (e.g. different devices, sizes and device deployment time), that allow their evaluation in order to determine the greatest effect of the stent-retriever angioplasty. Regarding the variables of endovascular treatment, there is a non-significant difference in favor of devices larger than 4 mm and placement longer than 5 min, as well as the administration of vasodilators through the guide-catheter. There is no data available in other papers already published, so this is an important issue for future research, in order to optimize the stent-retriever angioplasty.
To date, balloon angioplasty and intraarterial vasodilators are the most commonly endovascular treatment modalities used in refractory vasospasm due to aSAH. In most of the cases, those modalities are used in combination due to the assumption that balloon angioplasty is more useful in proximal stenosis and vasodilator drugs in distal stenosis. In fact, the discrepancy between clinical outcome and large-vessel stenosis treatment suggest that treating distal stenosis is equally important to improve clinical outcome.6
Balloon angioplasty is the most effective endovascular treatment available to date, in a recent review published by Hoh et cols,7 up to 62% (328/530) of patients treated with balloon angioplasty improved clinically. Both the improvement of the caliber of the vessel and the prevention of ischemic lesions were observed in several studies following balloon angioplasty.8,9 But balloon angioplasty is not without complications, Hoh et cols7 reported major complications in 5% of patients and vessel ruptured in 1.1% of the patients treated.
On a recently published paper,3 aggressive and early endovascular treatment in one cohort demonstrated better clinical outcome compared to a cohort with more restrictive selection for endovascular treatment in terms of delayed cerebral ischemia (20.8% vs 29%, p = 0.0001) and unfavorable outcome (44% vs 50.6%, p = 0.04) respectively. An important clinical implication is that we probably have to treat more patient in an early fashion, in order to prevent delayed cerebral ischemia and unfavorable outcome.
Due to the multifactorial nature of the pathogenesis of DCI2,10,11 and the lack of a strong causal relationship between vasospasm and functional outcome,12,13 we need to stagger the endovascular treatment in aSAH vasospasm from the least effective and with the least complications to the most effective but with the highest complication rate. At the same time, an endovascular treatment in awake patients with symptomatic vasospasm allows you to assess a clinical evaluation of the patient during treatment, in order to make a cause-effect correlation of the symptoms and to determine the treatment effectiveness.
For these reasons, we propose a two-step approach where the first-line treatment is a stent-retriever angioplasty in awake patients. The vasodilator drug is infused through the guide-catheter during the deployment of the stent-retriever in the vessel, in order to enhance the effect of both therapeutic strategies, targeting proximal and distal stenosis at the same time. We also recommend the use of push and fluff technique during the deployment of the stent-retriever in order to increase the radial force of the device against the vessel wall. After this first-line approach, if clinical symptoms and/or vasospasm did not improve or normalized, then we consider to perform the second-line treatment with balloon angioplasty of the proximal segments (ICA and/or MCA) under general anesthesia to improve the safety of the procedure. With this two-step approach, we stagger the endovascular treatment in aSAH vasospasm enabling the clinical evaluation of awake patients as the first-line of treatment, leaning to the safety and efficacy as primary objectives.
In conclusion, stent-retriever angioplasty as treatment for vasospasm secondary to aSAH is a safe and effective procedure that improves the CCT and vessel post-treatment stenosis. More data is needed to optimize stent-retriever angioplasty performance as treatment of vasospasm secondary to aSAH.
Please cite this article as: López-Rueda A, Vargas A, Piñana C, Chirife Ó, Werner M, Aja L et al. Angioplastia con stent recuperable como tratamiento del vasoespasmo secundario a la hemorragia subaracnoidea por aneurisma: un estudio multicéntrico de seguridad y eficacia. Radiología. 2022;64:103–109.