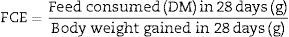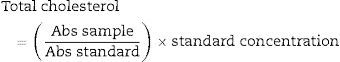The effects of avocado seeds (A) inclusion (2%, 4% and 8%) in diets on cholesterol and carbohydrate metabolism in normal rats Sprague Dawley (SD) fed on basal diet (BD) or high sucrose diet (HSD) and spontaneous hypertensive rats (SHR) fed on BD were studied. The inclusion of avocado seeds in the diet influences the feeding and growth performance in rats. The inclusion of A lowered (p<0.05) cholesterol at 2% and 4% doses and glucose at 2% in serum of SD rats fed on BD, whereas only serum cholesterol level was reduced at all the three doses in SHR. Increased liver glycogen (p<0.05) was noted in SD rats fed on BD with 8% A. All avocado seeds doses increased (p<0.05) liver glycogen storage in SHR fed on BD and SD rats fed on HSD. Avocado seeds can lower blood glucose and cholesterol and enhance liver glycogen storage in rats.
Los efectos de la inclusión de semillas de aguacate (A) (2%, 4% y 8%) en dietas sobre el metabolismo del colesterol y carbohidratos en ratas normales Sprague Dawley (SD) alimentados con dieta basal (BD) o dieta con alto contenido en sacarosa (HSD) se estudiaron las ratas hipertensas (SHR) alimentadas con BD. La inclusión de las semillas de aguacate en la dieta influye en la alimentación y el crecimiento en ratas. La inclusión de A disminuyó (p < 0,05) el colesterol a dosis del 2 y el 4% y glucosa al 2% en suero de ratas SD alimentadas con BD, mientras que solo el nivel de colesterol sérico se redujo en todas las 3 dosis en SHR. Se observó un aumento del glucógeno hepático (p < 0,05) en ratas SD alimentadas con BD con un 8% de A. Todas las dosis de semillas de aguacate aumentaron (p < 0,05) el almacenamiento de glucógeno hepático en SHR alimentado con BD y SD alimentadas con HSD. Las semillas de aguacate pueden reducir la glucosa y el colesterol en la sangre, y aumentar el almacenamiento de glucógeno hepático en ratas.
Dietary manipulation through the consumption of specific plant materials containing phytochemicals been proven to be effective treatment methods for the regulation of glucose and lipids in the blood.1 Avocado belongs to the flowering plant family of Lauraceae, genus Persea and specie Persea americana. It is commonly called avocado, alligator pear (English); aguacate, palta (Spanish). Avocado probably originated in southern Mexico but was cultivated from the Rio Grande to central Peru before the arrival of Europeans.2
The avocado fruit has a lot of nutrients. This includes its high content of essential minerals, potassium, vitamin b6, vitamin E and B complex. The avocado seed also contains various classes of natural products such as phytosterols and triterpenes, fatty acids with olefinic, acetylenic bonds, furanoic acid, dimmers of flavonols and oligomeric proanthocyanidins, β-d-glucoside of 8-hydroxyabscisic acid and epi-dihydrophaseic acid β-d-glucoside.3,4 Studies have shown that avocado can be used to reduce visceral fat accumulation and improve hyperlipidemia and hyperglycaemia in rat.5,6 In addition, the consumption of avocado based diets showed lower cholesterol levels.7
Avocado seed showed ameliorate the symptoms of type 2 diabetes, targeting peroxisome proliferator-activated receptor gamma in a similar manner as the thiazolinediones class anti-diabetic drugs.8 To date, there are intensive bioactivity studies on the pulp of the avocado. However, the avocado seed which basically considered a waste product is an under-utilized resource. The objectives of this study are to determine in vivo the effects of avocado seeds on parameters related to diabetics and hypertension such as blood glucose, cholesterol levels and glycogen in liver in three rat's models; normal rats, hypertensive rats and rats exposed to hyperglycaemic condition by feeding on high sucrose diet (HSD) during 4 weeks of feeding period. In addition, evaluate the feeding and growth performance of rats.
Materials and methodsMaterialsAnimal feedRat chow made from soybean meal was purchased from local feed manufacturer (Gold Coin Malaysia). Avocado (Persea americana) and soybean milk (Nutrisoy Inc.) powder were purchased from local markets.
Experimental animalsMale Sprague-Dawley rats (SD; n=56) and spontaneously hypertensive rats (SHR; n=28) out bred of Wistar-Kyoto rats, 6–8 weeks old were randomly selected from University of Malaya animal house. These animals were individually caged (600mm×380mm×200mm) at all-time had access to rat chow and fresh water ad libitum.
MethodsPreparation of ground avocado seedsThe pulp of fresh avocado fruits was removed and the seeds were washed out of residual flesh. A total weight of about 8kg of seeds from the fruits was obtained and the seeds were dried in the oven (50°C) and ground separately into fine particles of about 50μm particle size.
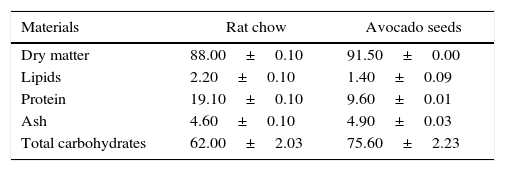
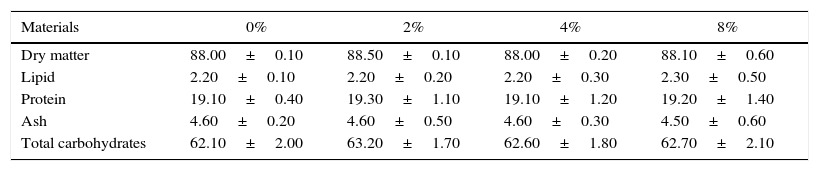
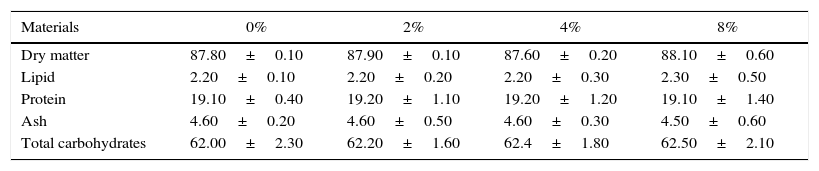
Preparation of diets containing ground avocado seedsThe basal diet (BD) was mixed with the ground avocado seeds at the following concentrations: 2%, 4% and 8% (wt/wt). High sucrose diet (HSD) was prepared by adding 30% (wt/wt) sucrose to the BD. Equivalent weight of soymilk was added into the diets to balance the difference in nitrogen and energy content as a result of the seeds inclusion. Proximate analysis of rat chow and fresh ground avocado seeds is shown in Table 1. Furthermore, proximate analysis of the basal diet and high sucrose diet containing different concentrations of avocado seeds are shown in Tables 2 and 3 respectively.
Proximate analysis of rat chow and fresh ground seeds (%).
| Materials | Rat chow | Avocado seeds |
|---|---|---|
| Dry matter | 88.00±0.10 | 91.50±0.00 |
| Lipids | 2.20±0.10 | 1.40±0.09 |
| Protein | 19.10±0.10 | 9.60±0.01 |
| Ash | 4.60±0.10 | 4.90±0.03 |
| Total carbohydrates | 62.00±2.03 | 75.60±2.23 |
Data are presented as the mean of four observations±standard error mean.
Proximate analysis of the basal diet containing different concentrations of avocado seeds.
| Materials | 0% | 2% | 4% | 8% |
|---|---|---|---|---|
| Dry matter | 88.00±0.10 | 88.50±0.10 | 88.00±0.20 | 88.10±0.60 |
| Lipid | 2.20±0.10 | 2.20±0.20 | 2.20±0.30 | 2.30±0.50 |
| Protein | 19.10±0.40 | 19.30±1.10 | 19.10±1.20 | 19.20±1.40 |
| Ash | 4.60±0.20 | 4.60±0.50 | 4.60±0.30 | 4.50±0.60 |
| Total carbohydrates | 62.10±2.00 | 63.20±1.70 | 62.60±1.80 | 62.70±2.10 |
Data are presented as the mean of four observations±standard error mean.
Proximate analysis of high sucrose diet containing different concentrations of avocado seeds.
| Materials | 0% | 2% | 4% | 8% |
|---|---|---|---|---|
| Dry matter | 87.80±0.10 | 87.90±0.10 | 87.60±0.20 | 88.10±0.60 |
| Lipid | 2.20±0.10 | 2.20±0.20 | 2.20±0.30 | 2.30±0.50 |
| Protein | 19.10±0.40 | 19.20±1.10 | 19.20±1.20 | 19.10±1.40 |
| Ash | 4.60±0.20 | 4.60±0.50 | 4.60±0.30 | 4.50±0.60 |
| Total carbohydrates | 62.00±2.30 | 62.20±1.60 | 62.4±1.80 | 62.50±2.10 |
Data are presented as the mean of four observations±standard error mean.
Three different and independent experiments were conducted. In the first experiment, SD rats (n=28) were randomly assigned into seven groups of four each and served with test diets (BD) containing avocado seeds at the various concentrations 2, 4 and 8%, respectively where 0% served as control in the group. In the second experiment, the same procedures were carried out except that each group was fed on HSD containing 2, 4, and 8% avocado seeds respectively and 0% avocado seeds served for control group. In the third experiment, SHR were assigned into seven groups accordingly and fed with BD. Initial body weight (IBW), body weight gain (BWG), feed intake (FI) and faecal output from each rat were measured at the beginning and every seven days thereafter for 4 weeks.
The animals were sacrificed by cervical dislocation at the end of the feeding period. Blood samples were collected into heparinized tubes and after centrifugation (2500×g; 10min 4°C) the plasma was harvested and kept cold at 4°C for further analysis. Liver samples of approximately 5g were collected and stored at −20°C for liver glycogen estimation.
Feeding performance analysis- (1)
The amount of weekly diet ingested was calculated as the difference in the total weight of feed offered at the beginning and the balance at the end of the week. The weekly data collected were then used to calculate daily feed intake according to Ennouri et al.9 with the following formula:
- (2)
Faecal dry matter (DM) was determined after drying faeces collected in 24h at 105°C to constant weight.9
- (3)
Macro nutrients digestibility was assessed as the difference between daily DM intake and 24h DM excretion in faeces according to Ennouri et al.9
- (4)
The feed conversion efficiency (FCE) was determined by the following formula.9
- (5)
The protein efficiency ratio (PER) is the weight gain of the growing rat divided by intake total protein intake during the feeding period9 according to the following formula:
The total cholesterol was determined using commercial kits from Chemo Lab (Malaysia). The cholesterol concentration was estimated by thoroughly mixing 10μl of serum or standard solution with 1.0ml of kit reagent. The mixed solutions were allowed to stand for 5min at 37°C prior to absorbance reading at 500nm.
The cholesterol concentration was calculated using the following formula:
Estimation of blood glucose concentrationSerum glucose concentration was determined according to modified Trinder method.9 Serum glucose content was determined by mixing 0.1ml of serum with 1.0ml of water, 1.0ml of 5.0% zinc sulphate and 1.0ml of 0.25N sodium hydroxide. The mixture was then centrifuged (2500×g, 10min) and the supernatant (1.0ml) was transferred into a test tube containing 1.0ml of alkaline copper reagent followed by boiling in water bath for ten minutes. The mixture was cooled by placing the tubes under running water for 3min. Arseno-molybdate reagent (1.0ml) was added to the resultant solution and the volume was made up to 10.0ml with water. The optical density was read at 500nm against a blank set at zero. The glucose concentration in the samples was then calculated from a glucose calibration curve which was also run at the same time with the glucose analysis.
Estimation of liver glycogen contentLiver glycogen content was determined according to Vats et al.10 Liver samples (200mg) were rinsed with ice-cold saline and then solubilized by incubating with 2ml of 30% potassium hydroxide at 55°C for 30min. The solubilized liver tissue (0.2ml) was placed on ice bath and then neutralized with 0.2ml of 1.0M HCl, 0.8ml of water and 2.0ml anthrone reagent (0.2g anthrone/100ml of 95% H2SO4). The mixture was then incubated at 100°C for 10min. Absorbance was measured at 620nm and the liver glycogen content (mg glycogen/g tissue) was calculated using glucose standard curve.
Statistical analysisData were analysed using paired t test. Mean values were obtained by averaging independent measurements. Data were presented as mean±standard error mean. Difference between control and experimental groups were considered significant at p<0.05.
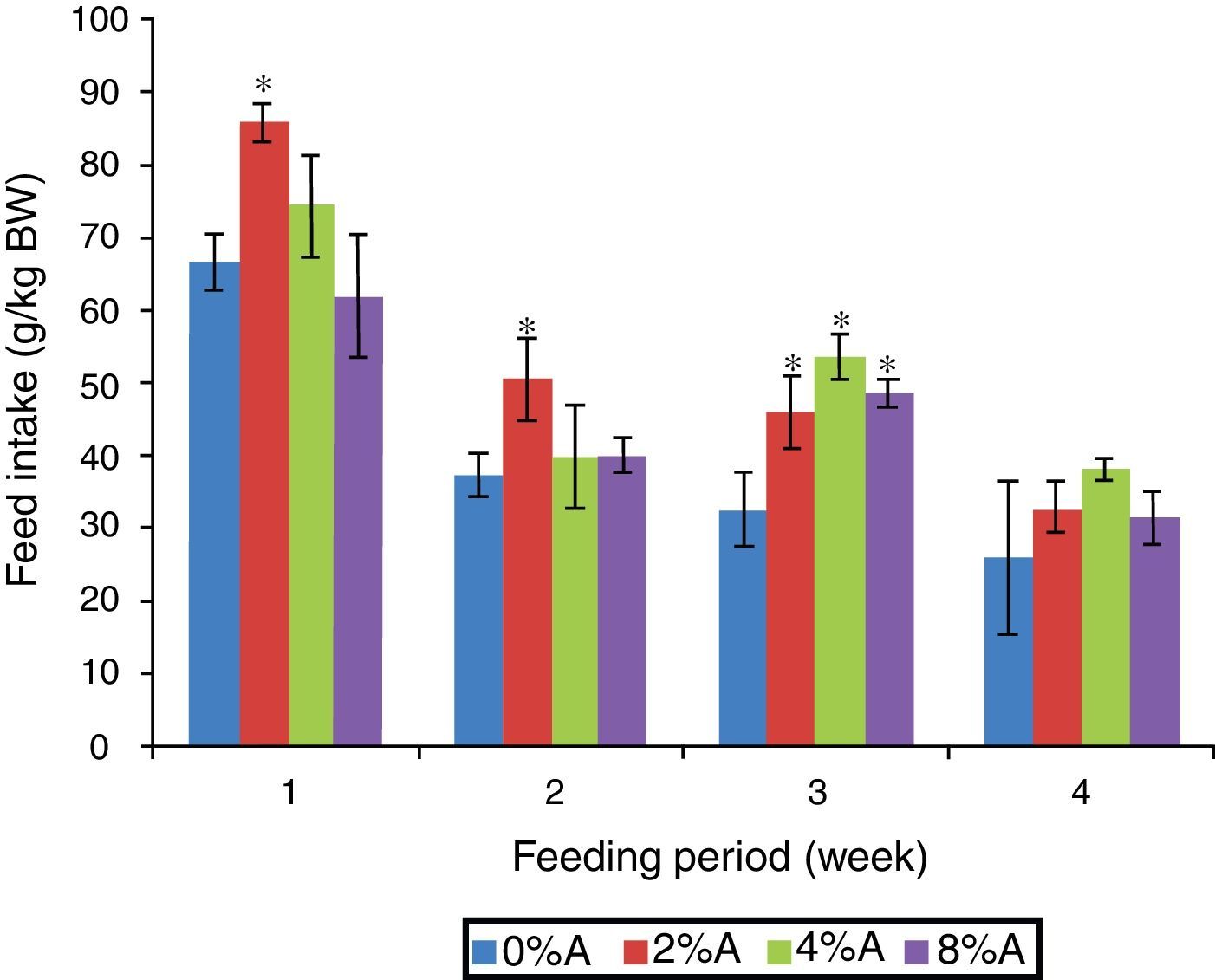
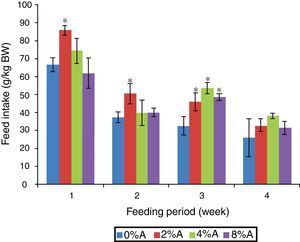
Results and discussionFeed intake of SD rats fed on BD containing avocado seedsThe FI of SD rats fed on control BD reduced gradually from 66 to 26g/kg BW during 4 weeks of feeding period (Fig. 1). The inclusion of avocado seeds (2%) to the BD tended to increase (p<0.05) FI of SD rats to 85g/kg BW and 55g/kg BW at the first and second week respectively. All the three concentrations of avocado seeds included in BD showed significant increase in FI of SD rats at the third week of feeding period compared to control group (Fig. 1). The lowest FI (28–38g/kg BW) was recorded on week 4 of feeding for all treated groups.
No significant difference (p>0.05) was observed in body weight gain between the treated groups and control group during entire feeding period (data not shown). All rats had 5–10% body weight gain during the feeding period. However, when expressed as average body weight gain (g) during the entire feeding period to initial body weight (kg IBW), it was found that the inclusion of avocado seeds resulted in increased average body weight gain compared to control (56.8±15g/kg IBW; data not shown). Nevertheless, increasing inclusion of avocado seeds concentrations into BD tended to reduce average body weight gain to 93.16, 85.77, and 58.47g/kg IBW for 2%, 4% and 8% of avocado seeds respectively (data not shown).
Avocado is known as a super food. It provides healthy nutrients to the body. However, avocado seed contained nearly 20 vitamins and minerals.4 In addition, it is high in unsaturated fats (75%) such as monounsaturated and polyunsaturated fats.4 These notations may partially influence the feed intake and growth performances, particularly when the effects appeared to be dose dependent.
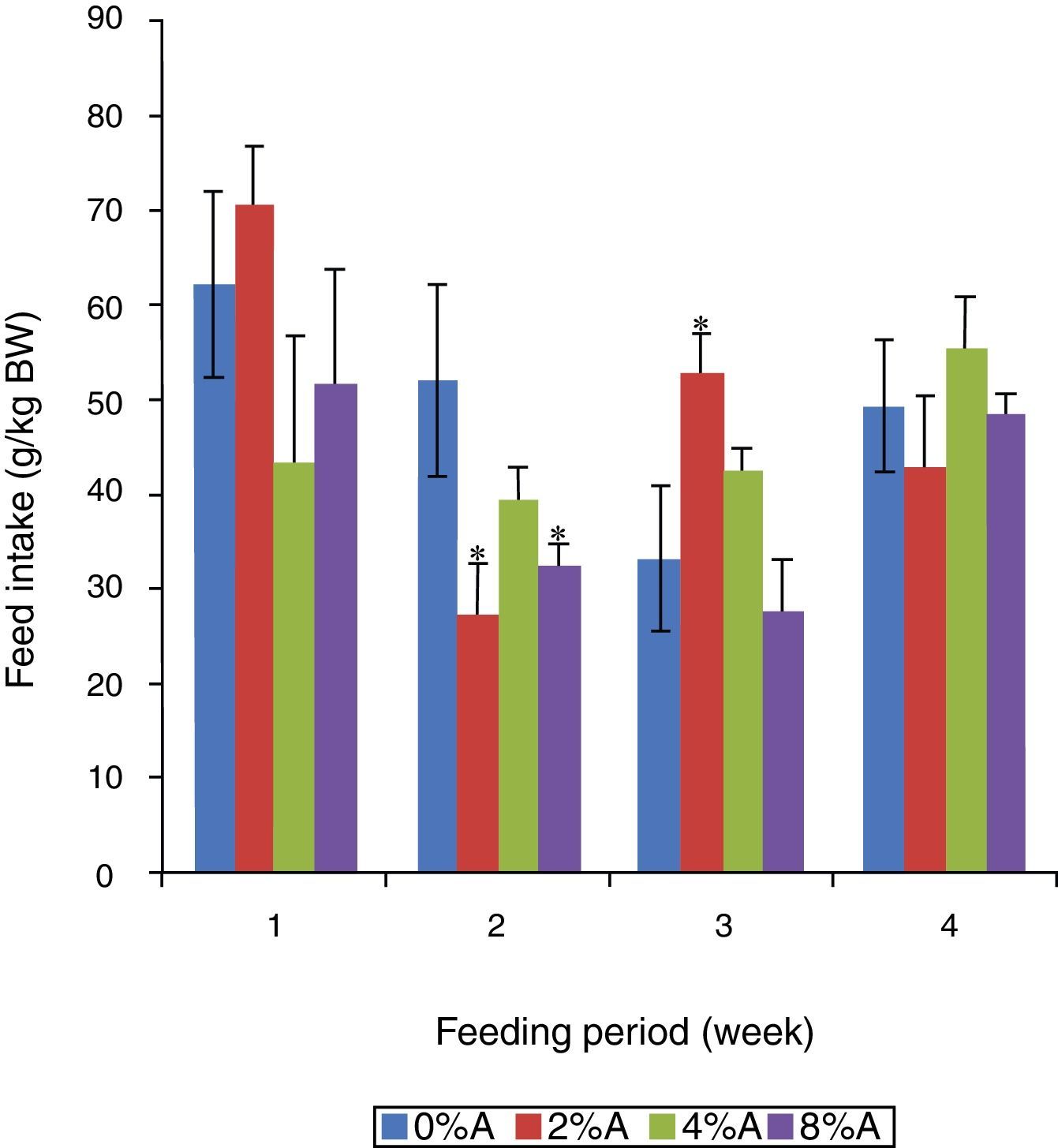
Feed intake of SD rats fed on HSD containing avocado seedsThe FI of the control group reduced gradually (p<0.05) from 62 to 33g/kg BW in the first three weeks of the experiment (Fig. 2). The inclusion of 2% of avocado seeds in the diet resulted in higher (p<0.05) FI by SD rats in the third week of the experiment, while 4% and 8% of avocado seeds had lower FI in the first two weeks of the experiment compared to control (Fig. 2).
No significant difference (p>0.05) was observed between control and treated groups during 4 weeks of feeding period (data not shown). The weight gain of SD rats when expressed to the initial body weight range from 15% to 25%. Inclusion of avocado seeds lowered the average body weight gain of the experimental groups (data not shown). Control rats (0% avocado seeds) had the highest body weight gain (281.97±36.39g/kg IBW) followed by 4%, 2% and 8% of avocado seeds included HSD (200.03±22.61, 174.34±75.47 and 151.53±24.83g/kg IBW respectively; data not shown).
Previous studies showed that rats fed on high carbohydrate meal (e.g. sucrose) gain more weight than rats fed on normal rat chow.11 However, the apparent beneficial effect of sucrose on body weight gain was lowered when avocado seeds were included in HSD. This indicated that the presence of secondary compounds in avocado seeds may have acted as anti-nutritive factors in HSD resulted in lower absorption and post digestive assimilation of nutrients into body mass of animals.12
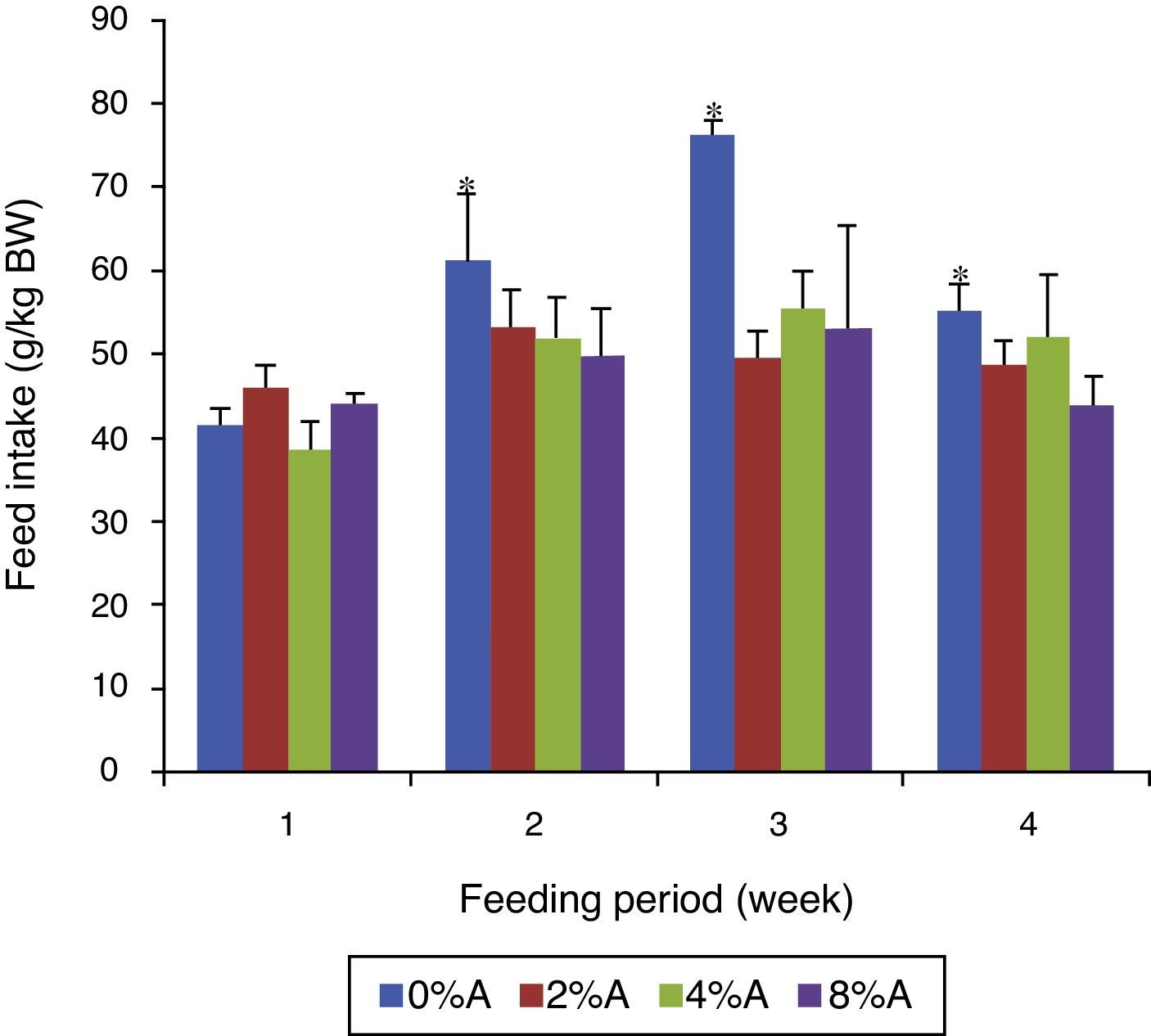
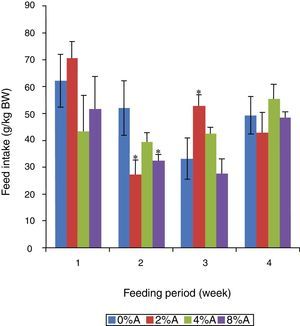
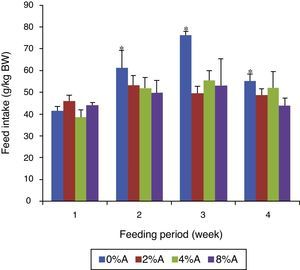
Feed intake of SHR fed on BD containing avocado seedsThe FI of control group increased gradually (p<0.05) from 42 to 76g/kg BW (80% increase) during the first three weeks before reducing to 53g/kg BW at week 4 of the feeding period (Fig. 3). The FI of BD containing avocado seeds was similar for all groups in week 1 of the feeding trial but increased by only 12% for the next three weeks. The average FI over the period for the tretaed groups fed on 2%, 4% and 8% of avocado seeds included BD were 49.45, 49.51 and 47.85g/kgIBW respectively, which were lower than control (58.75g/kg IBW; data not shown).
There was no significant difference (p>0.05) in body weight gain between control and treated groups during 4 weeks of feeding period (data not shown). The body weight gain of SHR ranged between 15% and 20% during feeding period. The average body weight gain (g) to initial body weight (kg IBW) during the feeding trial reduced with increasing inclusion of avocado seeds in the diet (data not shown). Control rats had average body weight gain of 134.06±20.53g/kg IBW whereas treated groups fed on 2%, 4% and 8% of avocado seeds had average body weight gain of 126.44, 118.30 and 112.15g/kg BW respectively (data not shown).
The presence of avocado seeds could have lowered the feed intake by SHR (Fig. 3) which resulted to lower weight gain. This suggested that there is an impaired assimilation of nutrients (reduced feed efficiency) from ingested feed by SHR when avocado seeds were added to the diet.
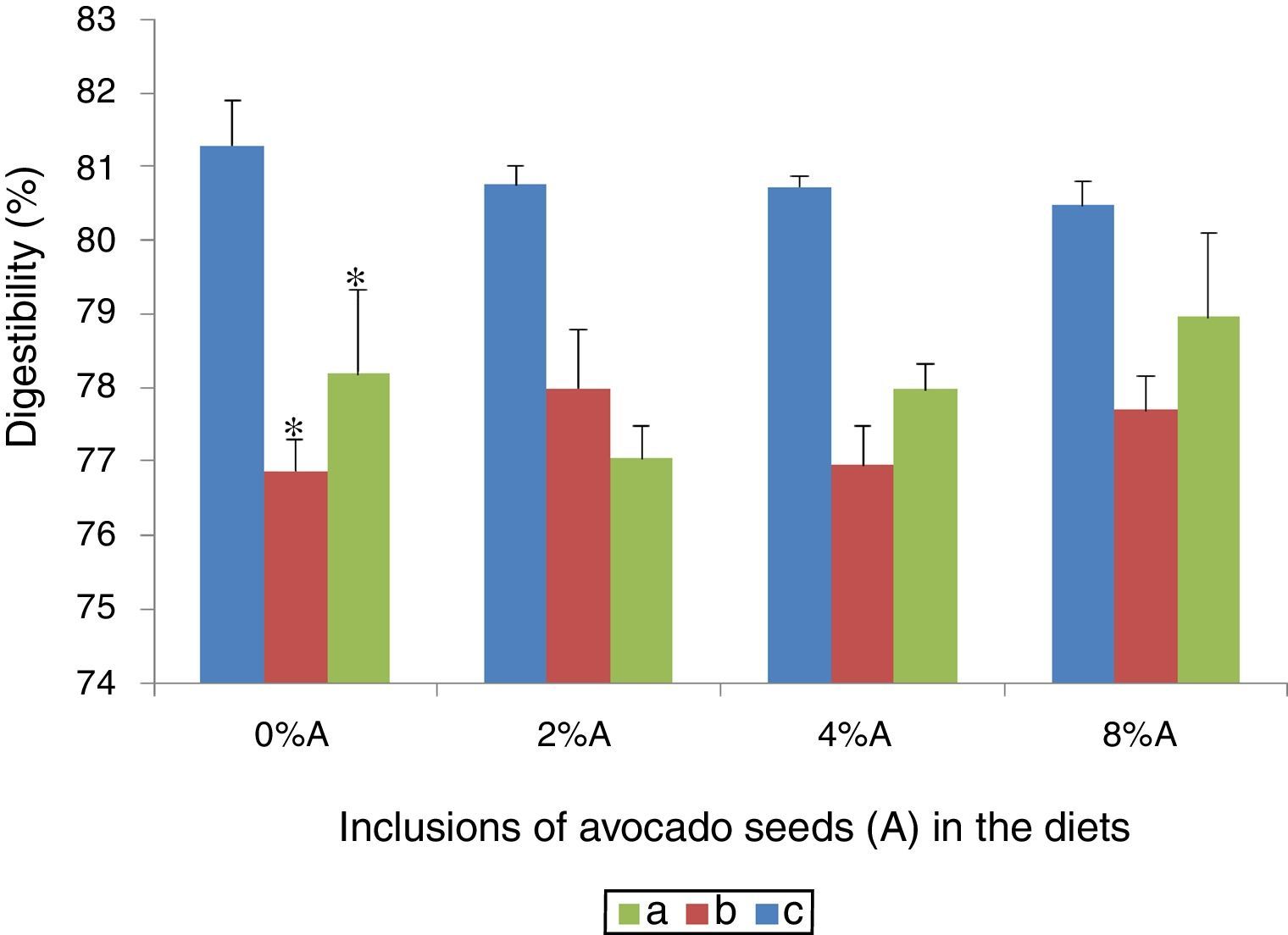
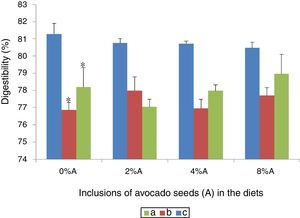
Effect of avocado seeds on the digestibility of diets in ratsThe effect of different concentrations of avocado seeds containing diets is shown in Fig. 4. BD consumed by SD rats (81.3%) was more digestible (p<0.05) than HSD consumed by SD rats (76.9%) and BD consumed by SHR (78.2%; Fig. 4). The addition of avocado seeds into both BD and HSD did not affect the digestibility of the diets in treated groups.
Digestibility is described as the difference in feed intake and faecal excretion in relation to feed intake.13 Thus when consumption of feed is high and faecal excretion is high, the value of digestibility is low i.e. feed is not properly digested and/or absorption is impaired. In the present study, the values of digestibility ranged from 77 to 81% which are comparable to previous report using normal rats 9 and hypercholesterolemic rats.13 To date there are no reports which indicated the negative effects of avocado seeds inclusion in diet on digestibility. The addition of avocado seeds in HSD may however provide additional beneficial effect of the secondary compounds (i.e. tannins) which was reported to improve protein digestibility at low concentration.14
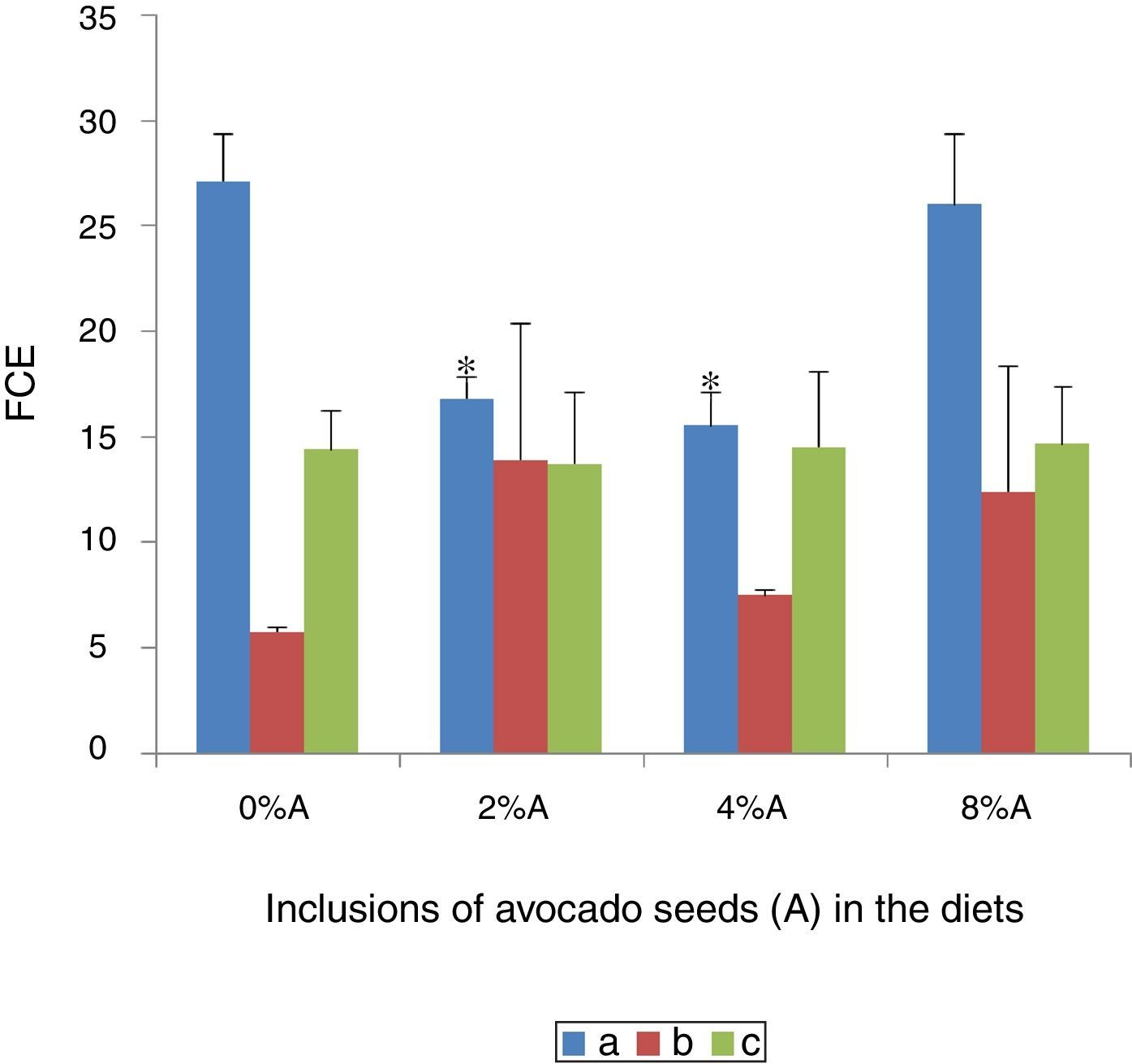
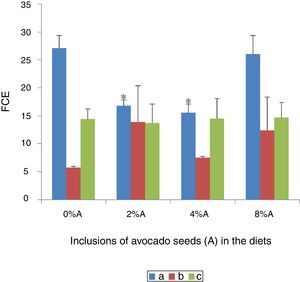
Effect of avocado seeds on feed conversion efficiency (FCE) of diets in rtasThe FCE was best in SD rats fed on HSD (5.8±0.04) whereas the FCE of BD was better when consumed by SHR (14.4±2.2) than by SD rats (27.1±2.3; Fig. 5). The FCE of BD consumed by SD rats improved (p<0.05) when avocado seeds were included at 2% and 4% (15.5±1.6 and 16.8±1.0 respectively) compared with control (Fig. 5). The inclusion of avocado seeds did not improve the FCE of BD and HSD when fed to SHR and SD rats respectively.
The feed conversion efficiency (FCE) of BD and HSD containing different concentrations of avocado seeds fed to SD rats and SHR. (a) SD rats fed on BD, (b) SD rats fed on HSD and (c) SHR fed on BD. Values are presented as mean±SEM (n=4). *p<0.05 compared to control (0% avocado seeds).
Low values of FCE imply high efficiencies and vice versa. The present study shows that the same diet may have different effect on animals of different physiological status and thus the same animal may perform differently on different diet as reported in several earlier studies.15,16 Phenolic compounds are known to improve the feed efficiency by reducing internal parasites.17 The high affinity of phenolic compounds i.e. tannins was suggested to bind to feed proteins and thereby reduces excessive breakdown of protein in the stomach.18 This increases availability of high quality proteins for absorption in the lower guts and thus increases the efficiency of protein utilization.19 However, regardless of sucrose can be easily digested and stored in various energy depots including glycogen in the liver, the beneficial effect of sucrose on FCE was reduced in the presence of avocado seeds.
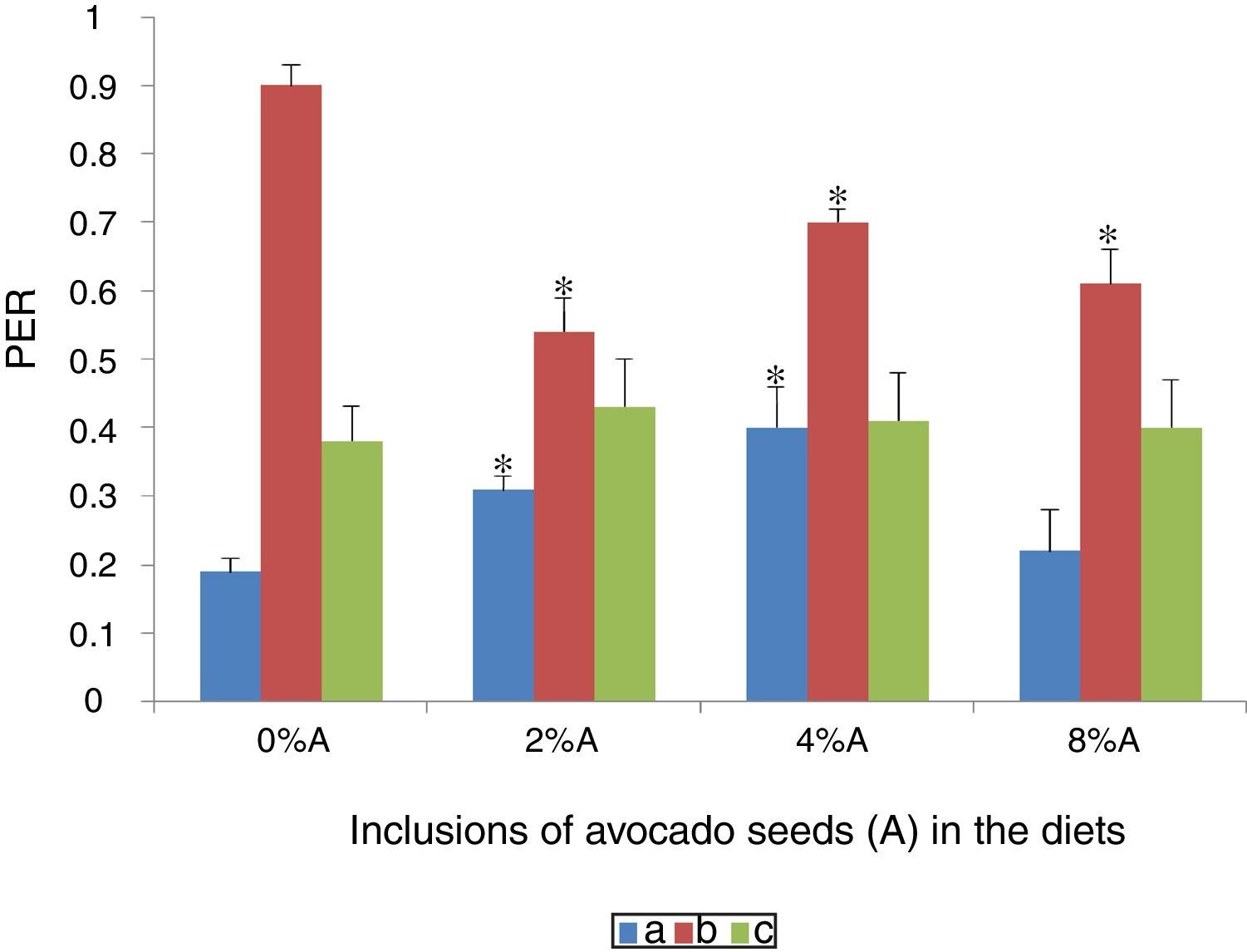
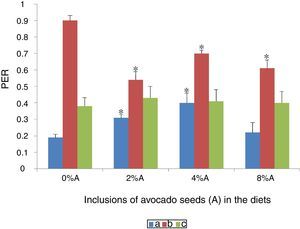
Effect of avocado seeds on protein efficiency ratio (PER) of diets in ratsPER of HSD consumed by SD rats was the highest (0.9±1.2) followed by BD consumed by SHR (0.4±2.3) and SD rats (0.19±0.05) respectively (Fig. 6). The PER was only improved (p<0.05) for BD contianing 2% and 4% of avocado seeds consumed by SD rats (0.31±0.04 and 0.40±2.4 respectively). Inclusion of avocado seeds at all the three concentrations did not cause any changes in PER of SHR fed on BD whereas PER of SD rats fed on HSD reduced (p<0.05) between 0.53 and 0.68 during the last three weeks of feeding period (Fig. 6).
Protein efficiency ratio relates the body weight gain over the protein consumed with the implication that a high PER value indicates an efficient feed as a protein source.9 Inclusion of plant phenolic compounds in diets has been reported to increase digestibility of protein.12 The presence of avocado seeds reduced PER of HSD-fed rats which are in agreement to the negative effects of avocado seeds on FCE as described earlier (Fig. 5).
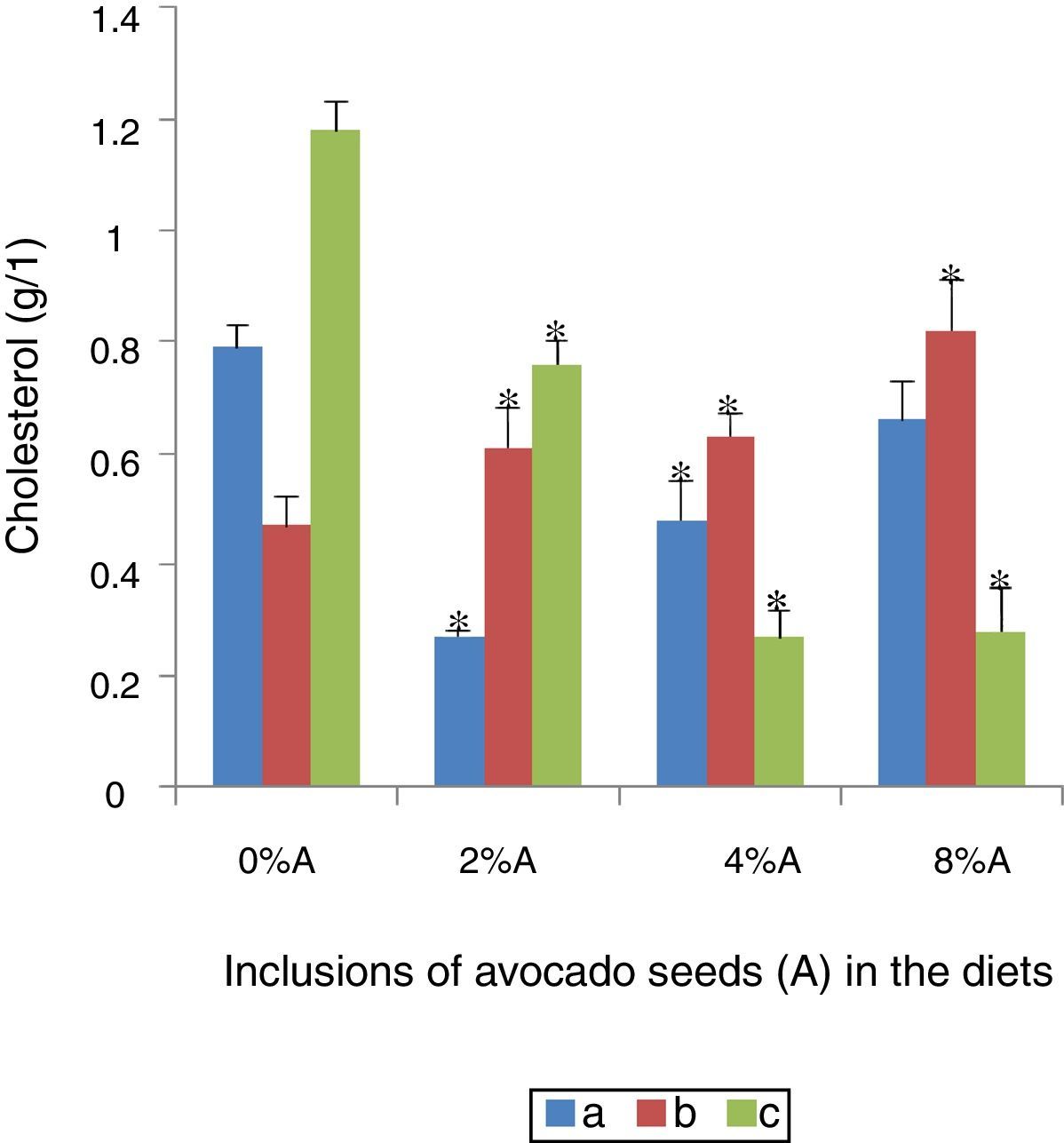
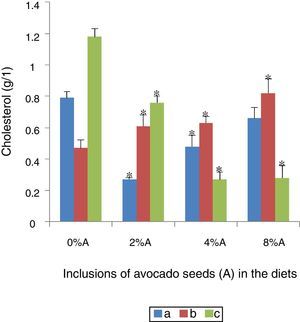
Serum cholesterol concentration of rats fed on BD and HSD containing avocado seedsThe blood cholesterol was the highest in SHR fed on BD (1.18±0.30g/l) followed by SD rats fed on BD (0.79±0.04g/l) or HSD (0.47±0.02g/l) respectively (Fig. 7). There was a significant reduction (p<0.05) in blood cholesterol of SHR fed on BD with increased incorporation of avocado seeds in the diet. Maximum reduction was achieved at 4% of avocado seeds (0.27±0.03g/l; Fig. 7). The inclusion of avocado seeds lowered the blood cholesterol of SD rats fed on BD with significant effect (p<0.05) occurred at 2% and 4% (0.27±0.05g/l and 0.48±0.07g/l, respectively) compared to control. The inclusion of avocado seeds increased (p<0.05) the blood cholesterol level in SD rats fed on HSD ranged from 0.6g/l to 0.8g/l (Fig. 7).
The lowering of blood cholesterol as a result of avocado seeds inclusion in the diet may be due to the phenolic compounds such as phytosterols in a concentration of 826mg/kg mg/kg dry weight.20 These phytosterols in particular the beta-sitosterols are known to induce a decrease in plasma lipoprotein and cholesterol levels21 by decreasing the cholesterol solubility and absorption across the intestinal barrier.22,23 This lowering effect is based on the fact that the higher phytosterols hydrophobicity is more readily to mix with bile salt and acid micelles24 than can animal cholesterol25 resulting in the excretion with the faeces a greater part of unabsorbed cholesterol, particularly the low density lipoprotein.26 There was an increase in the cholesterol levels of SD tars fed on HSD containing avocado seeds. These changes may be attributed to animal variations in responses to the presence of avocado seeds since SD rats fed on HSD are expected to be hypercholesterolemic as demonstrated in other studies of animals fed on high sucrose diet.11
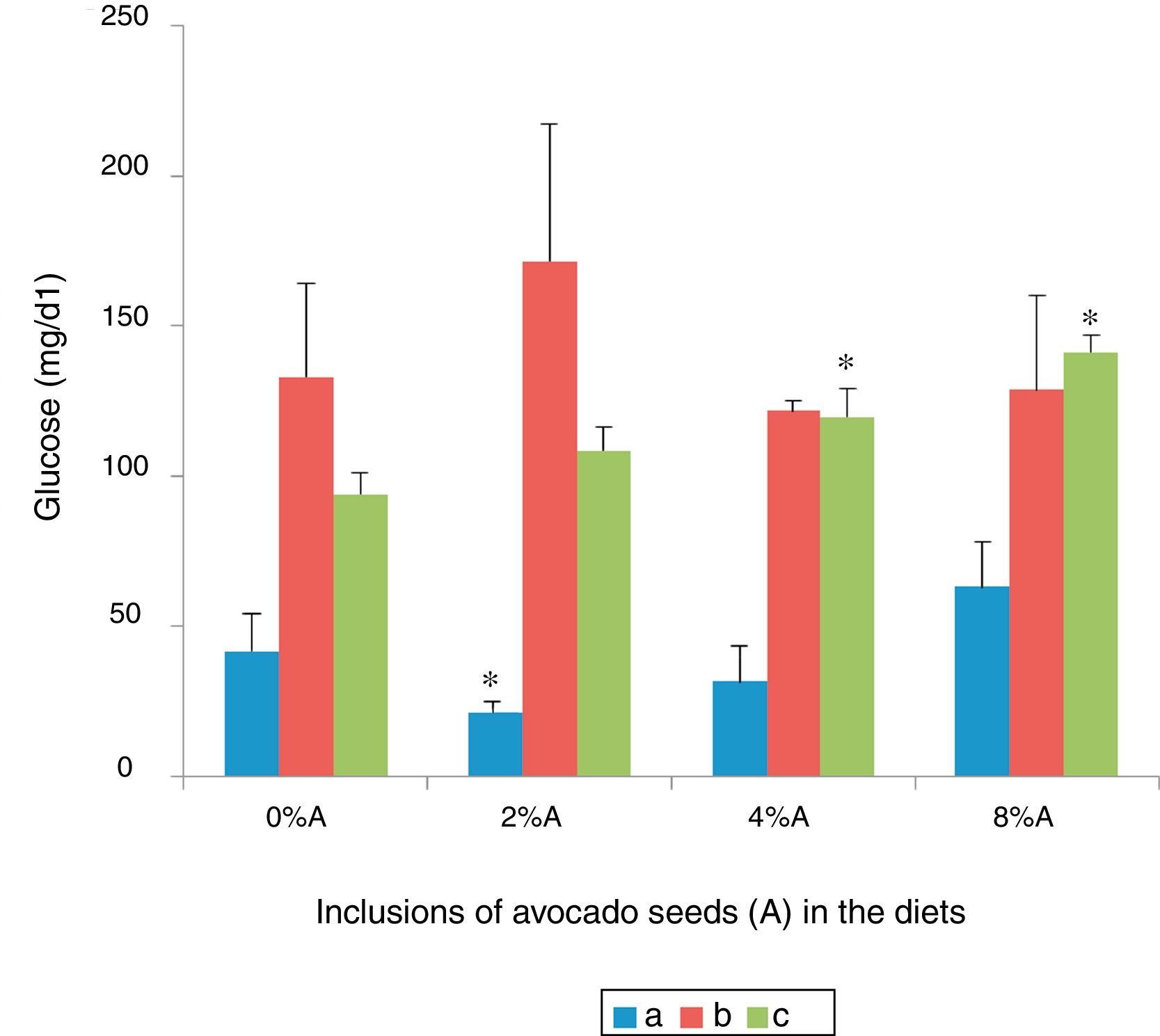
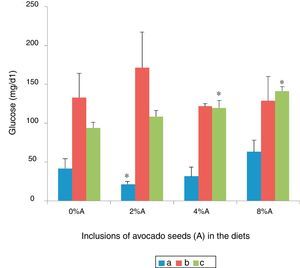
Serum glucose concentration of rats fed on BD and HSD containing avocado seedsThe lowest blood glucose was shown in SD rats fed on BD (41.7±12.5mg/dl) followed by SHR fed on BD (93.82±7.34mg/dl) and SD rats fed on HSD (133.08±31.23mg/dl) respectively (Fig. 8). Inclusion of avocado seeds (2%) in BD reduced (p<0.05) SD rats blood glucose (21.35±2.29mg/d). No significant effects (p>0.05) on blood glucose level of SD rats were observed at any concentrations of avocado seeds included in HSD. The addition of avocado seeds into BD increased (p<0.05) the blood glucose level of SHR at 4% and 8% (Fig. 8).
Phytochemicals in the form of phytosterols,27 polyphenols28 and flavonoids29 can have blood glucose lowering capacity9 and it was suggested to achieve this directly by inhibiting intestinal absorption of glucose.30 However, avocado seeds appeared not to be a strong suppressor of blood glucose elevation in SD rats fed on HSD and SHR fed on BD as compared to control. This could be due to the physiological conditions of the animals.31 The difference in response of these rats reflects the important of several facets of blood glucose homeostasis.
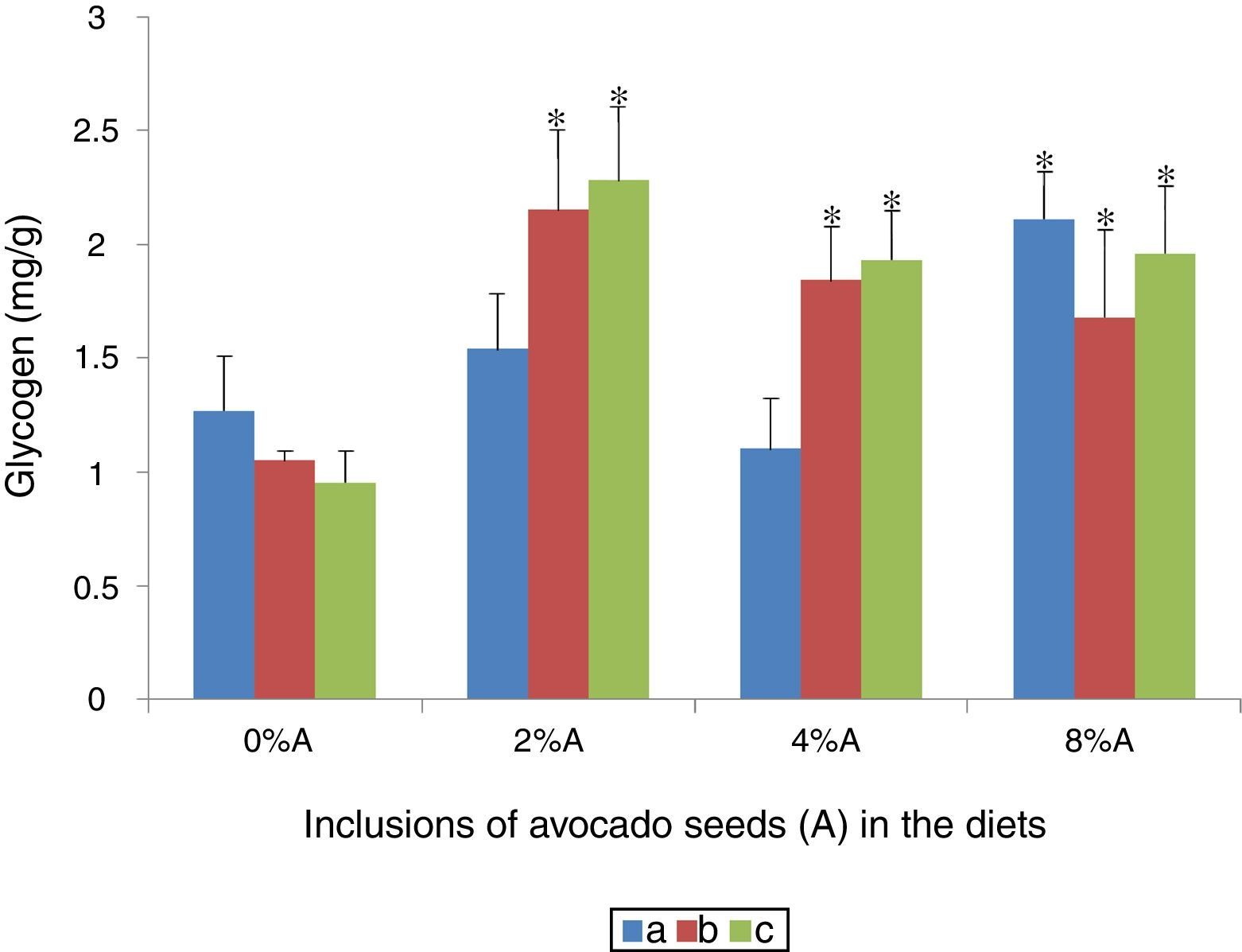
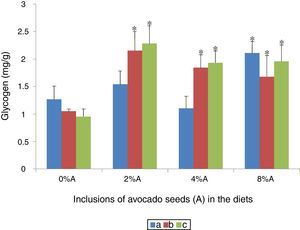
Liver glycogen content of rats fed on BD and HSD containing avocado seedsThere were no sgnificant differences (p>0.05) in liver glycogen content of SD rats and SHR fed on BD or HSD with values ranged from 0.9 to 1.3mg/g (Fig. 9). Increase in liver glycogen content attributed to avocado seeds inclusion in the diets occurred only at 8% in SD rats fed on BD (2.11±0.31mg/g; p<0.05) compared with control (1.27±0.24mg/g). All the three concentrations of avocado seeds included into BD or HSD increased (p<0.05) the levels of glycogen storage in SHR and SD rats with higher values were shown at 2% (Fig. 9).
Glycogen is stored in the liver or muscles32 and functions as secondary energy storage in animals. Glycogen storage could be enhanced by various processes such as increased glucose intake or use of additives or compounds i.e. phytochemicals.9 In the present study, the inclusion of avocado seeds had positive effects on glycogen storage. This improved in liver glycogen storage could be attributed to the presence of phytochemicals in avocado seeds which effective enough to enhance deposition of glucose into glycogen.
ConclusionsThe results of the present investigation have explored the promising values of seed components as source of energy, protein as well as bioactive phytochemical to stimulate growth and metabolism. The inclusion of avocado seeds in the diet may partially influence the feeding and growth performance particularly when the effects appeared to be dose dependent. The avocado seeds inclusion also lowered the cholesterol levels of the rats, suppress high blood glucose especially when sucrose was added and improved liver glycogen storage capability of the rats. It could be concluded that avocado seeds can be used to modulate carbohydrate and lipid metabolism and enhancement of liver glycogen storage capacity in rats. The present study has thus provided insights in the possible dietary uses of avocado seeds in people with hyperglycemia and/or hypercholesteremia. Further study is needed to investigate the effects of avocado seeds in diets on cholesterol and carbohydrate metabolism in Sprague-Dawley rats after inducing hypertension for a comparison with SHR.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe author declares that there is financial relationship with IPPP University Malaya. Amal Shori declares that she has no conflict of interest.
We are grateful to the Vice chancellor University Malaya, Prof Datuk Dr. Ghauth Jasmon and to the IPPP University Malaya for their dynamic and financial support. And also to the Dean Faculty of Science, University Malaya Professor Datin Dr. Saadah, Abdul Rahman for his kind gestures.