The aim of this research was to evaluate whether the application of two plant growth-promoting (rhizo)bacteria might reduce nitrogen fertilization doses in cotton. We used strains Azotobacter chroococcum AC1 and AC10 for their proven ability to promote seed germination and cotton growth. These microorganisms were characterized by their plant growth-promoting activities. Then, we conducted a glasshouse study to evaluate the plant growth promoting ability of these strains with reduced doses of urea fertilization in cotton. Results revealed that both strains are capable of fixing nitrogen, solubilizing phosphorus, synthesizing indole compounds and producing hydrolytic enzymes. After 12 weeks, the glasshouse experiment showed that cotton growth was positively influenced due to bacterial inoculation with respect to chemical fertilization. Notably, we observed that microbial inoculation further influenced plant biomass (p<0.05) than nitrogen content. Co-inoculation, interestingly, exhibited a greater beneficial effect on plant growth parameters compared to single inoculation. Moreover, similar results without significant statistical differences were observed among bacterial co-inoculation plus 50% urea and 100% fertilization. These findings suggest that co-inoculation of A. chroococcum strains allow to reduce nitrogen fertilization doses up to 50% on cotton growth. Our results showed that inoculation with AC1 and AC10 represents a viable alternative to improve cotton growth while decreasing the N fertilizer dose and allows to alleviate the environmental deterioration related to N pollution.
El objetivo de esta investigación fue evaluar si la aplicación de 2 (rizo)bacterias promotoras del crecimiento vegetal podría reducir la dosis de fertilizante nitrogenado en el cultivo de algodón. Se usaron las cepas Azotobacter chroococcum AC1 y AC10 por su habilidad para promover la germinación de semillas y el crecimiento del algodonero. Estos microorganismos fueron caracterizados sobre la base de sus actividades de promoción del crecimiento vegetal. Luego se realizó un estudio de invernadero con plantas de algodón para evaluar la capacidad de promoción del crecimiento vegetal de dichas cepas con dosis reducidas de urea. Los resultados revelaron que ambas cepas son capaces de fijar nitrógeno, solubilizar fósforo, sintetizar compuestos indólicos y producir enzimas hidrolíticas. Después de 12 semanas, el experimento de invernadero permitió observar que el crecimiento del algodón fue influido positivamente por la inoculación bacteriana con respecto a la fertilización química. En particular, se evidenció que la inoculación microbiana impactó más en la biomasa vegetal (p<0,05) que en el contenido de nitrógeno. Curiosamente, la coinoculación exhibió un mayor efecto positivo sobre los parámetros de crecimiento en comparación con la inoculación simple. Además, se observaron resultados similares, sin diferencias estadísticamente significativas, entre la coinoculación bacteriana más del 50% de urea y el 100% de fertilización. Estos hallazgos indican que la coinoculación de las cepas de A. chroococcum AC1 y AC10 permitiría reducir las dosis de fertilización nitrogenada del cultivo de arroz en hasta el 50% y aliviar, de esta manera, el deterioro ambiental relacionado con la contaminación por N.
Sustainable agricultural production requires new approaches to reduce the applications of polluting agrochemicals. In particular, the application of synthetic nitrogen fertilizers (SNF) represents an important environmental threat as it involves pollution due to its production and usage33. Within 40–50% of the total fertilizers involved in nitrogen uptake are not assimilated immediately by plants or are lost via leaching, denitrification, volatilization and are subject to conversion into unavailable forms. Poor assimilation of nutrients eventually leads to an increasing utilization of SNF, which reduces soil fertility, and biodiversity, contaminates groundwater, and consequently affects human health19. In Colombia, the unsustainable cotton production system is an example of the negative aftermath of SNF overuse. Low profitability for producers, lower export competitiveness, and environmental degradation are some consequences of this problem5. One potential solution to overcome this major challenge is the study of the biological processes involving plant growth-promoting (rhizo)bacteria (PGPR/PGPB) and their interaction with plants2.
The group of PGPR/PGPB encompasses several bacterial phyla, and although the direct molecular mechanisms associated to plant growth promotion remain poorly understood, it is widely accepted that their primary effect on plant growth is due to increasing availability of essential nutrients, plant growth stimulation, phytopathogen control, and decrease of negative effects in some abiotic stress involved in plant growth8,9. Several lines of evidence suggesting direct mechanisms of PGPR/PGPB involved in plant growth promotion are the following: (i) production of ACC deaminase, which reduces the level of ethylene in crop roots thus enhancing root length and density; (ii) symbiotic and associative nitrogen fixation, which increases the availability of soluble nitrogen in soil; (iii) nutrient solubilization and mineralization (e.g. P, K, Zn and Si), which increases the availability of those elements for plant uptake; (iv) synthesis of phytohormones such as indoles, gibberellins, abscisic acid and cytokinins which modulate plant growth and division; (v) ability to produce siderophores, hydrolytic enzymes and antibiotics, which renders the cells more competitive over niche colonization; (vi) quorum sensing signal interference and inhibition of biofilm formation, and (vii) enhanced resistance to stresses by synthesis of water soluble vitamins as niacin, thiamine and biotin4,12. Undoubtedly, the relationship among those bacterial attributes are associated to plant growth promotion in diverse studies, and hence their use is promising as a biotechnological tool to be integrated into the agricultural production systems13.
Within the PGPR/PGPB group, the nitrogen-fixing bacterium Azotobacter chroococcum has shown to promote the growth of different crops under different soil types and climatic conditions15. Indeed, recent literature suggests that A. chroococcum might be a potential alternative to ameliorate the negative impact of NaCl on maize growth29. Although their positive effects on plant growth have been reported, few studies have explored the potential of A. chroococcum as supplement of chemical fertilization. For example, Bonilla et al.5 found that the application of A. chroococcum AC1 and AC10 increases cotton biomass. Moreover, significant differences in all plant growth parameters were evidenced between the inoculated treatment and the full fertilizer rate (positive control). They suggested that the use of AC1 and AC10 could not completely replace nitrogen (N) fertilizer. The goal of this work was to determine whether the inoculation of these A. chroococcum strains might reduce N fertilization rates in cotton. For this purpose, we evaluated the integrated use among these microorganisms and different doses of urea on plant growth attributes and nitrogen content under glasshouse conditions. Moreover, we related the evidenced results with the plant growth-promoting traits measured in these bacteria.
Materials and methodsMicroorganisms and culture conditionsWe used the Gossypium hirsutum-associated bacterial strains A. chroococcum AC1 and A. chroococcum AC10 for their compatibility, and ability to promote seed germination and growth of cotton without fertilization5. AC1 and AC10 strains were obtained from the Soil Microbiology Lab at Corpoica, Colombia. For routine use, both strains were stored at −80°C in 50% (v/v) glycerol in MBR broth22, and grown on Ashby plates (composition in g/l: mannitol 10.0, K2HPO4 0.2, MgSO4 0.2, NaCl 0.2, CaSO4 0.1, CaCO3 5.0, agar 15.0, pH 7.5) at standard conditions (i.e. 30°C for 48h). All reagents used were from Merck, Millipore, USA.
In vitro screening of plant growth promoting featuresIn order to characterize the plant growth promoting (PGP) traits of AC1 and AC10 strains, the ability to fix nitrogen11, solubilize nutrients as P10 and potassium17, produce the ACC deaminase enzyme16, synthesize Indole Acetic Acid (IAA)6,14 and siderophores31, and secrete cell-wall-degrading enzymes (proteases and chitinases) and other enzymes such as pectinases, cellulases, amylases, and ureases7 were evaluated. Each assay was performed with at least two biological replicates, and three technical replicates.
Influence of A. chroococcum and fertilizers on cottonIn order to determine whether inoculation with AC1 and AC10 as single or mixed inoculation could reduce the fertilization dosage on cotton, we established a glasshouse experiment at Centro de Investigación Motilonia (CIM) of Corpoica, Agustín Codazzi, Colombia. The experiment was carried out in a completely randomized design (CRD) with six treatments. Each experiment was performed three times with six replicates. The fertilizer doses were 100, 75 and 50% (p/v) of urea (Ecofertil®, Colombia) with respect to the recommended application, each accompanied with 100% of both diammonium phosphate (DAP; Nutrimon®, Colombia) and KCl (Diabonos®, Colombia), as sources of N, P, K respectively; 100% of fertilizer corresponded to 75kg/ha–2.34g/pot for urea, 50kg/ha–1.56g/pot for DAP and 50kg/ha–1.56g/pot for KCl. We used A. chroococcum AC1, AC10, AC1+AC10 (1:1) strains, and sterile MBR broth as control treatment. In all cases, the biological treatments were applied along with 50% urea. Bacterial inoculum (∼1×109CFU/ml) was prepared by growing the cells in MBR broth, using a Rushton type turbine-agitated fermenter (Miniforms, INF-30174) at 300rpm, 1lpm, and 30°C during 24h. Soil was collected from cotton at CIM and its preparation was performed according to Rojas-Tapias et al.29. Cotton seedlings of DP ACALA 90 were surface-sterilized using 1% (v/v) NaClO for 10min and rinsed three times in deionized-sterilized water. The surface-sterilized seeds were inoculated (∼4×108CFU/ml/seed) by soaking with 10ml of inoculum or in sterile MBR broth as a control for 30min. Two inoculated seeds were planted in 600g of soil/pot. Five ml of each bacterial suspension (∼1×109CFU/ml/pot) were re-inoculated in the rhizosphere per treatment after 20 and 40 days of establishment. Soil chemical and physical properties are listed below: pH (7.15), organic matter (3.02%), effective cationic interchange coefficient (15.23cmol/kg), P (215.93ppm), S (15.08ppm), Ca (13.37cmol/kg), Mg (1.8cmol/kg), K (0.63cmol/kg), Na (0.05cmol/kg), Fe (38.5ppm), Mn (2.2ppm), Cu (1.7ppm). Plants grown at 25–35°C, 16:8 day/night regime with daily water application. After 90 days, flowering stage, root and shoot length were measured. Samples were dried at 50°C for 72 days and subsequently, dry weights were taken (boll, shoot and root). In addition, nitrogen (N) content was estimated over the total plant tissue in the LISSALAB Laboratory at Corpoica.
Data analysisStatistical analysis was performed using ANOVA (α=0.05) and the Tukey's HSD test. Associations among variables were examined by a simple correlation analysis. These analyses and graphics were performed using the GraphPad prism 7 software (Graphpad, San Diego, CA).
Results and discussionInoculation with bacteria improves plant growth regardless of chemical treatmentRhizosphere is a highly dynamic environment in which complex interactions take place between plants and microorganisms. The understanding of those biological interactions represents a potential opportunity for biotechnological developments and could increase crop yield while reducing the use of agrochemicals28. In this study, the biofertilizer potential of Azotobacter strains with N fertilizer doses were evaluated in a glasshouse experiment to determine whether inoculation could replace N fertilization rates in cotton growth.
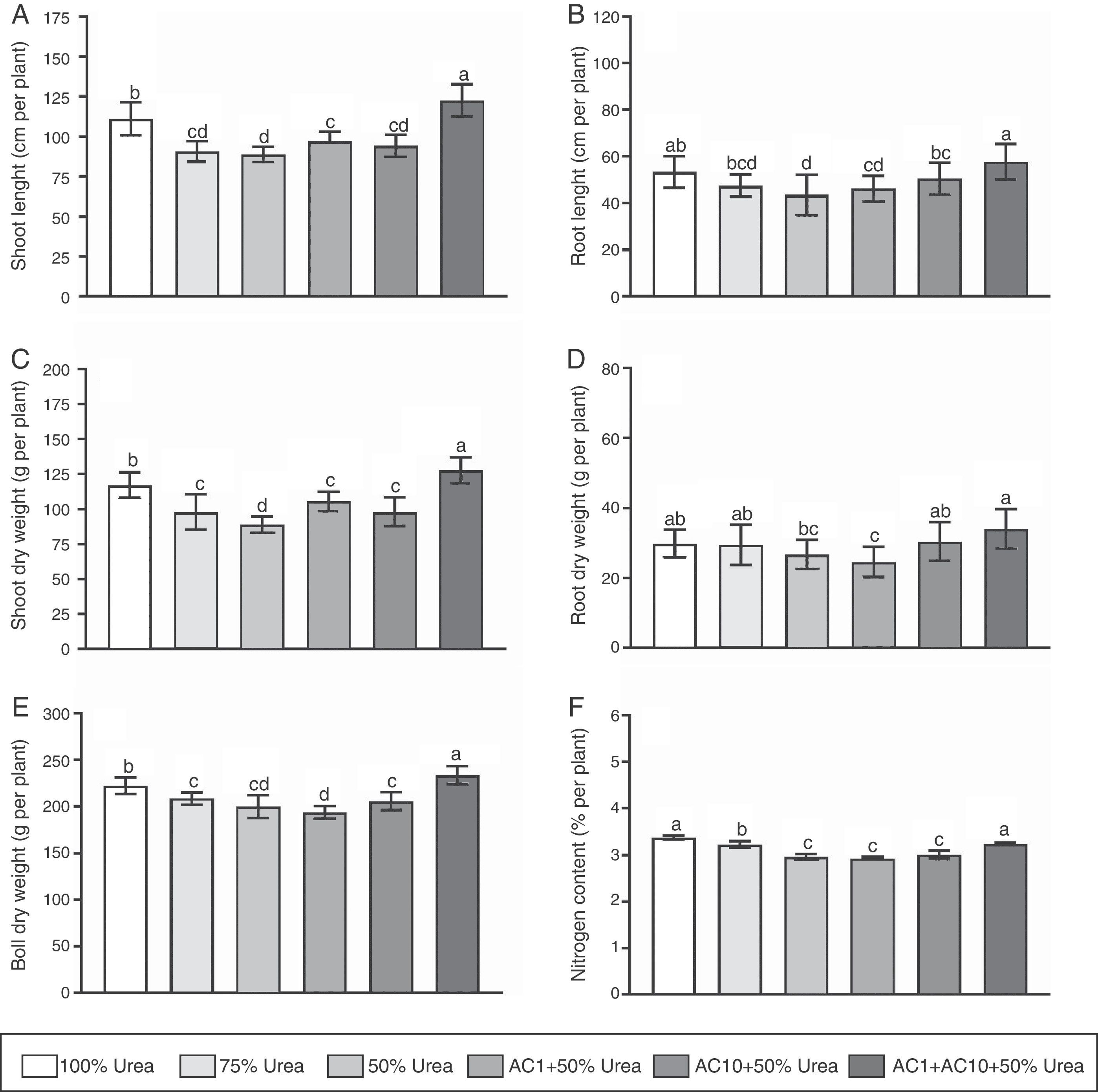
The results showed inoculation with bacteria caused a significantly increase (p<0.05) in plant growth compared to controls (Fig. 1). For example, when concentration of the chemical fertilizer was 50% of the recommended dosage, we observed inoculation with AC1, AC10 and their co-inoculation significantly (p<0.05) increased shoot length to 9, 7 and 38% and root length to 6, 16 and 32%, respectively. In dry weight, root, shoot, and boll were significantly (p<0.05) enhanced to ∼13, 20 and 25%, respectively. We also studied the effect of inoculation on N content. In general, microbial application led to a slight increase in N content. Therefore, significantly improvements in N content by bacteria were only exhibited when mixed inoculation was applied, which indicates that bacterial inoculation has a greater influence on plant biomass respect to N content. The present findings suggest plant–bacteria interaction might be able to improve nutrient use efficiency of the fertilizer and/or bacterial inoculation might directly benefit plant growth1. It is important to note that the effect of AC1 was more pronounced on shoots than on roots, while AC10 had a greater effect on roots. A positive correlation (R=0.65) was observed between root length and N content on whole-plant tissue solely when AC10 was present. Kumar et al.18, who found that A. chroococcum had the ability to settle in cotton roots, support our findings by its chemotactic response to root exudates. Furthermore, Adesemoye et al.3, proposed a combination of the activities of the plant and the inoculants as model for PGPR/PGPB-enhanced nutrient uptake in plants. We concluded that the increase in root length by PGPR/PGPB might contribute to an increased rate of N uptake in the plant.
Influence of Azotobacter chroococcum strains with different urea doses on growth parameters of cotton: shoot length (A), root length (B), shoot dry weight (C), root dry weight (D), boll dry weight (E), and N content (F). Media and standard errors are the result of six replicates per treatment and three independent experiments. Different letters indicate significant differences based on the HSD Tukey test.
In this experiment, we observed that mixed inoculation with AC1 and AC10 increased plant growth with respect to single inoculations. Indeed, we evidenced an increase ranging between 8 and 53% in all parameters of plant growth, suggesting that microbial co-inoculation has a higher potential to promote plant growth (Fig. 1). The observed improvement in plant growth could be explained by a positive and additive contribution of each microorganism compared to single inoculations. Noteworthy, other investigators have observed similar results when using bacterial co-inoculations to promote plant growth2,9,20. Rojas-Tapias et al.30, in contrast to our observations, reported that co-inoculation did not lead to any additional effect compared to single inoculations of PGPR/PGPB when studying maize growth. This contrasting observation, however, might be associated to the evolutionary relationship among different plant and bacterial species, and soil chemical–physical properties26.
Co-inoculation with A. chroococcum AC1 and AC10 strains allow to replace nitrogen fertilizationNotably, we found co-inoculation plus 50% urea caused a significantly effect on cotton growth in comparison with 75 and 50% of chemical fertilization, respectively. Furthermore, we evidenced 100% fertilization and 50% fertilization+co-inoculation produced similar results without statistical differences. In the presence of co-inoculation plus half urea doses, promotion of shoot length, shoot dry weight and boll dry weight were 10, 9 and 5% with respect to the complete fertilization program. In contrast, root length and N content were higher under full urea rates for 0.6 and 4%, respectively. This observation strongly suggests co-inoculation partially replaced the normal doses of nitrogen fertilization, which means that a reduction in 1.17g/pot for urea or 37.5kg per ha of urea is possible due to biological fertilization. Maheshwari et al.21 reported similar findings, with Sesamun indicum showing a fertilizer reduction of 50% using A. chroococcum TRA2. In cotton, Pereg and McMillan24, described that the bacterial genera most commonly used to increase the efficiency of chemical fertilizers are Azospirillum sp., Methylobacterium sp., Bacillus sp., Pseudomonas sp. and combination of A. chroococcum with arbuscular mycorrhizal fungi with comparable results on growth, yield and quality of fiber with respect to the complete fertilization program.
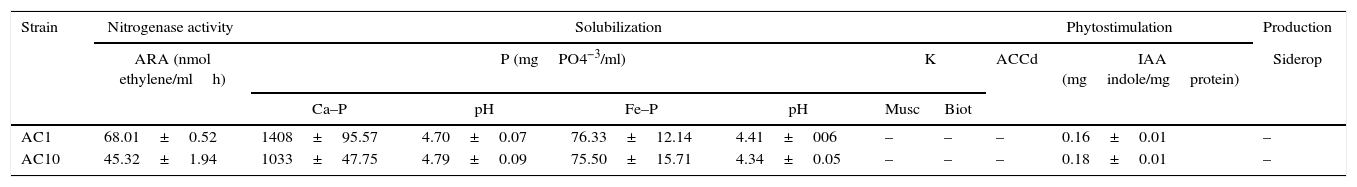
AC1 and AC10 possess PGP traitsBacterial inoculation led to increased plant growth. In order to understand the potential mechanisms involved in plant growth promotion, we characterized some of the PGP features of AC1 and AC10 using in vitro experiments. The results revealed that each strain exhibits more than one PGP attribute, which indirectly might explain the observed results in the glasshouse experiments. We observed that AC1 and AC10 were able to fix nitrogen, as expected in this bacterial genus. The observed values of acetylene reduction (AR) ranged between 1033 and 1408nmol ethylene/mlh (Table 1). Interestingly, we found an inverse relationship among AR results and N content in total plant tissue in both microorganisms.
Multiple plant growth promotion traits in vitro
| Strain | Nitrogenase activity | Solubilization | Phytostimulation | Production | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ARA (nmol ethylene/mlh) | P (mgPO4−3/ml) | K | ACCd | IAA (mgindole/mgprotein) | Siderop | |||||
| Ca–P | pH | Fe–P | pH | Musc | Biot | |||||
| AC1 | 68.01±0.52 | 1408±95.57 | 4.70±0.07 | 76.33±12.14 | 4.41±006 | – | – | – | 0.16±0.01 | – |
| AC10 | 45.32±1.94 | 1033±47.75 | 4.79±0.09 | 75.50±15.71 | 4.34±0.05 | – | – | – | 0.18±0.01 | – |
Data are presented as mean±standard error. Media and standard errors are the result of three replicates per biochemical analysis and results are representative of two independent experiments. (+/−) indicates presence/absence of trait. ARA: acetylene reduction assay; Ca-P: tricalcium phosphate; Fe–P: iron phosphate; Musc: muscovite; Biot: biotite; IAA: indole-3-acetic acid; ACCd: aminocyclopropane-1-carboxylate deaminase; Siderop: siderophore.
With respect to nutrient solubilization, we observed that strains were able to solubilize P from tricalcium phosphate at pH 7.5 and iron phosphate at pH 5.0. Negative correlations (R=0.88) between pH and P solubilization were obtained in both P sources. This biological capability of strains was assessed under similar soil pH conditions and P insoluble sources. This result showed that AC1 and AC10 strains were more efficient in solubilizing Ca–P than Fe–P under our study conditions. Phosphate solubilization is associated to soil phosphorus availability increase, possibly conducted by the bacterial secretion of organic acids, acid phosphates and/or chelating agents into the growing medium23. However, negative results, were observed in K solubilization from muscovite and biotite.
Our results also demonstrated that none of the strains was able to produce iron siderophore complexes in CAS agar and did not possess ACC deaminase activity. To our knowledge, no evidence of the ability of Azotobacter to express ACCd activity has been not previously reported. Based on our observations, we associated root development with IAA production by the strains. Our cultures produced and excreted IAA to the medium in the presence of L-Tryptophan. Plant roots produce organic compounds including L-Tryptophan, which can be employed by PGPR/PGPB for IAA biosynthesis under natural conditions. IAA is the most physiologically active auxin in plants involved in cell enlargement and division, tissue differentiation, and responses to light and gravity32. Ultimately, we found production of hydrolytic enzymes such as proteases, urease, and pectinase in our strains (Table 2). These bacterial features not only confer ability to inhibit phytopathogens or to compete against soil microbial populations since their cell walls will be degraded by extracellular enzymes and their deleterious effects suppressed, but also contribute in the process of nutrient mineralization25.
Bacterial plant growth promotion is a well-established and complex phenomenon, which is often achieved by activities of more than one PGP characteristic exhibited by plant-associated bacteria27. In our study, both strains exhibited more than four PGP traits. Although several studies have focused on biological nitrogen fixation of Azotobacter as the primary influence on plant growth, these findings lead us to consider the possibility that improvement of cotton growth could be more related to other PGP activities of our strains than N uptake due to the ability to fix nitrogen.
We conclude that co-inoculation of A. chroococcum strains allow to reduce nitrogen fertilization doses up to 50% on cotton growth in its flowering stage under glasshouse conditions. Furthermore, biochemical analyses showed that both strains possess multiple PGP features, which indirectly might explain the observed results. Although our work does not establish whether bacterial mechanisms were responsible by the positive effect of co-inoculation, this study provides a starting point for future research in which molecular tools in different metabolic pathways will allow to discern the mechanisms associated with the results reported here. Therefore, field tests should be carried out to obtain information regarding the response of cotton plants in terms of yield and cotton fiber quality, when inoculated with AC1 and AC10 strains. We consider that a reduction in the amount of agrochemicals used in cotton crop is paramount to reduce its impact on environmental health and contribute to the mitigation of climate change.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was financed by Ministerio de Agricultura y Desarrollo Rural of Colombia.
Conflict of interestThe authors declare that they have no competing interest in relation to work presented in this manuscript.
We thank to Mauricio Barón for the support in the glasshouse experiment, Daniel Bravo for help with determining K solubilization, Jorge Arguelles for statistical analysis, and Diego Rincón and Juan Pablo Hernández for critical review on the manuscript.