Controlled ovarian stimulation is an essential part of in vitro fertilization (IVF) cycles. The aim of this process is to permit follicular aspiration of approximately 8–10 oocytes. Individual women have different ovarian responses based on their ovarian reserves. Low doses of exogenous follicle-stimulating hormone (FSH) may lead to cancelation of an IVF cycle as a result of insufficient response, and high doses may also lead to cancelation due to the risk of ovarian hyperstimulation syndrome. Knowing the patient's ovarian response permits the use of individually tailored doses of gonadotropin, resulting in decreased occurrence of inappropriate ovarian responses.
ObjectiveTo conduct a systematic review of antral follicle count (AFC) performance in adjusting the dose of gonadotropins to prevent inadequate responses in IVF cycles.
MethodA systematic review was conducted of studies published in the last 13 years that appraised AFC performance in adjusting the dose of gonadotropins to prevent inadequate responses in IVF cycles. The databases consulted were Medline, LILACS, SciELO and Pubmed. Search descriptors were “antral follicle count” and “ovarian hyperstimulation syndrome”.
Results131 articles were found. Five articles published between 2000 and 2013 were selected.
ConclusionAFC appears to perform well in adjusting the dose of exogenous gonadotropins to prevent inappropriate responses in IVF cycles.
A estimulação ovariana controlada é parte essencial de ciclos de fertilização in vitro (FIV). O objetivo deste processo é permitir a aspiração folicular de aproximadamente 8–10 oócitos. As mulheres têm respostas diferentes e individuais, baseadas em suas reservas ovarianas. Baixas doses exógenas de hormônio folículo estimulante (FSH) podem levar ao cancelamento do ciclo de FIV como resultado de resposta insuficiente e altas doses também podem levar ao cancelamento devido ao risco da síndrome de hiperestimulação ovariana. O conhecimento da resposta ovariana das pacientes permite o uso de doses individuais adaptadas de gonadotrofinas, resultando em diminuição da ocorrência de respostas inadequadas do ovário.
ObjetivoRealizar revisão sistemática do desempenho da contagem dos folículos antrais (CFA) no ajuste da dose das gonadotrofinas para evitar respostas inadequadas em ciclos de FIV.
MétodoRealizou-se revisão sistemática de estudos publicados nos últimos 13 anos que avaliaram o desempenho da CFA no ajuste das doses de gonadotrofinas para evitar respostas inadequadas em ciclos de FIV. As bases de dados consultadas foram Medline, LILACS, SciELO e Pubmed. Os descritores de pesquisa foram “contagem de folículo antral” e “Síndrome de hiperestimulação ovariana”.
ResultadosForam encontrados 131 artigos. Cinco artigos publicados entre 2000 e 2013 foram selecionados.
ConclusãoA CFA parece ter um papel importante no ajuste das doses de gonadotrofinas exógenas para evitar respostas inapropriadas em ciclos de FIV.
Controlled ovarian stimulation is an essential part of in vitro fertilization (IVF) cycles; the objective of this procedure is to obtain a reasonable number of oocytes that can be fertilized. In clinical practice, specialist physicians generally rely on their experience to select the initial dose of gonadotropins to be used in the cycle. There is no consensus in the literature about the optimal dose of follicle stimulating hormone (FSH) in follicular stimulation to retrieve an acceptable number of oocytes. Reports in the literature have considered the recovery of 8–10 oocytes (range: 5–14) per cycle of stimulation1–3 to be adequate.
Women differ markedly in their ovarian reserves, and consequently present different responses to pharmaceutical stimulation of the ovaries. The occurrence of a low response to gonadotropins may result in cancelation of the cycle. Administration of higher doses of exogenous FSH may also lead to cancelation of the cycle due to the risk of ovarian hyperstimulation syndrome (OHSS).1,4
Cytokines and high levels of vascular endothelial growth factor (VEGF) released by the corpus luteum in the stimulated follicles lead to an increase in vascular permeability which is characteristic of OHSS.5–9 This disorder is usually self-limiting but may extend over a long period, mainly in cycles with conception.6 Its incidence has increased with the expansion of assisted reproduction techniques.7 Mild and moderate OHSS forms occur in 20–33% and 3–6% of all ovarian stimulation cycles, respectively, while the severe form of the syndrome may occur in 0.1–2% of IVF cycles.4
An estimate of ovarian response is possible by ovarian reserve tests (ORT). Among the various ORT available, the oldest is dosage of basal FSH. Antral follicle count (AFC) and serum levels of anti-Müllerian hormone (AMH) were introduced more recently.4
AFC can be used as a screening test to detect probable poor responders, normal responders, or hyper responders, and has the best predictive value for the number of oocytes that will be retrieved in IVF cycles. AFC does not appear to undergo significant changes during a menstrual cycle, especially when only the small antral follicles (2–6mm) are counted.10–13
With knowledge about the patient's ovarian response, individually tailored doses of gonadotropin can be used, resulting in reduced occurrence of inappropriate ovarian responses, fewer canceled cycles, reduced occurrence of OHSS, performance of fewer cycles with little chance of success, and improvements in pregnancy rates and the overall cost-effectiveness ratio for IVF programs.4 The objective of this study is to review the performance of antral follicle counting in adjusting the dose of gonadotropins to prevent inappropriate responses in IVF cycles.
MethodA systematic review was conducted of studies published from January 2000 to December 2013 in English, Portuguese and Spanish. The following databases were consulted: Medical Literature Analysis and Retrieval System Online (MEDLINE), Literature Latin American and Caribbean (LILACS), Scientific Electronic Library Online (SciELO), and US National Library of Medicine (PubMed). The descriptors used were: “antral follicle count” and “ovarian hyperstimulation syndrome”.
Among the studies identified, prospective studies, systematic reviews, meta-analyses and retrospective studies that addressed AFC as a predictor of ovarian response and individualized optimal dose of FSH to reduce inappropriate responses in an IVF cycle were selected. Inclusion criteria were studies with a sample composed of women <40 years of age with regular menstrual cycles, without ovarian anatomical changes and with causes of infertility treated by assisted reproduction techniques (ART).
The studies were selected independently and blindly by two authors according to the inclusion and exclusion criteria. Where there was disagreement between the two authors, the opinion of a third author was employed.
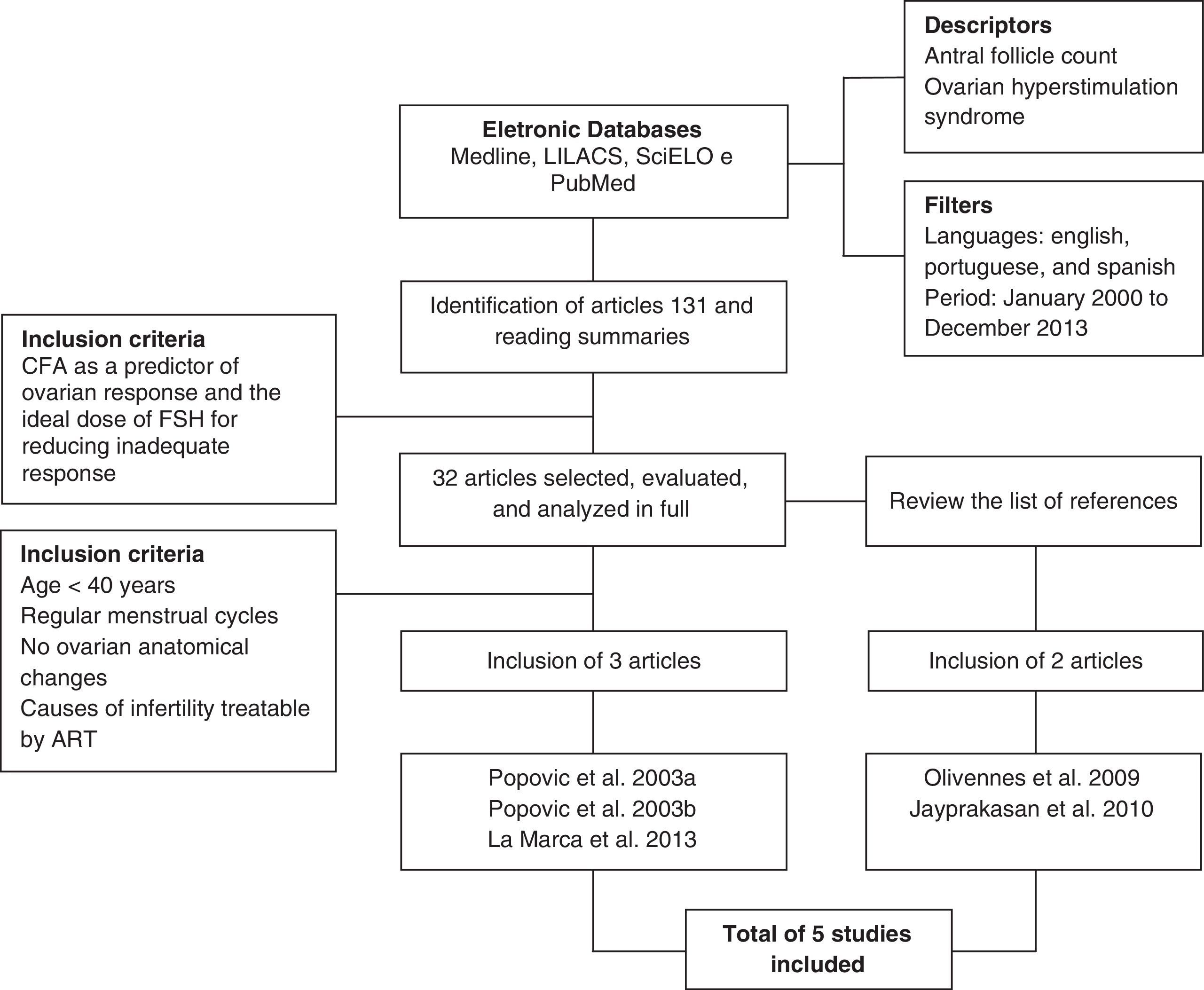
One hundred and thirty-one published articles were identified from the descriptors and filters used. Ninety-nine articles were excluded by the title, by reading the abstracts, or because of repetition in the databases, and 32 articles were selected that related AFC as a predictor of ovarian response to individualized optimal dose of FSH to reduce inappropriate responses. From these 32 articles, three that respected the inclusion criteria defined for this study were selected. The reference lists of the selected articles were analyzed to investigate the existence of new articles addressing the topic that could be incorporated. Two more articles fitting the proposed inclusion criteria were included, making a total of five articles analyzed in this study. Fig. 1 shows the flowchart summarizing the strategy adopted to identify and include the studies.
Because this study used only data published in the literature, approval by an institutional review board was not required.
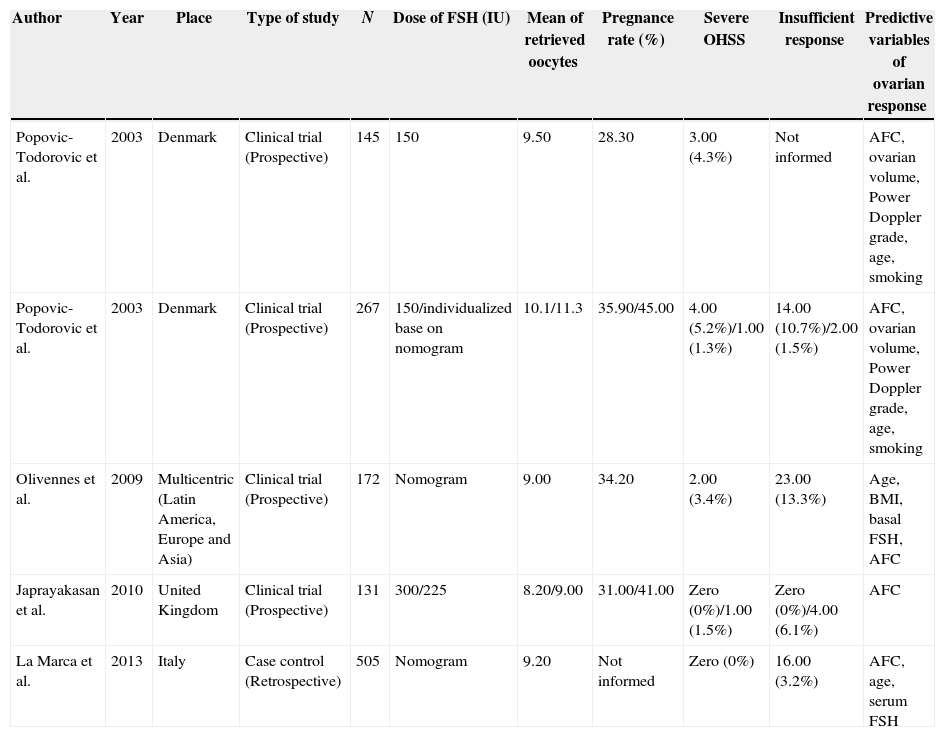
ResultsUsing the data from the articles, Table 1 was constructed for comparative analysis. Four of the five articles analyzed were prospective studies dealing with clinical trials, and one was a case–control study with retrospective design.
Summary of data found in the systematic review.
| Author | Year | Place | Type of study | N | Dose of FSH (IU) | Mean of retrieved oocytes | Pregnance rate (%) | Severe OHSS | Insufficient response | Predictive variables of ovarian response |
|---|---|---|---|---|---|---|---|---|---|---|
| Popovic-Todorovic et al. | 2003 | Denmark | Clinical trial (Prospective) | 145 | 150 | 9.50 | 28.30 | 3.00 (4.3%) | Not informed | AFC, ovarian volume, Power Doppler grade, age, smoking |
| Popovic-Todorovic et al. | 2003 | Denmark | Clinical trial (Prospective) | 267 | 150/individualized base on nomogram | 10.1/11.3 | 35.90/45.00 | 4.00 (5.2%)/1.00 (1.3%) | 14.00 (10.7%)/2.00 (1.5%) | AFC, ovarian volume, Power Doppler grade, age, smoking |
| Olivennes et al. | 2009 | Multicentric (Latin America, Europe and Asia) | Clinical trial (Prospective) | 172 | Nomogram | 9.00 | 34.20 | 2.00 (3.4%) | 23.00 (13.3%) | Age, BMI, basal FSH, AFC |
| Japrayakasan et al. | 2010 | United Kingdom | Clinical trial (Prospective) | 131 | 300/225 | 8.20/9.00 | 31.00/41.00 | Zero (0%)/1.00 (1.5%) | Zero (0%)/4.00 (6.1%) | AFC |
| La Marca et al. | 2013 | Italy | Case control (Retrospective) | 505 | Nomogram | 9.20 | Not informed | Zero (0%) | 16.00 (3.2%) | AFC, age, serum FSH |
FSH, follicle-stimulating hormone; IU, international unit; OHSS, ovarian hyperstimulation syndrome; AFC, antral follicle count; BMI, body mass index.
The earliest study was published in 2003 by Popovic-Todorovic et al., in Europe, while the most recent was published by La Marca et al., in 2013; this study was conducted in Italy, and has the largest sample, with 505 patients. The smaller study, with 131 women, was conducted in the UK by Jayaprakasan et al., in 2010.
Combining the populations studied in all the articles, 1220 patients were analyzed; of these, 407 underwent controlled ovarian stimulation with a fixed dose of recombinant follicle stimulating hormone (FSHr), an exogenous FSH, and 813 received individualized doses of FSHr.
DiscussionThe main goal of individualized treatment in IVF cycles is to provide every patient with therapy based on their unique characteristics, thereby permitting a greater chance of success with lower risks from ovarian stimulation.14 Although basal FSH has been used for decades, the criteria for selecting the appropriate initial dose of exogenous FSH have not been fully defined. The initial dose of exogenous FSH is usually chosen according to the patient history and clinical criteria, most important of which is the result of a prior IVF cycle. When there is no previous cycle, in other words, when performing the first cycle in a particular patient, age, body mass index (BMI), and markers of ovarian reserve are the principal variables used to determine dosage.15
Older studies were not based on ovarian reserve to assist in selecting the dose of gonadotropin used for ovarian stimulation in an IVF cycle.16–19 Measurement of serum basal FSH in women who would undergo an IVF cycle was one of the earliest markers of ovarian reserve to be used.20 Since the concept of ovarian reserve was introduced, studies using AFC began to appear. The initial studies we encountered that used AFC and nomograms, relating the idea of FSH dose individualization for ovarian stimulation, were the studies by Popovic-Todorovic et al., in 2003.2,3
One of the studies proposed a nomogram for clinical use to determine the optimal dose of FSH in an IVF cycle. This study used a sample of women <40 years of age, without ovarian changes, with regular menstrual cycles, and normal serum basal FSH levels. Using a starting dose of 150IU of FSHr, it was possible to define the variables which could predict ovarian response. The number of oocytes retrieved could be predicted by AFC, total Doppler score, smoking, and level of serum testosterone. However, the most significant variables in this study for predicting the number of oocytes retrieved were AFC and ovarian volume.3
The study proposed a nomogram based on ovarian ultrasound parameters (AFC, ovarian volume and Power Doppler score) and clinical data (age and smoking). The proposed optimal dose of FSHr to achieve an adequate yield of oocytes was 150IU in 30–35-year old non-smoking women, with an average number of antral follicles, average ovarian volume, and normal Doppler score. The optimal dose of FSHr proposed in the study was 100IU in a non-smoking woman <30 years of age with large ovaries, many antral follicles, and a high Doppler score. A dose of 250IU was more appropriate in the study for a smoking woman >35 years of age with few antral follicles, small ovaries, and a low Doppler score. During assembly of the nomogram, 3 cases of OHSS were observed when the initial dose of 150IU of FSHr was used. The study did not report any cases of cancelation of the IVF cycle as a result of poor ovarian response (less than 5 oocytes retrieved).3
In 2003, Popovic-Todorovic et al. used the nomogram in a randomized, double-blind prospective clinical trial to evaluate the results of using an dose of FSH of between 100 and 250IU/day. The sample was divided into a control group or standard dose (150IU) and an experimental group or individualized dose. There was a significant difference in outcome between the groups: in the experimental group, 77.1% of women had an adequate response to ovarian stimulation (defined as 5–14 oocytes retrieved) compared with 65.6% in the control group.2
Furthermore, the study showed that lower doses of FSH in patients with low ovarian reserve generated inappropriate responses, increasing rates of IVF cancelation. In the group receiving the individualized dose of FSHr, 1.5% of the cases were canceled due to insufficient ovarian response; in the fixed-dose group, cancelation was noted in 10.7% of cases. It was also observed that patients undergoing individualized doses of FSHr did not require gonadotropin dosage adjustments, and also presented fewer cases of OHSS, suggesting that each patient has an optimal dose.2
In 2009 a more marked tendency to individualize doses of exogenous FSH began to appear. The Consort study applied the Consort algorithm for individualized dosing of exogenous FSHr in normo-ovulatory women aged 18–34 years. The algorithm considered four parameters from the literature: age, BMI, basal FSH, and AFC, assigning increments of 37.5IU of FSHr according to these characteristics. This study supported studies showing that hormonal assessments such as the levels of inhibin B, anti-Müllerian hormone (AMH), and FSH may add to but not replace AFC, which seems to be more valuable for evaluating follicular reserve.1,3
This study suggested that low doses of gonadotropins are associated with higher incidence of canceled IVF cycles and consequent failure to retrieve oocytes. The study presented fewer cases of OHSS when compared with studies in which fixed-doses of FSHr are used, as seen in the study by Popovic-Todorovic et al. (2003). The results corroborate the importance of individualizing the dose of exogenous FSH used in assisted reproduction.1,3
In 2010, Jayaprakasan et al. used AFC as a predictor of ovarian response to compare fixed doses of gonadotropins (225 and 300IU) and did not observe a significant difference in the number of oocytes retrieved in women undergoing these doses during an IVF cycle, corroborating the studies of Henk et al., and the Latin-America and Puregon IVF Study Group report that compared the influence of fixed doses of 150IU and 250IU of FSHr in the number of retrieved oocytes.
These studies also did not show a significant difference in the number of oocytes retrieved when comparing doses of 150IU and 250IU. These doses did not exhibit significant differences in the number of OHSS cases reported. In the study by Jayaprakasan et al., 6.1% of cycles were canceled because of insufficient response in the group that received 225IU of FSHr, while no cancelation was observed in the group receiving 300IU of FSHr.1,3,16,17,21,22
In 2013, the retrospective study by La Marca et al. aimed to develop a nomogram based on age and markers of ovarian reserve to calculate the appropriate starting dose of exogenous FSH to be applied in IVF cycles in order to reduce the extremes of ovarian response. The analysis showed that the number of oocytes retrieved was predicted by age, BMI, smoking status, serum basal FSH, and AFC, but statistical significance was achieved only by age, basal FSH, and AFC. These predictors were used to construct the nomogram which can be easily used in clinical practice.
The study confirmed that ovarian response to FSH dose depends primarily on ovarian reserve. Moreover, it shows that individualizing the dose of gonadotropin used in ovarian stimulation reduces the number of cases of inadequate ovarian response. No cases of OHSS were reported in the study, and it was observed that only 3.2% of cases were canceled due to insufficient ovarian response.15 It was seen that in the studies using individualized doses of exogenous FSH, rates of cycle cancelation as a result of insufficient response are lower.
In addition to the study by La Marca et al., the studies by Popovic-Todorovic et al., CONSORT, and Ocal et al. demonstrate the significance of AFC as a predictor of ovarian response.23–25 The same concept is supported by the Optimist 2012 study proposal. The objective of this study is to perform two tests, one with poor responders and one with hyper-responders, sorted by AFC. The expectation of this proposal is an increase of 30–41% in the rate of live births after adjusting the dose of FSH; the study will also integrate the results into a decision model to compare the cost-effectiveness of three strategies for adjusting the dose of exogenous FSH.1–4,15,22,25
ConclusionEven with the small number of articles, this review highlights the fact that AFC appears to perform well in adjusting the dose of exogenous gonadotropins to prevent inappropriate responses in IVF cycles. Initial studies tended to use a standard dose of exogenous FSH. Current studies about ovarian reserve and AFC show a tendency to apply individualized doses of exogenous gonadotropins. The use of nomograms containing AFC may assist in clinical practice. Further studies are now being conducted to evaluate the potential of AFC in preventing hyperstimulation syndrome and inadequate responses in in vitro fertilization cycles.
Conflicts of interestThe authors declare no conflicts of interest.
Research conduced at Infertility Clinic, Faculty of Medicine, Pontifícia Universidade Católica de Goiás (PUC-Góias), Goiânia, GO, Brazil.