Neuroimaging studies play a fundamental role in the diagnosis and evaluation of epilepsy. Technological advances in neuroimaging techniques have made it possible to obtain functional as well as structural information. The pathophysiology of epilepsy consists of an abnormal increase in cerebral activity that can be appreciated on neuroimaging techniques such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT). In patients with epilepsy that is refractory to drug therapy, the main aim of surgery is to control seizures and thus to improve the quality of life. In the preoperative workup of these patients, fMRI has an increasingly important role, evaluating the location of functional areas that must be safeguarded during surgery.
Los estudios de neuroimagen tienen un papel fundamental en la evaluación y diagnóstico de la epilepsia. Los avances tecnológicos que se han producido en las técnicas de neuroimagen permiten en la actualidad obtener información no solo estructural, sino también funcional. La fisiopatología de la epilepsia consiste en un aumento anómalo de la actividad cerebral que puede llegar a apreciarse mediante técnicas de neuroimagen, como por ejemplo la RM funcional (RMf), la tomografía por emisión de positrones (PET) y la tomografía por emisión de fotón simple (SPECT). En pacientes con epilepsia farmacorresistente, la cirugía tiene como objetivo principal el control de las crisis, y por tanto mejorar la calidad de vida. La RMf juega un papel cada vez más relevante en la valoración prequirúrgica de estos pacientes, evaluando la localización de áreas funcionales que deben evitar lesionarse durante el acto quirúrgico.
Patients with refractory epilepsy can benefit from surgery, provided that the location of the region where the seizure originates can be determined. Neuroimaging techniques play a major role in epileptogenic focus localization, and are particularly effective in high definition structural studies, which allow for the localization of epileptogenic focus in almost 90% of patients with temporal lobe epilepsy (TLE).1 However, outcomes are not so accurate when imaging patients with extratemporal epilepsy.1
The objective of epilepsy surgery is the complete resection or complete disconnection of the epileptogenic zone (EZ), defined as the area of cortex indispensable for the generation of clinical seizures, with the preservation of the eloquent cortex,2 since the ultimate goal is to improve patients’ quality of life.
Although structural studies using magnetic resonance imaging (MRI) remain the main diagnostic imaging modality for refractory epilepsy, functional neuroimaging techniques are gaining growing relevance, particularly in the localization of eloquent areas that may be involved during surgery as well as in the evaluation of functional changes, since these technique may, in some patients, allow the localization of EZs that show normal structural imaging.
Functional MRI (fMRI) is a non-invasive technique that is increasingly gaining acceptance in the preoperative evaluation of patients with epilepsy because it indirectly helps in the localization of eloquent areas associated with the EZ. Hippocampus resection is the most widely used surgical procedure in patients with refractory partial epilepsy. For this reason, it is essential to establish hemispheric lateralization of language and memory dominance prior to surgery.
Some techniques, such as ictal SPECT and ictal fMRI, provide information about the changes that occur during a seizure. In contrast, other techniques, such as PET, obtain information during the so-called “interictal period”.
The purpose of this review is to provide an update of the new applications of functional neuroimaging studies to epilepsy. We will focus on fMRI studies used for mapping, language dominance, and memory evaluation, as well as on ictal fMRI and the role played by nuclear medicine functional imaging modalities (PET and SPECT) in patients with epilepsy.
Functional magnetic resonance imagingThe current standard fMRI technique is BOLD, which relies on the signal changes that occur in the venous flow as a result of excessive deoxyhemoglobin following a rise in perfusion during brain activation. Signal changes occurring during brain activation are very subtle, and thus it is advisable to use high-field MRI systems. In clinical practice, most fMRI studies can be performed on a 1.5-T scanner, although 3-T scanners are recommended if available. In 3-T fMRI systems, the BOLD signal increases 4–5% in comparison to 1.5-T systems, and although susceptibility artifacts increase with high field strengths, they can be reduced by using high-resolution images, reducing acquisition times with parallel-imaging techniques.3
Two important aspects must be taken into consideration before conducting an fMRI study. The first one is the type of study design. Although block designs are the standard method, some authors suggest that event-related designs provide more reliable outcomes for memory evaluation in fMRI studies.4 For the design of cognitive paradigms, such as language and memory paradigms, it is necessary to take previously validated neuropsychological studies as a reference. The second aspect to be considered is the analysis of the acquired images. Signal changes that occur between the activation and rest periods are subtle, and must be distinguished from signal changes that are not caused by brain activation processes, such as image noise. Therefore, statistical analysis of the data is required to determine if the signal changes identified have a pattern similar to that of the hemodynamic changes expected to occur during brain activation.
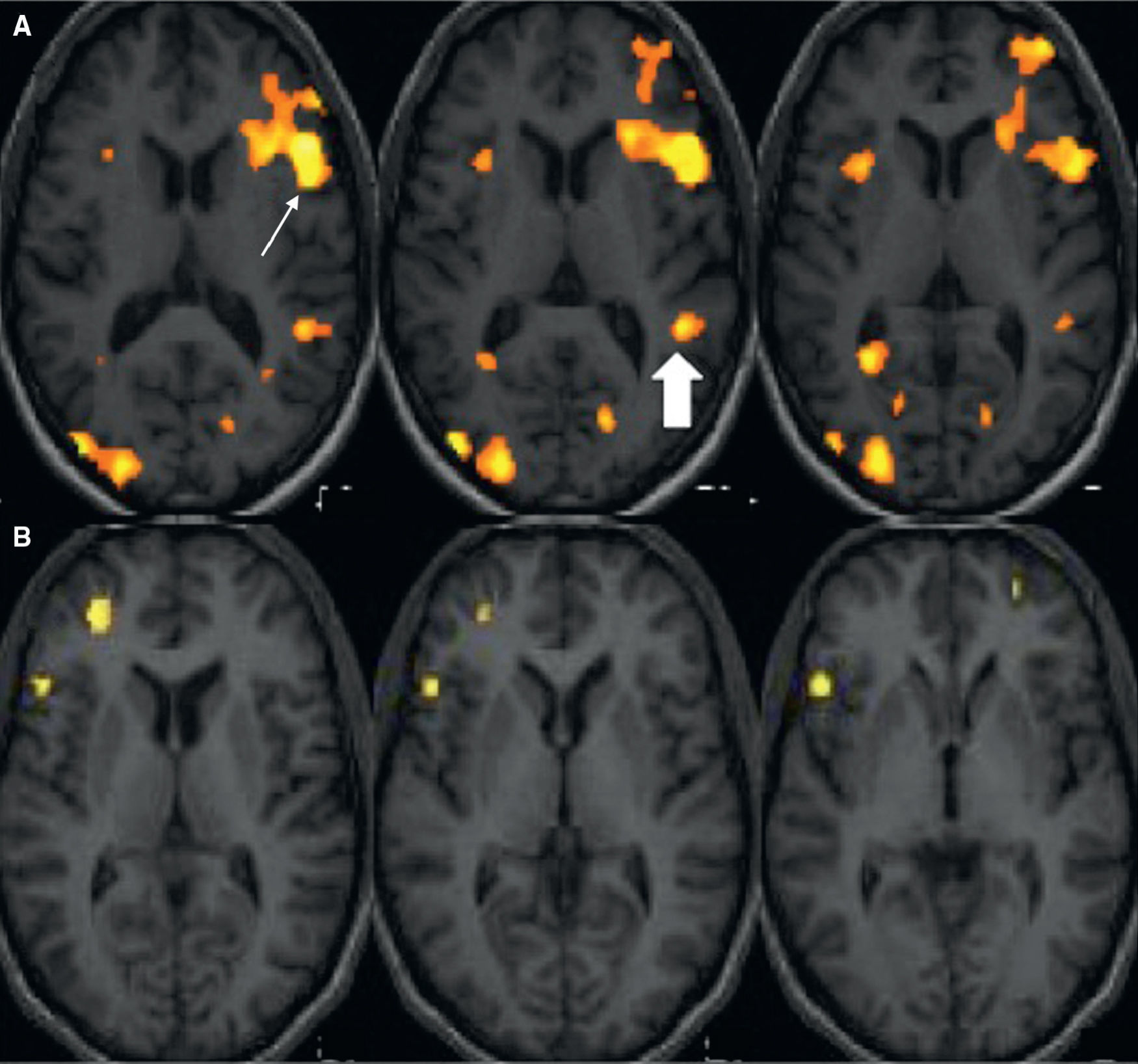
Functional magnetic resonance imaging for language lateralization and mappingThe most common clinical indication of fMRI is the determination of language hemispheric dominance. Resection of the anterior region of the temporal lobe and of mesial structures, such as the hippocampus, is the most common surgical procedure in refractory epilepsy. This resection may result in specific language deficits, such as dysnomia, and/or to memory deficits, such as amnesia, depending on the language hemispheric dominance. Verbal memory is located in the language dominant hippocampus. In patients with limited functional memory storage due to chronic epilepsy, resection of the language dominant hippocampus increases the risk of amnesia. It has been shown that there is a rise in the number of left-handed patients or patients with atypical language lateralization in the epilepsy population5–7 (Fig. 1). Consequently, it is crucial to determine the language hemispheric dominance in these patients, particularly when they are to undergo surgery in the theoretically dominant hemisphere (the left hemisphere in the majority of the population). Furthermore, in pediatric patients with hemispheric lesions, such as cerebral infarctation and encephalitis, brain functions may be reorganized or transferred to the nonaffected hemisphere. For this reason, dominant hemisphere determination is critical for functional hemispherectomy (i.e. disconnection of one hemisphere from the contralateral one)8,9 (Fig. 2). Some authors have reported interesting cases in which Broca's area of certain patients is located in one hemisphere and Wernicke's area is located in the contralateral one. This condition, known as “interhemispheric dissociation of frontal and temporal regions”, would suggest incomplete language tranfer.10,11
Language functional MRI with a word-generation paradigm. Structural axial images fused with the activation maps in the frontotemporal region. (A) Right-handed subject. Activation is observed in the left inferior frontal gyrus (Broca's area) (arrow) and in the left posterior temporal gyrus (Wernicke's area) (filled arrow) demonstrating left-hemispheric language dominance. (B) Left-handed subject. Activation is obtained in the right inferior frontal gyrus and dorsolateral right cortex, showing right-hemispheric language dominance.
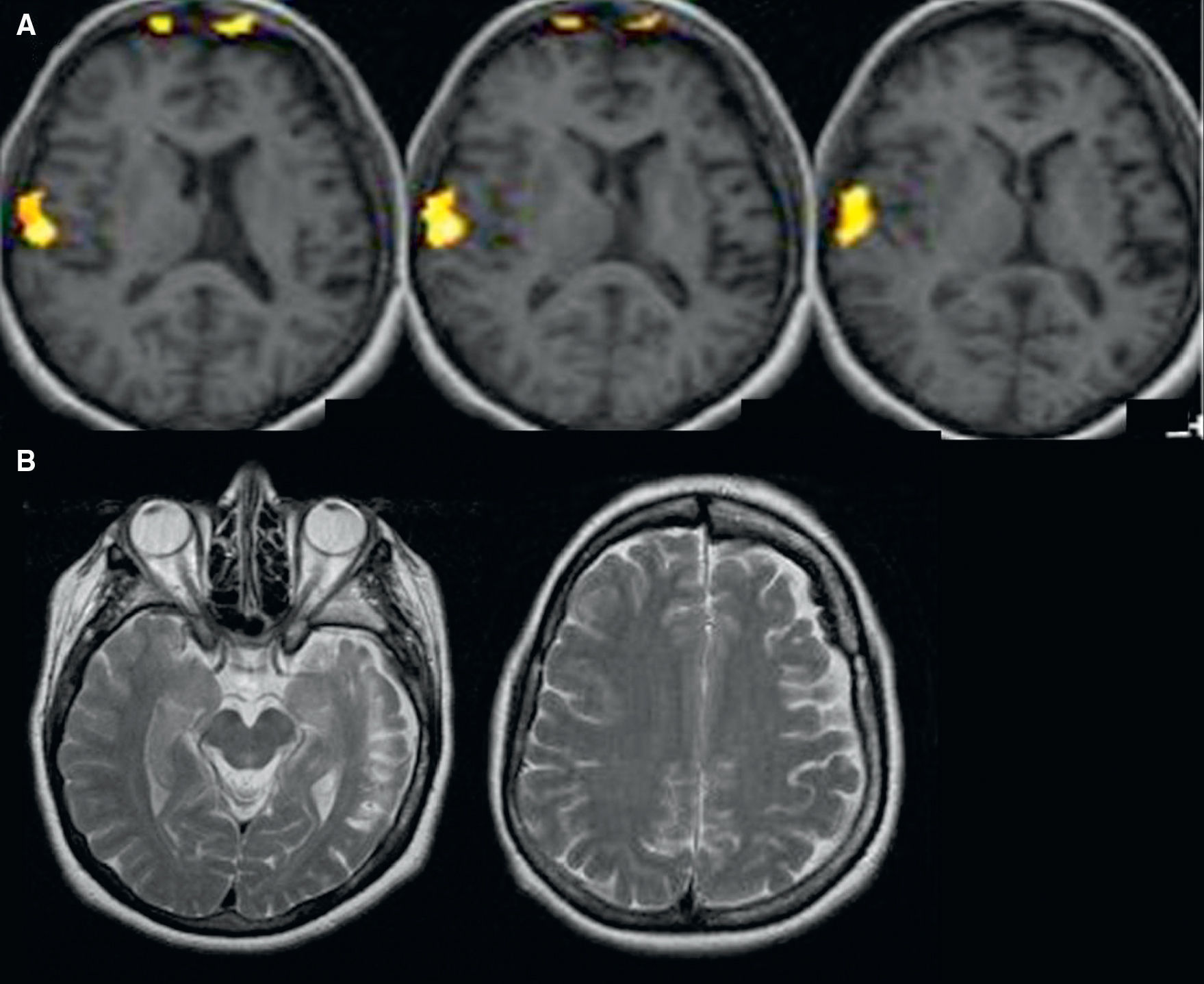
(A) Language functional MRI with an auditory comprehension paradigm. Structural axial images fused with the activation maps in the frontotemporal region in an epilepsy patient with a history of meningitis at the age of three. Activation is obtained in the posterior temporal region, demonstrating right-hemispheric language dominance. (B) Structural axial T2-weighted images show cortical atrophy of left hemisphere and signal changes of the left temporal lobe.
Until recently, the Wada test, also known as “intracarotid amobarbital procedure”, was the method of choice to determine language hemispheric dominance. In this test, one hemisphere undergoes local anesthesia with intracarotid amobarbital, which enables to determine what cognitive functions are affected. In addition to being an invasive technique, the Wada test is a questionable procedure, particularly because it is not possible to determine whether the theoretically anesthetic-free hemisphere becomes contaminated by collateral circulation between anterior circulation and posterior circulation.
The technique of choice to localize primary language areas is intraoperative cortical mapping or intracranial electrode stimulation. However, this is a very aggressive technique, which is only used when the area for surgical resection is very close to the theoretical areas associated with language.2
Over the last years, there has been a growing interest in the use of fMRI as an alternative to more invasive techniques. A number of studies that compare the Wada test with language fMRI show a very strong correlation between both techniques.12–17
Benke et al.12 reported on a series of 68 patients with chronic intractable TLE who underwent the Wada test and fMRI scanning based on a semantic decision paradigm to determine language lateralization. Findings showed a very strong congruence between both techniques in patients with right TLE (89.3%), and a reasonably good congruence in patients with left TLE (72.5%).
All these findings confirm the clinical usefullness of fMRI to determine language lateralization, and suggest that more invasive techniques, such as the Wada test, should be reserved for those cases showing discordance between clinical, neuropsychological, and fMRI findings.
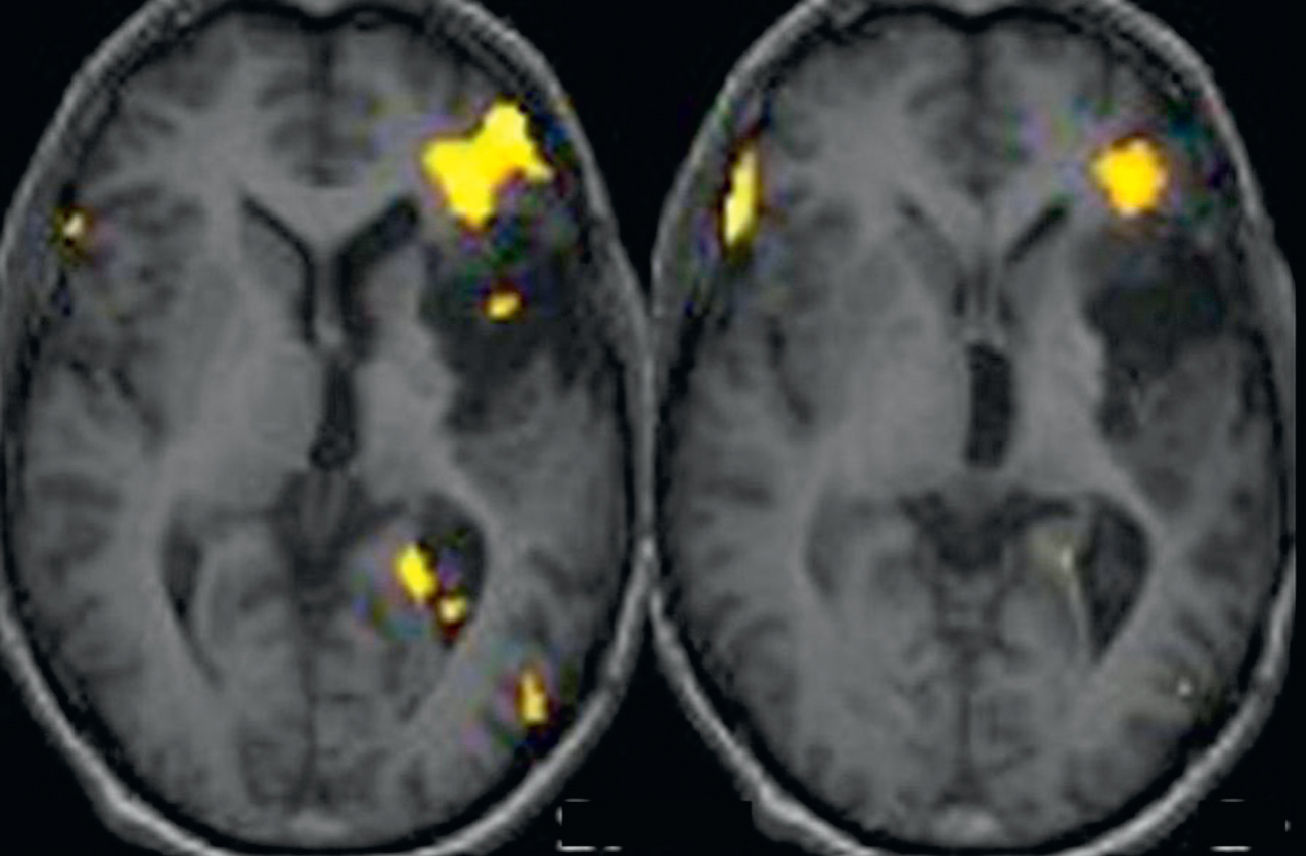
fMRI studies have also been used for the anatomical localization of eloquent language areas. Exact localization of the regions that control language functions varies significantly between subjects, and should be determined prior to surgery (Fig. 3). Correlation between fMRI and cortical mapping of the primary motor cortex has been demonstrated in a number of studies.18–21 However, only a few studies have described the correlation between fMRI and intraoperative mapping of language areas. Roux et al.22 only found correlation between fMRI and the areas involved in motor language process. However, in a more recent retrospective study, Petrovich et al.23 reported good correlation between fMRI and intraoperative cortical mapping in the inferior frontal gyrus, especially if the fMRI study involved the use of a vocalized speech task to test verbal fluency.
Language functional MRI with a word-generation paradigm in an epilepsy patient with a partially resected left astrocytoma in the insular region of the brain. Structural axial images fused with the activation maps in the frontotemporal region. Activation is observed in the left inferior frontal region adjacent to the mass.
It should be noted that there is a high incidence of atypical language lateralization in epilepsy patients, and particularly, in child-onset epilepsy patients.5,6,24 Therefore, in epilepsy surgery of eloquent language areas, fMRI can help in the localization of these cortical areas, but invase techniques such as intraoperative mapping and cortical stimulation with invasive electrodes are still essential to prevent language deficits in these patients.
Functional magnetic resonance for memory evaluationThe evaluation of the memory function is essential in patients with TLE. It has been demostrated that mesial sclerosis may lead to visual or verbal memory deficit, depending on the language dominant hemisphere.25
The standard technique to assess memory lateralization is neuropsychological evaluation. Invasive procedures, such as the Wada test, are reserved only for those cases involving a high risk of postoperative amnesia.
Great interest exists regarding the value of fMRI as a technique for memory evaluation, not only in epilepsy but also in other conditions, such as dementias.
Memory evaluation by fMRI poses a major challenge and there are a number of aspects concerning this technique that must be taken into consideration. Anatomic location of the hippocampus increases the magnetic susceptibility artifacts produced by the nearby bone and air of the paranasal sinuses, especially if fMRI sequences are used. This increase may mask hippocampal activation.26,27 Susceptibility artifacts can be partially reduced with parallel acquisition techniques.
Memory processing is a complex cognitive function. For this reason, developing a paradigm for memory is much more difficult than for language. In memory processing, different components are involved, such as encoding and retrieval of the information to be memorized. Furthermore, depending on the type of presented material (visual or verbal), different brain areas are activated (non-dominant hemisphere for visual memory and dominant hemisphere for language). Other brain areas are also involved in memory processing, such as prefrontal regions, which are associated with attention functions.28
In the literature, there is a large number of different fMRI experiments for memory assessment using different stimuli, including words, faces, objects, scenes and routes, memory functions (retrieval and encoding), and paradigms (block or event-related paradigms). The majority of these studies have shown activation of the prefrontal cortex and mesial temporal structures. The posterior body of the hippocampus, the parahippocampal and fusiform gyri are the mesial temporal regions that show higher activation. The absence of activation of the hippocampal head could be explained by the presence of susceptibility artifacts, very common in this intracranial region.27
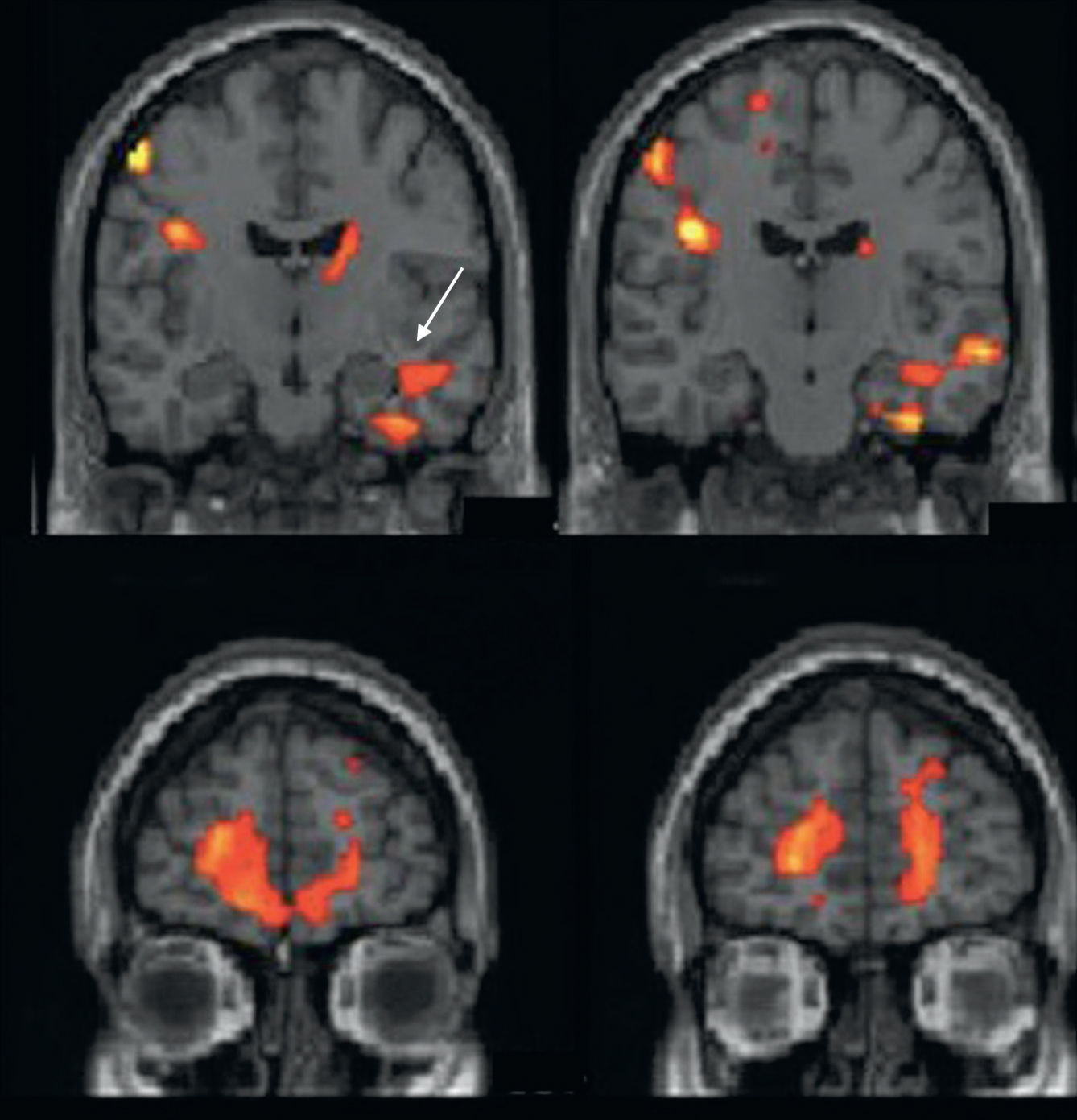
Although some authors have reported material-specific lateralization in memory processing activation with fMRI experiments,28,30 bilateral hippocampal activation is mostly observed when using encoding or recall tasks29,31,32 (Fig. 4).
Bilateral activation of mesial temporal structures could suggest that visual as well as verbal strategies are being used during encoding or recall tasks.
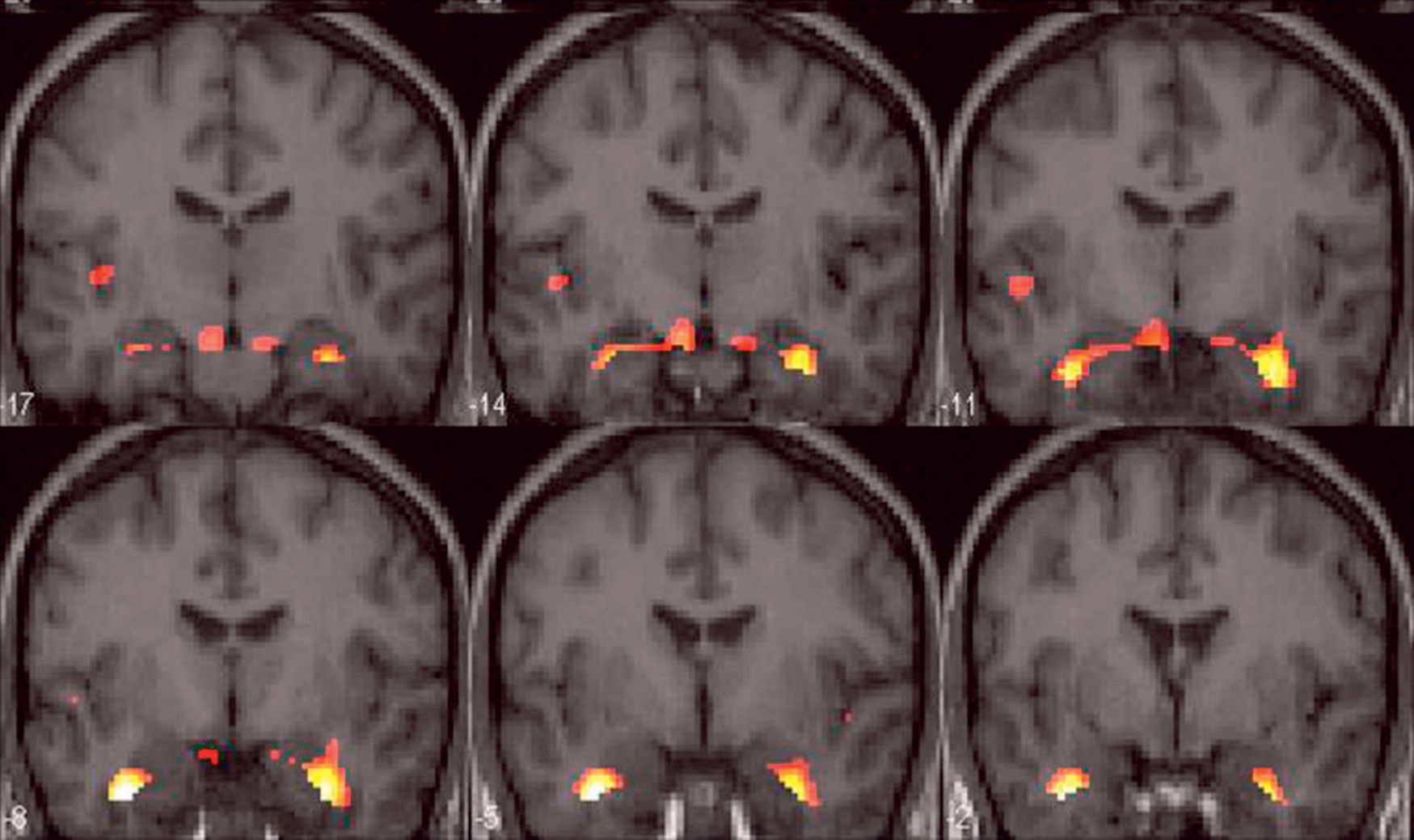
It should be pointed out that much of the data obtained from memory paradigms with fMRI refers to group studies, and thus, these results cannot be accommodated in clinical practice, because to yield robust results as a diagnostic or prognostic tool, it is mandatory to obtain both group and single-subject level data from fMRI memory paradigms. However, some authors have begun to study the usefulness of fMRI for memory assessment in patients with refractory TLE. Few studies have compared memory fMRI and Wada test performance. However, these studies show encouraging results.25,32,33 More studies have been performed comparing fMRI activation for memory evaluation between control and TLE patients. While control subjects usually show bilateral activation of mesial temporal structures, patients with mesial sclerosis mostly show unilateral activation in the unaffected hippocampus32,34–37 (Fig. 5).
Memory functional MRI with a retrieval paradigm. Group study in patients with right temporal epilepsy. Structural coronal images fused with the activation map show unilateral activation in left mesial temporal structures (arrow) and bilateral—though right-predominant—activation of the prefrontal cortex.
There are very few studies that look into the role played by memory fMRI to predict postsurgical memory deficits following temporal lobe resection. However, very promising results have been achieved.35,38,39 Some authors have recently demonstrated that language fMRI studies—particularly semantic comprehension studies, which are easy to reproduce—could predict postsurgical memory deficits. Memory paradigms of this type, which are less complex both for acquisition and analysis purposes, could facilitate research. However, further information is required to draw conclusions.40,41
Therefore, the role of memory fMRI in the presurgical evaluation of TLE remains unclear. Obviously, fMRI is less costly, non-invasive, and far more accessible than the Wada test. Nevertheless, the aspects previously discussed would explain why fMRI has not been validated yet as a diagnostic tool in clinical practice.
Ictal functional magnetic resonance imaging for localization of the epileptogenic focusIn recent years, a number of studies have been conducted that use fMRI in epilepsy patients in order to determine the location and extension of the cortical area involved in the onset of epileptic seizures.40,42–45
BOLD contrast used in fMRI studies shows the hemodynamic changes related to brain activity. Therefore, when a seizure is generated, giving rise to excessive brain activity, there will be changes in the BOLD signal of the areas involved. It has been demostrated that there is a decrease in BOLD signal in areas distant from the EZ some seconds before the electrical changes occur. This decrease is followed by a very significant increase at ictal onset that co-occurs with the electrical changes. Increases in BOLD signal spread to other brain areas, helping to identify the seizure propagation pattern.46
However, performance of ictal fMRI is complex. The image artifacts produced by motion of patients during seizure are a major drawback. In fact, only patients with partial—and preferably non-motor—seizures are candidates for this technique. Furthermore, an electroencephalography (EEG) recording is necessary during the fMRI acquisition period to correlate functional data with electrical brain activity. EEG recorders must be MRI compatible and their results must be analyzed with filters a posteriori because the magnetic field interferes significantly with the recording. Finally, the design and statistical analysis for image assessment are complex.45,46
The studies using ictal fMRI are mostly isolated or they include limited series. These studies describe focal signal changes that correlate with structural lesions.47–49
Our experience with ictal fMRI has provided good results (Fig. 6). However, this is such a complex technique that it will be very difficult to implement as a routine method in clinical practice.
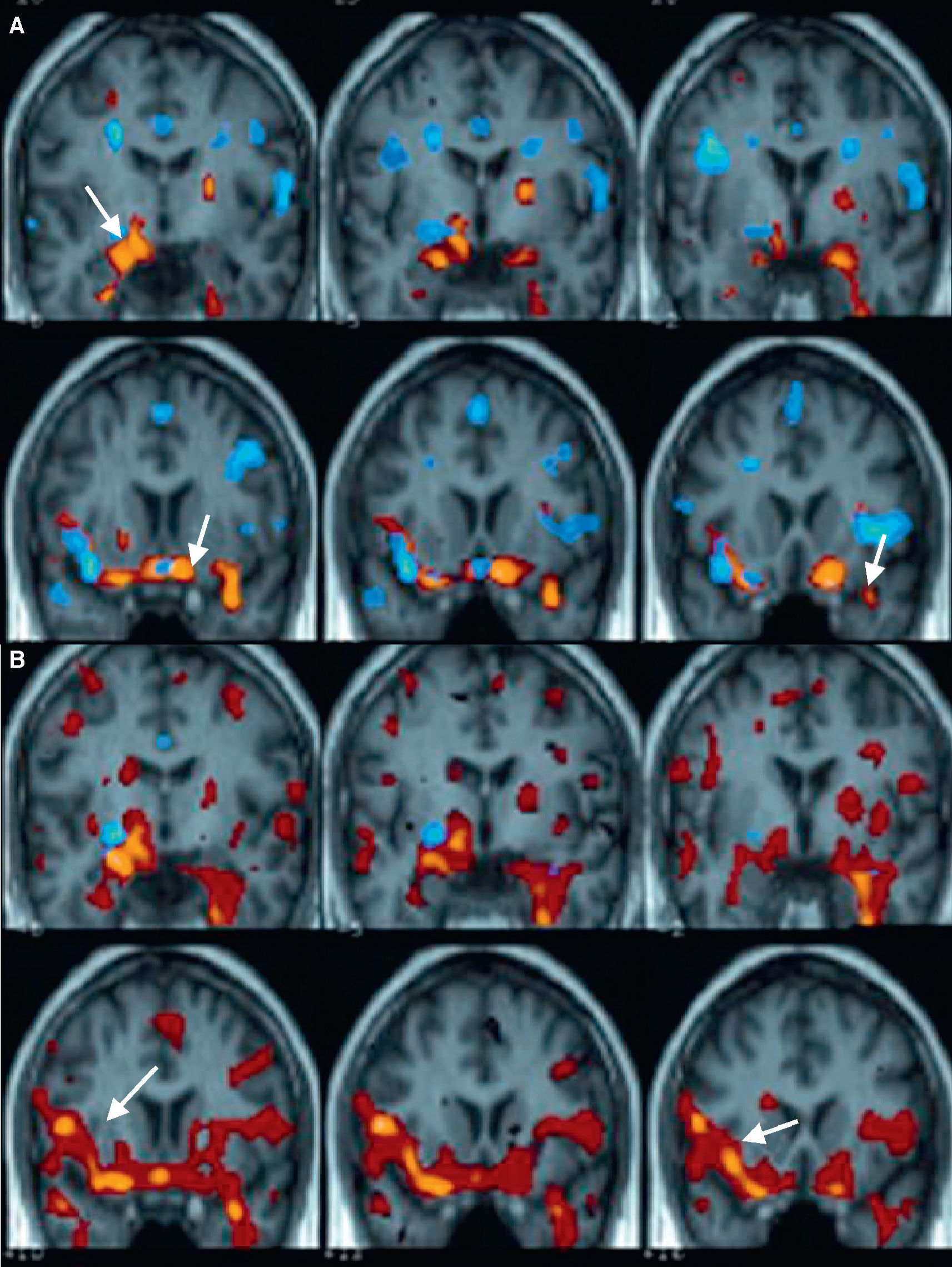
Ictal functional MRI. Patient with epilepsy seizures that have a right mesial temporal onset. Structural coronal images fused with the activation map (reduction in activation is shown in blue; activation is shown in red). (A) At the onset of the electroencephalographic changes, bilateral mesial temporal activation is observed (arrow) (likelihood of rapid propagation to the left cerebral hemisphere). (B) Three seconds after seizure onset, there is propagation to the right insula (arrows) and marked right-predominant temporal activation are observed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
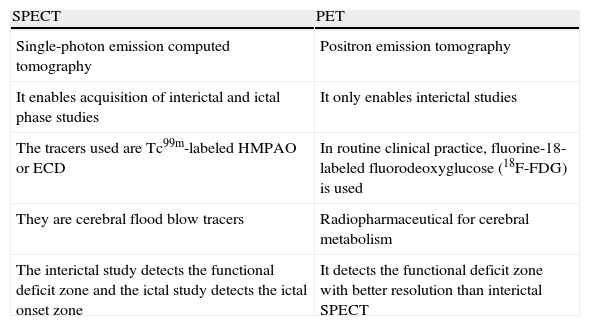
The nuclear medicine examination techniques that have been shown to be valid for localization of the EZ are PET and SPECT. The main features of and differences between both these techniques are detailed in Table 1.
Features of SPECT and PET.
| SPECT | PET |
| Single-photon emission computed tomography | Positron emission tomography |
| It enables acquisition of interictal and ictal phase studies | It only enables interictal studies |
| The tracers used are Tc99m-labeled HMPAO or ECD | In routine clinical practice, fluorine-18-labeled fluorodeoxyglucose (18F-FDG) is used |
| They are cerebral flood blow tracers | Radiopharmaceutical for cerebral metabolism |
| The interictal study detects the functional deficit zone and the ictal study detects the ictal onset zone | It detects the functional deficit zone with better resolution than interictal SPECT |
ECD, ethylcysteinate dimer; HMPAO, hexamethylpropyleneamine oxime; PET, positron emission tomography; SPECT, single-photon emission computed tomography; Tc99m, technetium-99m.
Brain perfusion SPECT imaging is a functional brain examination technique that relies on tracers that have the ability to cross the blood–brain barrier and distribute inside brain cells in proportion to cerebral blood flow. These tracers are hexamethylpropyleneamine oxime (HMPAO) and ethylcysteinate dimer (ECD), both labeled with technetium-99m (Tc99m). In patients with complex partial seizures, brain SPECT shows different patterns of tracer uptake and distribution depending on the location of the epilepsy (temporal, mesial or neocortical) and on the moment at which the perfusion tracer is injected (ictal or postictal).
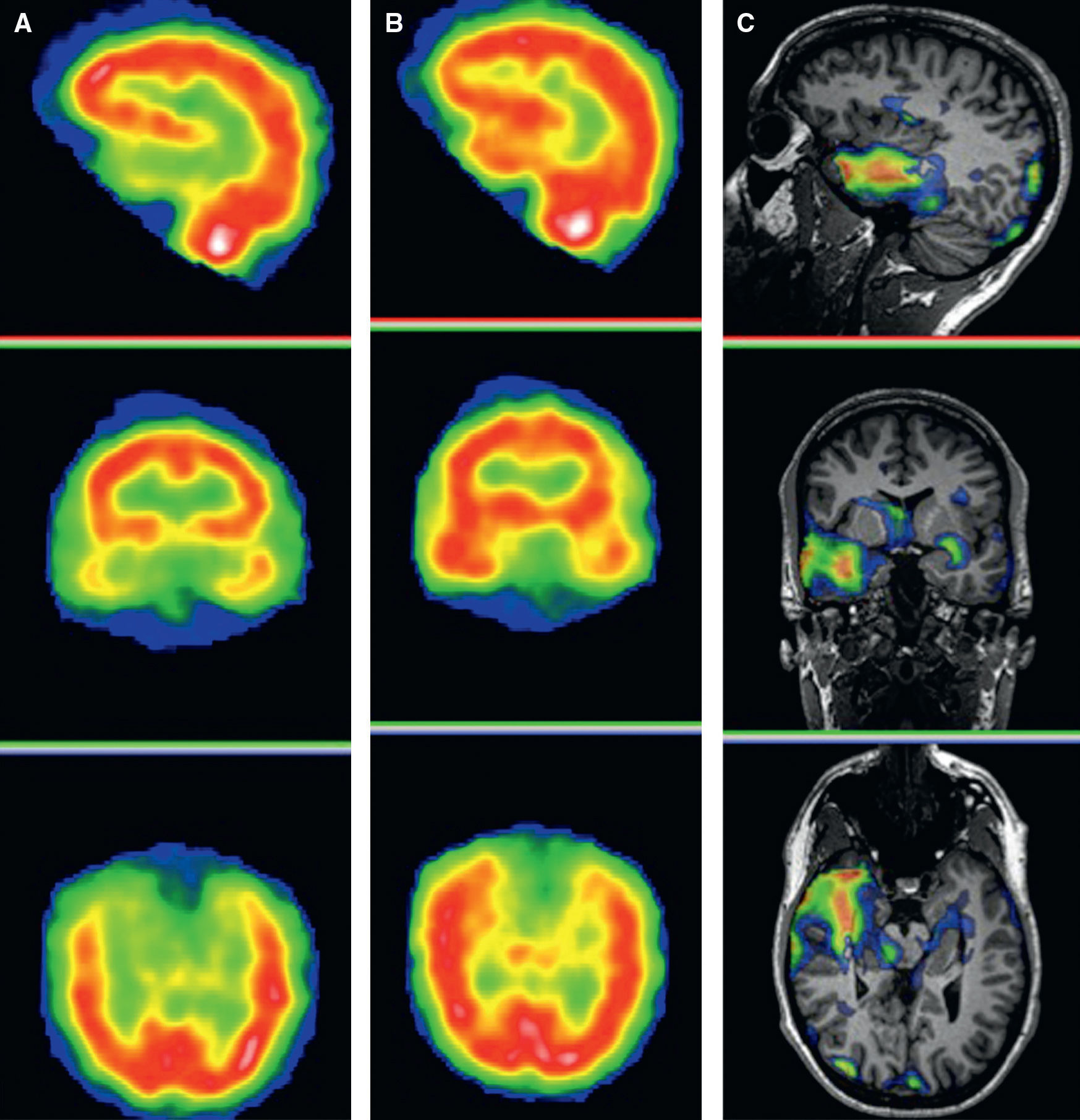
Brain single-photon emission computed tomography in mesial temporal epilepsyInterictal SPECT involves injecting the tracer with the patient in baseline condition, at rest, and seizure-free for over a 24-h period. Interictal SPECT shows the EZ as a hypoactive focal area, which means a low-perfusion region. In TLE, interictal SPECT shows decreased perfusion in the temporal region (Fig. 7A), which is usually localized in the mesial area of the anterior horn of the temporal lobe.
(A) Sagittal, coronal and axial interictal SPECT of a patient with complex partial seizures in the right temporal lobe. SPECT image shows a decrease in perfusion in the right anteromesial temporal region. (B) Sagittal, coronal and axial brain SPECT images of the same patient, who was injected the tracer during a complex partial seizure, which was localized in the right temporal lobe. SPECT image shows an increase in perfusion in the right temporal lobe, exactly where the EZ is located. (C) Images showing fusion of the ictal-interictal SPECT subtraction coregistered to the MRI of the same patient. An increase in perfusion in the anterior pole of the right temporal lobe with mesial region predominance is observed.
In the literature, interictal SPECT sensitivity to lateralize the EZ in complex partial seizures of the temporal lobe varies significantly (35–80%), with a mean value of 50%. According to our experience,50 interictal SPECT sensitivity to localize the EZ in complex partial seizures of the temporal lobe was 68%.
Ictal SPECT involves tracer injection during the epilepsy seizure and the scintigraphic images can be acquired up to 2h later, once the seizure has been controlled. Comparison of brain perfusion between ictal and interictal SPECTs performed on the same patient in two different situations may assist in localizing the EZ with a diagnostic sensitivity>90% in patients with temporal lobe seizures.51 In these conditions, brain SPECT shows an increase in radiopharmaceutical uptake in the EZ secondary to an increase in the regional cerebral blood flow during the seizure (Fig. 7B).
Tracer injection is usually delayed for some seconds, being performed once ictal activity has ended. The SPECT image acquired, known as “postictal SPECT”, usually shows the temporal EZ as focal tracer hyperperfusion in the mesial temporal cortex and as diffuse lateral temporal hypoperfusion. As time between the end of seizure and tracer injection increases, the hyperperfusion area decreases and the hypoperfusion area extends to involve almost the entire temporal lobe. Sensitivity of postictal SPECT for EZ detection is 75% in TLE.51
Brain single-photon emission computed tomography in neocortical epilepsyThe diagnostic accuracy of both interictal and ictal SPECT significantly decreases in neocortical epilepsy, which includes extratemporal epilepsy and extralimbic temporal epilepsy. Sensitivity of interictal SPECT to localize hypoactive areas in the EZ ranges from 15% to 30%, and it would not be therefore recommended for this clinical situation. The only application of interictal SPECT in neocortical epilepsy would be the comparative analysis between interictal and ictal images.
Ictal SPECT is the most accurate diagnostic imaging technique for localization of the EZ in extratemporal epilepsy. Sensitivity of ictal SPECT is >75% in almost all the series found in the literature.52 As with temporal epilepsy, ictal SPECT in neocortical epilepsy detects a focal perfusion increase in the EZ.
However, ictal SPECT scans are not easy to acquire due to brevity of extratemporal seizures. In most cases, it is postictal SPECT that is performed in extratemporal epilepsy, yielding very poor results because sensitivity drops to 20–50%.51
SISCOM (Subtraction Ictal Spect Co-registered to Magnetic Resonance Imaging) has been recently implemented to optimize surgical outcomes by combining SPECT and MRI images. SISCOM improves the diagnostic performance of SPECT and MRI by co-registering MRI morphologic data to SPECT functional data. A SISCOM image results from fusing the difference image between ictal SPECT and interictal SPECT with the MRI image of the same patient. The SISCOM methodology improves localization of the EZ, and provides an accurate anatomic localization of the EZ (Fig. 7C).53
The main clinical indications for SISCOM are neocortical and temporal epilepsies free of MRI-positive structural lesions; extensive or multiple epileptogenic lesions, such as bilateral mesial sclerosis and bilateral cortical dysplasias; epilepsies showing discordance between examinations (video-EGG and MRI); and epilepsies in patients with postsurgical seizure relapse.
In general, the SISCOM technique can assist in localizing the area under examination in all cases suggesting subdural electrode placement, improving the accuracy of this invasive technique. SISCOM plays a crucial role in treating patients with malformations of cortical development (MCD), since it has been demostrated that the dysplastic area is not entirely epileptogenic. Concretely, SISCOM could help in determining the surgical resection area.54–56 O’Brien et al.57 showed that ictal SPECT with SISCOM and non-localizing MRI with SISCOM localized the EZ in 19 out of 22 patients with dysplasia (86%) and in 8 out of 10 patients with dysplasia, respectively.
Positron emission tomographyThe basis of fluorine-18-labeled fluorodeoxyglucose (18F-FDG) PET is that intracraneal glucose distribution equals cerebral metabolism. 18F-FDG PET is used in interictal studies for presurgical evaluation because ictal studies are difficult to obtain due to slow brain glucose uptake and the short decomposition time of 18F. Interictal PET detects a focal decrease in glucose uptake, which is described as hypometabolism reflecting a focal functional brain deficit associated with the EZ. The main advantage of 18F-FDG PET over interictal SPECT is that 18F-FDG PET provides a higher spatial resolution and higher diagnostic sensitivity for EZ localization.
Brain single-photon emission computed tomography in mesial temporal epilepsyPET sensitivity in mesial temporal epilepsy ranges from 80% to 90%, and can detect focal temporal hypometabolism in patients free of MRI-positive mesial sclerosis.58 PET-positive anterior temporal hypometabolism in patients with mesial temporal sclerosis is usually more extensive than the MRI-positive lesion itself. This may be because the PET-positive hypometabolic area not only includes the MRI-positive lesional area, but also the functional deficit zone.
PET is particularly indicated in cases of suspected mesial temporal epilepsy, with discordant video-EEG and MRI, and in MRI studies that do not show structural changes.
Brain positron emission tomography in neocortical epilepsyThe role played by PET in neocortical epilepsy seems to be more significant since this disorder is more difficult to diagnose. PET findings with 18F-FDG can be particularly useful in patients with very subtle or ill-defined lesions on MRI, such as malformations of cortical development (MCD), and in patients with normal or non-localizing MRI studies. MRI sensitivity in cortical dysplasias ranges from 30% to 80%, whereas PET sensitivity ranges from 70% to 90%.55 In patients with MCD, the hypometabolism detected on PET can reveal a more extensive cortical dysplasia than that detected on MRI. In other cases, PET assists in confirming the diagnosis of very subtle or undefined lesions on MRI or it can even localize dysplasias in patients with normal MRI.
Nonetheless, PET studies in extratemporal epilepsy are still scarce and most of them focus on patients with childhood epilepsy. PET successful performance in childhood epilepsy mar be due to a higher percentage of neocortical epilepsy cases (>50%) or to a higher incidence of cortical dysplasias, where the diagnostic accuracy of MRI is lower.
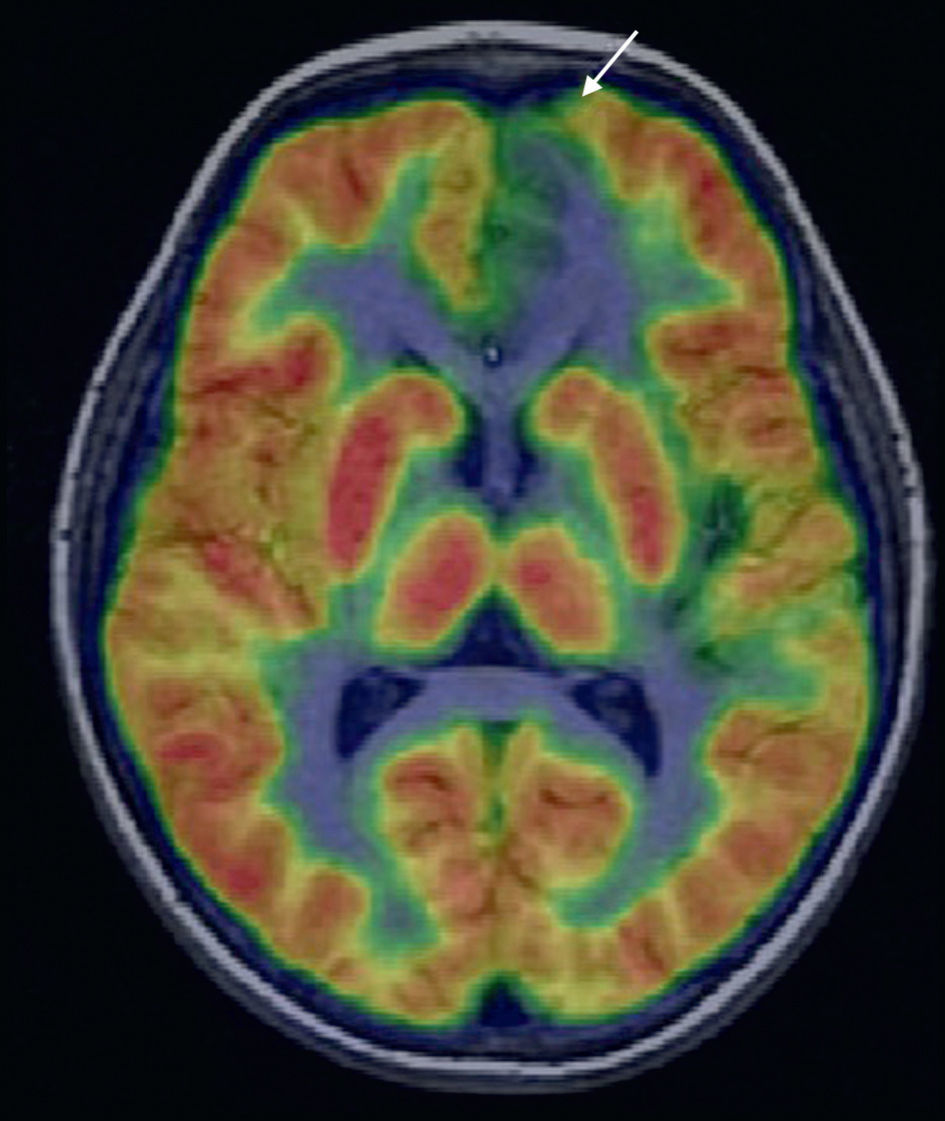
A recent study by Salamon et al.59 has demostrated that a methodological variant of PET involving overlaying of tomographic images in a color scale on the MR images of the patient by coregistration, significantly improves PET diagnostic accuracy in cortical dysplasias. With this methodological variant (Fig. 8), PET sensitivity in cortical dysplasias can reach 70–80%. PET/MRI coregistration allows for localization of a hypometabolic lesion in 33% of patients with discordant or negative EEG and MRI findings. For these reasons, in patients with extratemporal epilepsy and normal MRI or with an undefined lesion, PET and PET/MRI coregistration may, in addition to improve the diagnostic accuracy of MRI, help to delimitate the extension of the surgical resection, thus avoiding incomplete resections of the EZ.60
ConclusionThe main objective of both structural and functional (PET, SPECT, ictal fMRI) neuroimaging studies is to localize the EZ, and thus, provide the best surgery planning to improve the quality of life of patients with epilepsy. In temporal epilepsy, both structural MR neuroimaging and functional (PET and SPECT) techniques provide high diagnostic accuracy. It is in extratemporal epilepsy with no structural lesion where functional neuroimaging techniques play a crucial role because they can provide information on the localization of the EZ.
It has been demostrated that language fMRI is an effective tool to determine language hemispheric dominance. Although the usefulness of fMRI in the determination of cognitive memory storage has not been scientifically proven yet, this technique will eventually become a routine tool for the clinical management of patients with drug-refractory epilepsy.
Authorship- (1)
Responsible for the integrity of the study: NB
- (2)
Conception of the study: NB, XS
- (3)
Bibliographic search: NB, XS
- (4)
Drafting of the paper: NB, XS
- (5)
Critical review with intellectually relevant contributions: NB, XS
- (6)
Approval of the final version: NB, XS
The authors declare no conflict of interests.
Please cite this article as: Bargalló Alabart N, Setoain Parego X. Imagen en epilepsia: estudios funcionales. Radiología. 2012;54:124–36.