In the present work, a yeast strain Pichia kudriavzevii was identified on the basis of 18S rDNA, showing maximum growth at 30°C and pH 7.0. Among all the complex polysaccharides used, wheat bran proved to be the best substrate as indicated by the maximum growth of the yeast strain. The yeast isolate was capable of producing xylanase both intra- and extra-cellularly, the dominant form being extracellular. The maximum enzyme activity was determined at pH 5.0 and at 50°C. Na+, Mg2+ and Fe2+ presence caused a substantial increase in enzyme activity while a slight decrease (4.5%) was observed in the presence of Mn2+, Zn2+ and Cu2+. Pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) activities were assayed to confirm the presence of the ethanol pathway and PDC activity was much more pronounced (73%) compared to ADH activity (51%). The yeast strain can be employed to utilize hemicellulose containing agroindustrial residues for ethanol production.
En el presente estudio se identificó en aguas residuales de una zona industrial de Pakistán una cepa de la levadura Pichia kudriavzevii sobre la base del 18S ADNr, dicha cepa mostró un crecimiento máximo a 30°C y a pH 7. Entre todos los sustratos de crecimiento evaluados para esta cepa, que incluyeron residuos industriales y medios definidos, el salvado de trigo demostró ser el mejor en función del crecimiento máximo alcanzado. Este aislado de levadura fue capaz de producir xilanasa intracelular y extracelular, esta última fue la forma predominante. Dicha capacidad enzimática mostró ser óptima a un pH de 5 y a 50°C. La presencia de Na+, Mg2+ y Fe2+ causó un incremento sustancial de la actividad enzimática, y hubo un ligero descenso (4,5%) en presencia de Mn2+, Zn2+ y Cu2+. Se evaluaron también las actividades de piruvato descarboxilasa y alcohol deshidrogenasa para confirmar la presencia de la vía del etanol. La actividad de la piruvato descarboxilasa fue mucho más pronunciada (73%) en comparación con la de alcohol deshidrogenasa (51%). Esta cepa de levadura puede emplearse para aprovechar los materiales hemicelulósicos de los residuos agroindustriales en la producción de etanol.
The requisition of development of alternative energy resources has grasped spectacular attention due to increasing energy consumption along with rising global population and the subsequent depletion of finite natural fossil fuels20. Moreover, the burning of conventional fossil fuels contributes to long-term global warming in the form of soaring atmospheric burdens of carbon dioxide in past decades2,5. In addition to climate pollution, unconstrained use of limited fossil fuels has resulted in a decline of energy resources and it has been predicted that the annual oil production may drop dramatically by 205011,18. Therefore, many countries have shown great concern in exploring both new alternative and renewable energy resources against petroleum-based transportation fuels that would not contribute to greenhouse gases to the atmosphere3,23. Thus, in order to resolve the energy exhaustion crisis, researchers have been urged to produce fuel ethanol from bioconversion of environmentally friendly biomass4,6.
Bioconversion of environmentally friendly feedstock to produce bioethanol, a clean, safe and renewable resource, has been considered an efficient alternative to petroleum-derived transportation fuels, as the use of food grains for fuel production may incite a direct competition between bioethanol and limited agricultural land needed for food and feed production. To resolve such controversies, the most promising approach would be to use agricultural residues and feedstocks that have been derived from inedible parts of food crops, for their bioconversion into renewable fuels. Thus, from both the energetic as well as the environmental point of view, it could be highly advantageous to develop second generation bioethanol from lignocellulose biomass in collation with the first generation from starch and molasses30,33.
Cellulose, hemicellulose and lignin make up the lignocellulosic biomass which is highly invulnerable to chemical and biological conversion. Large amounts of residual plant biomass considered “waste” are being discarded annually, imposing additional burdens on economy and the environment in the form of scarcity of places and costlier disposal. Hence, an upsurge of interest has been observed in the utilization of lignocellulose materials such as woody biomass and agronomic residues for ethanol production17,22. The demand for microbial enzymes is increasing due to their potential to be used in various applications in a wide variety of processes. A wide variety of microbial strains have been reported to produce xylanolytic enzymes including bacteria, yeast, filamentous fungi and actinomycetes.
The present study aims to isolate and characterize xylanase-producing yeast from the local environment and to optimize various parameters indispensable for high production of xylanase enzyme. Ethanol production was confirmed by pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) assays. The effect of agroindustrial residues on yeast growth and cell physiology (ethanol production) was also determined.
Materials and methodsSample collection and culture mediaWastewater samples were collected from the industrial areas of Raiwand and KotLakhpat (Pakistan). Some physiochemical characteristics (temperature, pH, and color) of wastewater samples were also noted. The samples were serially diluted and plated onto yeast peptone dextrose (YPD) agar medium, composed of 10g/l yeast extract, 20g/l peptone, 20g/l dextrose and 20g/l agar for yeast cultivation. Antibiotics such as streptomycin sulfate, benzyl penicillin and novidat ciprofloxacin were added into the medium at a concentration of 0.2, 0.2 and 2μl/ml, respectively, to minimize bacterial contamination. One milliliter of serially-diluted samples was spread in YPD agar plates and incubated in an inverted position at 30°C for 2–4 days.
Screening of xylanase-producing yeastsTwenty-eight yeast isolates obtained were further screened for their ability to consume diverse carbon sources such as xylose, sodium pyruvate, acetaldehyde, and carboxymethyl cellulose (CMC). For this purpose, YPD agar plates were supplemented with the abovementioned substrates as the sole carbon and energy source whose concentrations were increased gradually from 0.2% to 3%.
Growth on agro-industrial residuesThe agro-industrial residues used in this study were acquired from an agricultural area near Muridke, Sheikhupura, district of Punjab, Pakistan. All four agro-residues could be used as a promising substrate for bioethanol production after dilute acid pretreatment. Prior to acid pretreatment, each type of agro residues was sieved through a 1–1.5mm sieve for keeping particle size uniform and then impregnated with 5% sulfuric acid placed in a rotary shaker at 37°C for 24h. The acid hydrolyzates thus obtained were recovered by filtering through a double-layered Whatman filter paper and pH values were adjusted at around 7.
For xylanase production, mineral salt medium [MSM] (g/l); MgSO4, 0.1; (NH4)2SO4, 1.0; CaCl2, 0.086; FeSO4, 0.028; KH2PO4, 0.10 and K2HPO4, 0.15) supplemented with 1% of the abovementioned agro-industrial residues was used. The selected yeast isolates were cultivated at 30°C in this production medium for 48h.
Crude enzyme preparationTo produce stress conditions, the selected yeast isolates were cultivated in 250ml Erlenmeyer flasks with 100ml of the production medium supplemented with 1% (w/v) glucose. The flasks were shaken at 150rpm at 30°C for 7 days. The cell culture was harvested by centrifugation (6000rpm for 10min) and supernatant was saturated with ammonium sulfate up to 60%. The mixture was left overnight for precipitation and the precipitate was recovered by centrifugation at 14000rpm at 4°C for 10min. The pellet along with 3ml of supernatant was used as crude enzyme to measure xylanase activity of the respective yeast isolates.
Enzyme assayXylanase activity was determined by measuring the release of reducing sugar groups by the dinitrosalicylic acid method13. The reaction mixture contained 2.5ml of 50mM sodium phosphate buffer (pH 6.0) with 1% xylan and 0.4ml crude enzyme and was incubated at 40°C for 10min. The reaction was stopped by adding 100μl 3,5-dinitrosalicylic acid (DNS) and was boiled for 10min. The reaction mixture was allowed to cool at room temperature prior to taking optical density at 540nm.
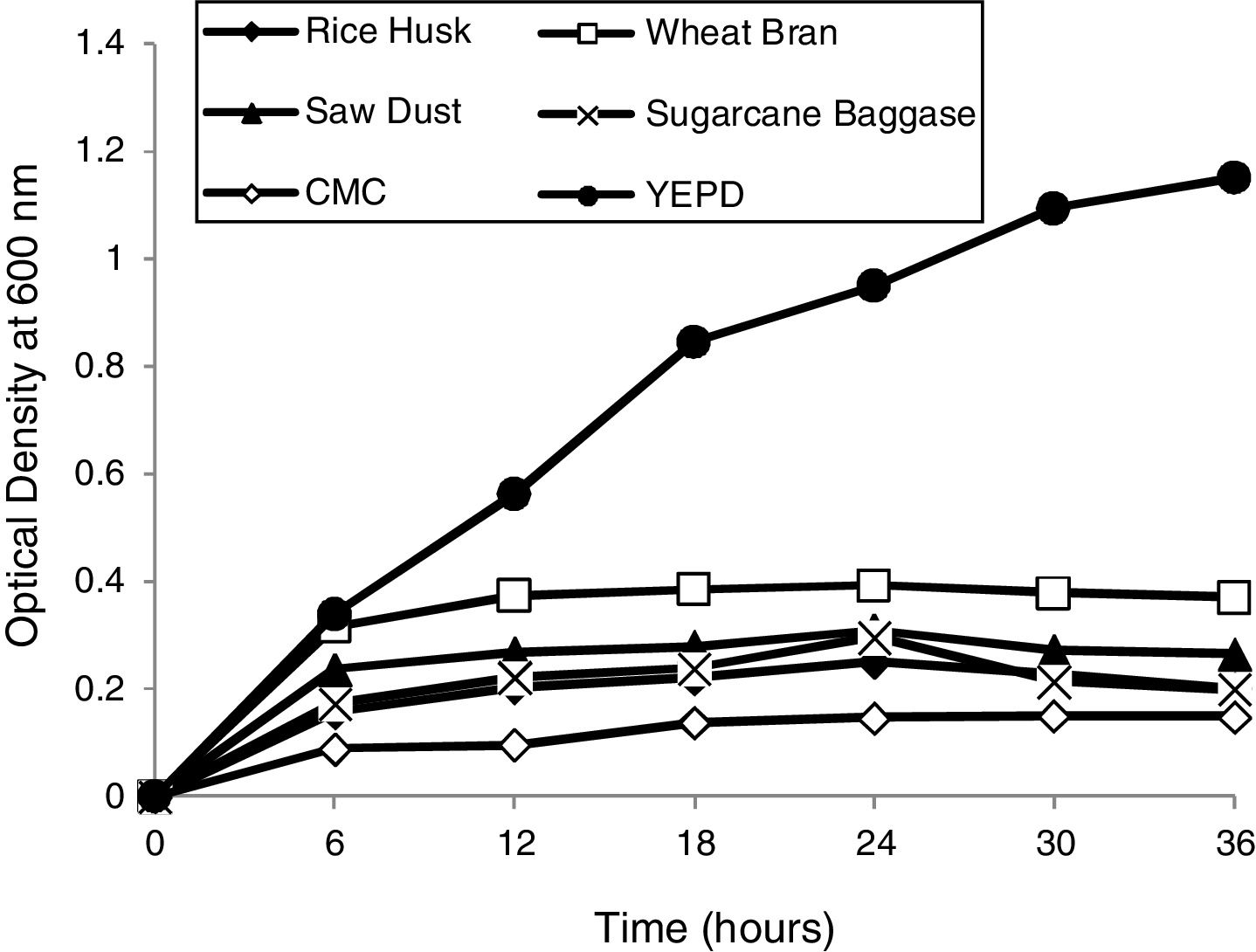
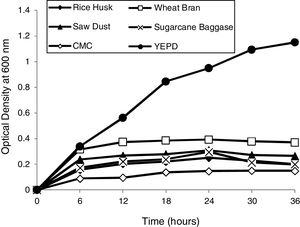
Determination of optimum growth conditions and growth curvesOptimal growth conditions (temperature and pH) of the yeast isolate were determined. Growth curves of yeast isolate 2-KLP1 were determined by growing the yeast isolate in YPD medium (control) as well as in the MSM with addition of 1% substrate, i.e., rice husk, wheat bran, sawdust, sugarcane bagasse, and carboxymethyl cellulose (experimental) and optical density was measured at 600nm with the interval of every 6h up to 36h of growth.
Molecular characterization of the yeast isolateDNA isolation was done according to Masneuf-Pomarède et al.24. PCR amplification was performed by using universal fungus-specific primers (5-GGAAGTAATAACAACG-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) in order to amplify the 5.8S ribosomal DNA region27. Amplification reactions were performed in 20μl of distilled water containing 2μl of each primer (20pmol), 2.5μl of genomic DNA (5μg/ml) (Amersham Pharmacia, Piscataway, NJ, USA). PCR was performed by initial denaturation at 94°C for 4min, followed by 35 thermal cycles, each of them at 94°C for 2min, 55°C for 2min and 72°C for 2min, with a final extension at 72°C for 10min. The amplicon of approximately 580bp thus obtained was cleaned up using a Fermentas purification kit (#K0513) and then sequenced with the genetic analysis system model CEQ-800 (Beckman) Coulter Inc., Fullerton, CA, USA. Basic local alignment search tool (BLAST) analysis was performed to determine nucleotide sequence similarities in the database.
Intracellular versus extracellular xylanase activityThe yeast isolate was grown in 100ml salt medium containing 1% xylose in 250ml for 6 days. The culture was harvested by centrifugation at 6000rpm for 10min. The pellet was washed twice with phosphate buffer and then subjected to sonication for 3 times for 15s with an interval of 60s. After sonication, the pellet was centrifuged at 14000rpm for 10min. The supernatant obtained was used for assaying the intracellular enzyme. Both intra- and extra-cellular enzyme assays were performed according to Jalal et al.13.
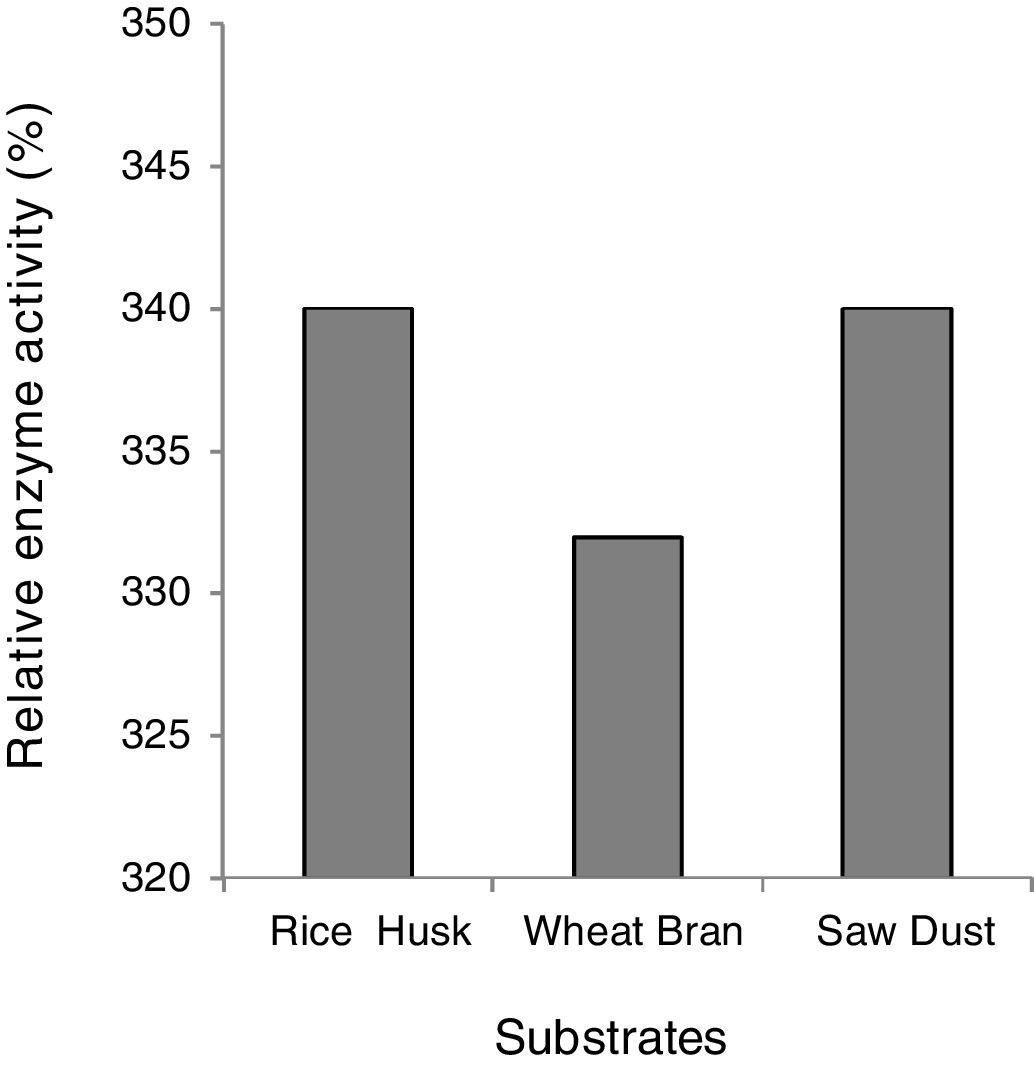
Effect of carbon sources on xylanase activityThe effect of carbon sources on xylanase production was evaluated by cultivating the yeast isolate in 250ml Erlenmeyer flasks with 100ml mineral salt medium supplemented with 1% rice husk, wheat bran, and sawdust added separately to the medium. After a five-day incubation at 30°C in shake flasks, the cultures were centrifuged at 4°C (6000rpm) for 15min. The crude enzyme was prepared and xylanase activity was estimated according to Jalal et al.13.
Determination of time profile for xylanase productionTo determine maximum xylanase production, the isolate was grown in 100ml MSM with 1% wheat bran in a rotary shaker at 130rpm. The culture was harvested after 2, 3, 4, 5, 6, and 7 days and the crude enzyme was prepared as described above. Xylanase activity was determined according to Jalal et al.13.
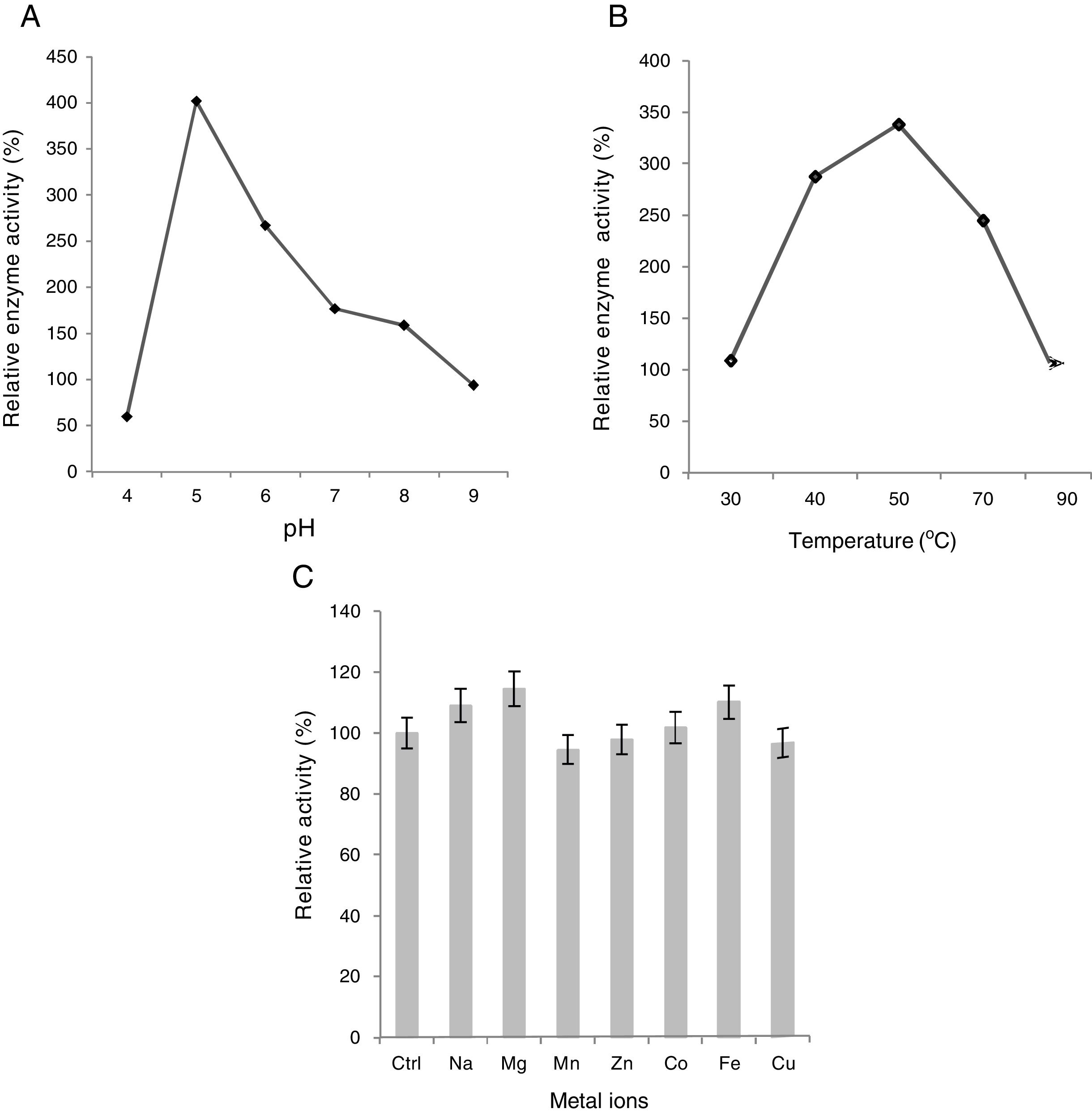
Effect of pH, temperature, and metals on xylanase activityTo investigate the optimum pH for xylanase activity, the reaction mixture consisted of the crude enzyme (0.4ml) in different buffers ranging in pH values from 4.0 to 9.0, supplemented with 1% Birchwood xylan and incubated for 10min. Buffers used were acetate buffer for pH 4, sodium acetate for pH 5–6, Na2HPO4 for pH 7–8, and Tris-HCl for pH 9. The reaction was performed as described by Jalal et al.13.
For determining xylanase optimal temperature, enzyme assays were performed under standard conditions at temperatures ranging from 30°C to 90°C. The reaction mixture, prepared in 50mM sodium phosphate buffer (pH 6.0) with 1% Birchwood xylan solution, was incubated with crude enzyme extract at various temperatures, i.e., 30, 40, 50, 70, and 90°C and enzyme activity was determined as described by Jalal et al.13.
The metal ion effect on xylanase activity was determined by adding chloride salts of Na+, Mg2+, Mn2+, Zn2+, Co2+, Fe2+, and Cu2+ in the form of CuSO4, to a final concentration of 0.1mM. The reaction mixture was prepared in 50mM sodium phosphate buffer (pH 6.0) supplemented with 1% Birchwood xylan solution and was incubated for 30min. For the control assay, no metal ion was added to the enzyme reaction mixture and xylanase activity was evaluated by Jalal et al.13.
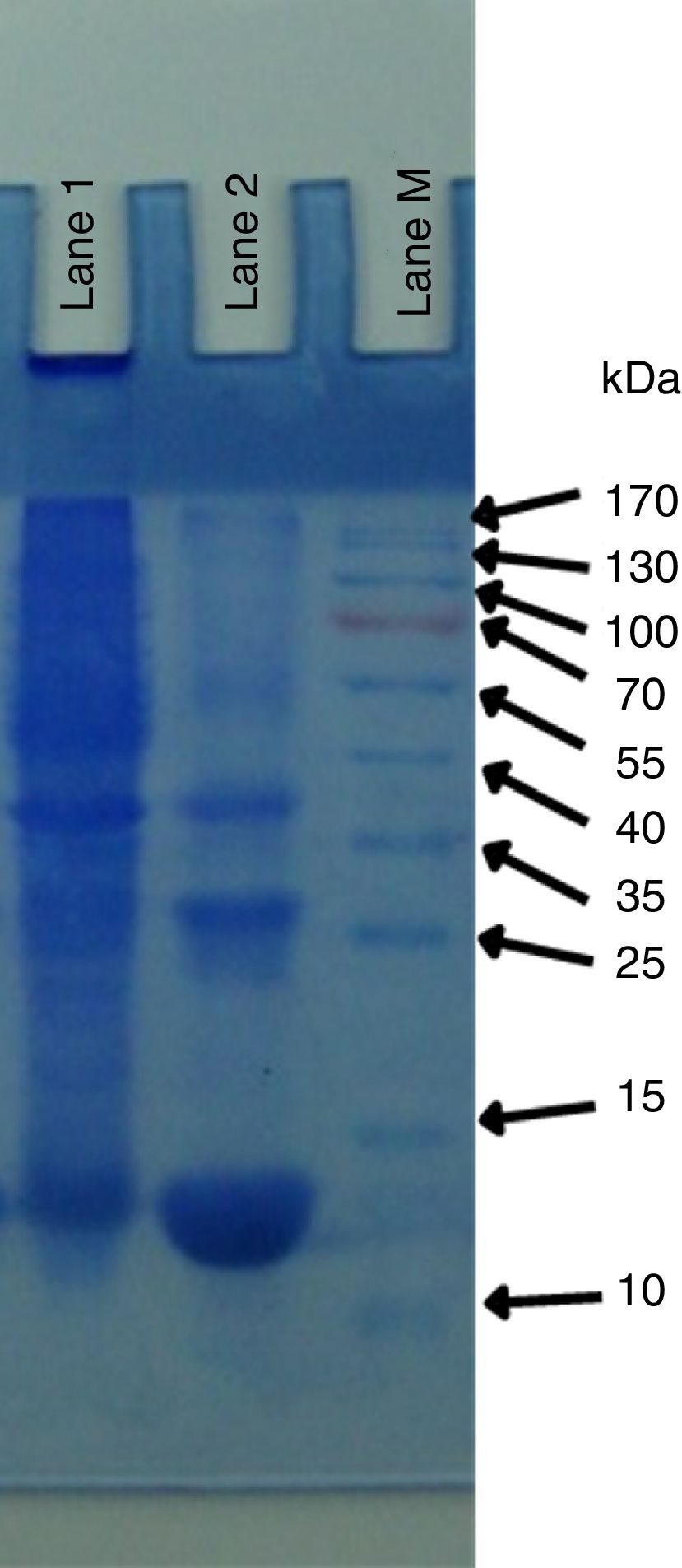
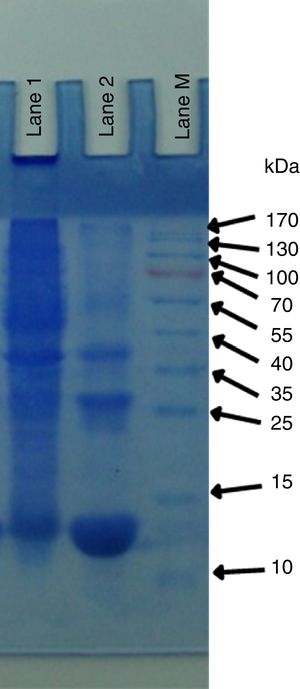
Yeast protein isolationYeast protein profiling was done according to Laemmli19. Briefly, protein purification was carried out at 4°C and the protein sample was dialyzed (Cellu·Sep membrane cat no 5-5050-34; pore size 34mm for MW of protein 50000) against 10mM sodium tartrate buffer (pH 5.5) overnight. The dialyzed sample was centrifuged at 6000g for 1.5h in centricon Ultracel YM-100 membrane- (100000 NMWL) just to remove and concentrate the protein having a molecular weight of more than 100kDa. The protein solution above the centricon was discarded and flow-through was taken, having proteins less than 100kDa. This protein solution was further used in Ultracel YM-30 membrane (50000 NMWL) to remove proteins having MW 50 or greater than 50kDa. The above protein solution was further used in Ultracel YM-50 membrane (50000 NMWL) to remove the protein with MW 50kDa. The protein solution in the upper chamber of the centricon was further concentrated in a concentrator at 4°C and finally concentrated up to 1.5μg/μl. Purified samples were finally run on SDS-PAGE.
Ethanol fermentationFermentation at a large scale was done in a 4l fermenter (Bioengineering Fert. Datum-IMS Art-Nr 332071). The fermentation medium consisting of 1.8l MSM with 1% wheat bran was inoculated with log phase yeast cell (200ml) and incubated at 30°C. The other parameters for the fermentation experiment were adjusted as pH 7.0 with 0% oxygen being agitated at 100rpm for 6 days. After 3 and 6 days, the culture was harvested by centrifugation at 6000rpm for 10min and the crude enzyme (both intracellular and extracellular) was prepared as described in enzyme assay section to perform the following experiments.
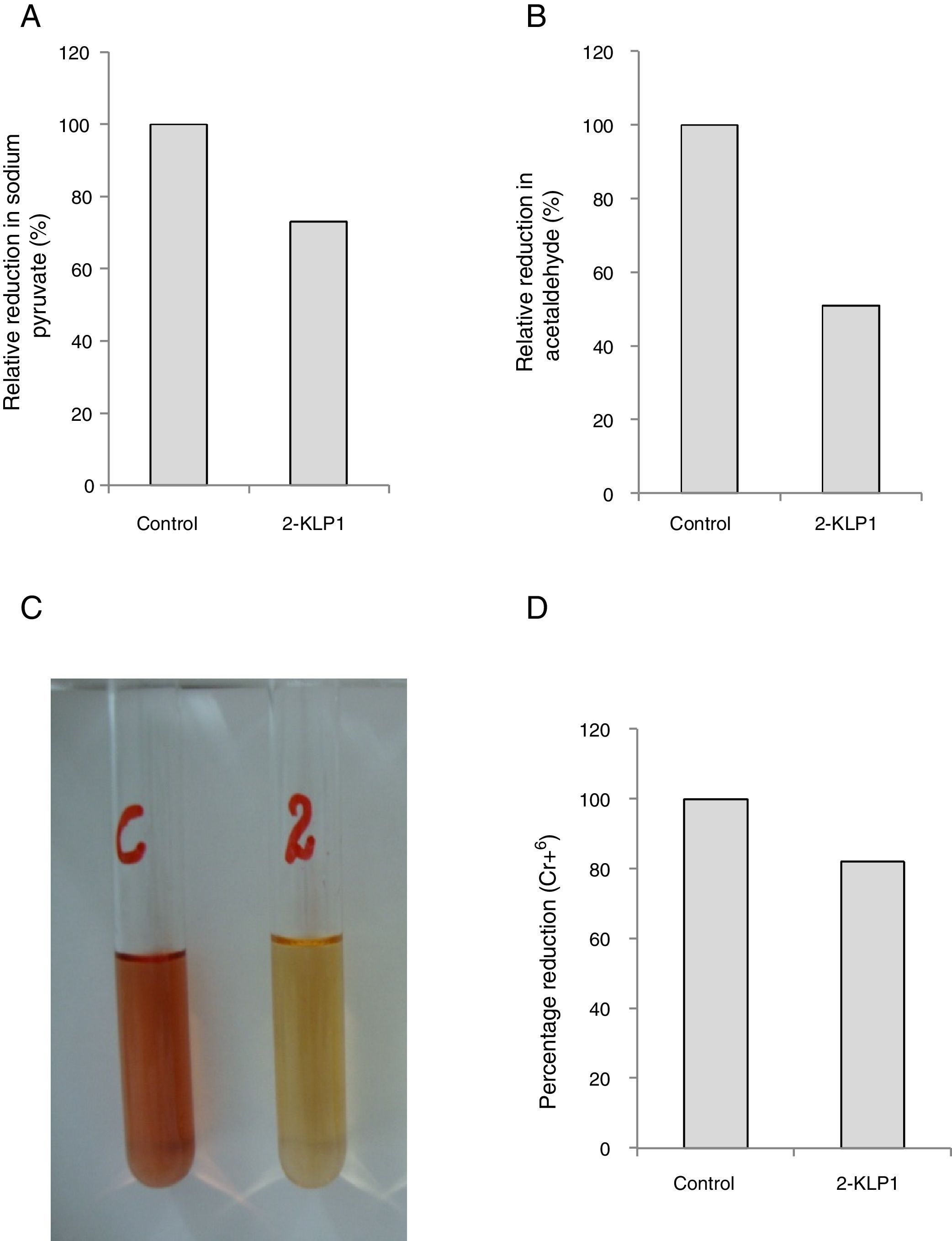
Pyruvate decarboxylase and alcohol dehydrogenase activitiesPDC and ADH activities were assayed by measuring the reduction of 2,6-dichlorophenol indophenol (DCPIP) spectrophotometrically. The reaction mixture, consisted of 0.5ml of crude enzyme, 0.5ml of 0.1mM DCPIP, 0.5ml of 100 mM Tris-HCl (pH 7.5), and 0.5ml of 1% sodium pyruvate, was incubated at room temperature and optical density was taken at 600nm after 15 and 60min. Percentage reduction of sodium pyruvate and acetaldehyde by the enzyme was calculated.
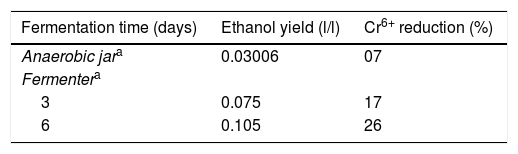
Ethanol production assayTo confirm ethanol production during fermentation, the supernatant of 2-KLP1, which was cultivated in MSM supplemented with 1% wheat bran extract under anaerobic condition, was allowed to react with hexavalent chromium (1% K2Cr2O7) and determine the decrease in hexavalent chromium concentration (Table 1). For every 100μl treated supernatant, 1ml diphenylcarbazide solution (0.25g diphenylcarbazide in 100ml acetone) was used. The reaction mixture (containing 100μl treated supernatant, 1ml diphenylcarbazide solution and 1–2 drops of phosphoric acid) was allowed to incubate at room temperature for 60min and then optic density (OD) was measured at 540nm. Any change in hexavalent chromium concentration was calculated with the help of an already prepared standard curve.
Results and discussionScreening of xylanase-producing yeastTwenty-eight different yeast isolates obtained from wastewater samples were cultured on YPD medium supplemented with the above mentioned substrates starting from a concentration of 0.2% and then increasing concentrations systematically up to 3%. Thus, on the basis of carbon source consumption, only 5 isolates (1-KLPI, 2-KLP1, 13-S1A, 26-KLP3 and 29-S1B) were selected. For further screening of the best yeast strains, two approaches were carried out, one was the culture of the screened yeast isolates in MSM supplemented with 1% carbon sources either with xylose, sodium pyruvate, acetaldehyde, CMC, or 1% agro residues. The other approach was the estimation of xylanase activity assayed through sugar liberation using the DNS method. On the basis of these results, only one yeast isolate 2-KLP1 was selected for further research. According to Wickerman32, for all yeast isolates the optimum growth temperature was 30°C, which was in agreement with our results as Pichia kudriavzevii 2-KLP1 showed maximum growth at 30°C and pH 7. Among all agro-industrial wastes, very slight differences in growth rate were observed when yeast cells were grown in wheat bran and sawdust. The maximum growth rate of yeast cells was determined in rice husk.
Identification of the yeast isolateThe yeast was found to be round in shape, 3–4mm in size, creamy-off white in color and budding in nature (Table S1). The partially amplified and sequenced ITS rDNA region from the local isolate (2-KLP1) was uploaded to the NCBI (National Center for Biotechnology Information) website. The BLAST query revealed that this gene is highly identical to an already reported gene of P. kudriavzevii. The ITS sequence coding for the 18S rRNA gene of P. kudriavzevii was submitted to the GenBank database under accession number JN 009854.
Optimum growth conditions of the yeast isolateP. kudriavzevii 2-KLP1 showed maximum growth at 30°C and at pH 7.0. To scrutinize the growth pattern of P. kudriavzevii, the yeast was cultured in MSM broth supplemented with 1% complex polysaccharides, i.e., rice husk, wheat bran, sawdust, CMC and sugarcane bagasse (experimental), against its growth in YPD (control). Among all these complex polysaccharides, wheat bran supported the highest growth whereas the least growth was observed in the presence of CMC (Fig. 1).
Intracellular versus extracellularBoth intra- and extra-cellular enzyme assays were performed to determine the most dominant form of the enzyme. The result clearly showed that almost 50% of the activity increase was determined in the extracellular assay in comparison to the intracellular enzyme assay (Fig. S1).
Time course xylanase activityTime course of xylanase production was investigated for 7 days at 50°C and the results showed that although xylanase production started readily after 48h, the maximum enzyme production was obtained after a 5-day incubation (Fig. S2). Incubation after 5 days did not show any increment in xylanase yield but led to decline in growth and enzyme production. This is in close agreement with the results recorded by Tallapragada and Venkatesh31 who reported a 6 day-requirement for maximum xylanase production. However, this is in disagreement with the time reported by Kamra and Satyanarayana14, which is only 72h. The reason behind this decrease is probably due to nutrient depletion in the media and a surge in toxic byproducts or may be proteolysis. The shift of pH toward alkalinity was observed after regular intervals (24h) during the time course of xylanase production. This increase in pH may render unfavorable growth conditions for the organism to cultivate happily and produce the enzyme31.
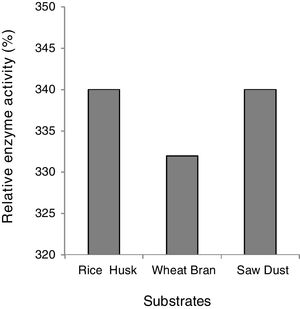
Effect of the substrate on xylanase activityThe effective induction of xylanase was investigated by using xylan containing three natural lignocellulosic biomasses namely; wheat bran, rice husk and sugarcane bagasse, maintaining other physical parameters and nitrogen sources constant. All the agro-residues showed great potency for xylanase induction and there was only a marginal decrease in the case of wheat bran (Fig. 2). In the literature, several substances have been reported as suitable carbon sources for xylanase-producing microorganisms, namely, oat wheat, Birchwood xylan, oat spelt xylan, bagasse xylan, wheat bran and rice bran14,15. The high level of xylanase production on xylan and xylan-containing substrates (rice husk, wheat bran, sawdust) suggests that xylan is necessary for effective induction. However, the low activity of xylanase produced constitutively showed that the production of extra cellular xylanase was inducible and controlled by catabolite repression. Inducible production of xylanase has been reported widely10,21 nevertheless, there are only few reports on constitutive production of xylanases by microorganisms7,8,28.
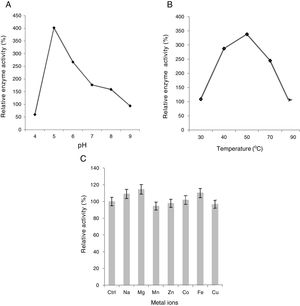
Effect of pH, temperature and metal ions on xylanase activityIt was found that xylanase enzyme exhibited the highest activity at slightly acidic pH, i.e., 5.0 (Fig. 3A). Moreover, it still retained equal to or more than 60% of maximum activity over a range of pH 4.0–6.0. However, above pH 7.0, the enzyme activity declined significantly with a sharp drop at pH 9.0, at which the enzyme showed considerably low activity (only about 23%). Our results are in good agreement with the study by Phongdara and Tumsuwan29 who worked on Pichia stipites, showing the same results. Enzyme activity is dramatically influenced by pH because the charge distribution on the substrate and particularly enzyme molecules often play a key role in substrate binding with enzymes and the subsequent catalysis26.
The optimal temperature for higher xylanase activity was 50°C at pH 5.0 (Fig. 3B). However, the enzyme showed great stability and retained more than 70% of the maximum activity between 40°C and 70°C but plummeted sharply at 90°C where its low activity was exactly similar to what was observed at 30°C. Our results are in good agreement with the works by Fujimoto et al.9 and Khanna et al.16.
Seven different metal ions with final concentration of 0.1mM were tested. When compared to control, the presence of Na+, Mg2+, and Fe2+ caused a substantial increase in enzyme activity thus acting as strong enhancers while only a slight rise was observed in the presence of Co2+. Moreover, Mn2+, Zn2+, and Cu2+ inhibited the enzyme activity only slightly (up to 4.5%), Zn2+ being the weakest of all as the enzyme retained more than 97% of its original activity (Fig. 3C). The results are in good agreement with the study by Phongdara and Tumsuwan29 with the exception of Zn2+ that increased the enzyme activity in P. stipites. A slight drop in xylanase activity was observed in the presence of CuSO4 (3.54%) in contrast to the study by Mohana et al.25 who reveals a significantly large decrease (70%) in relative enzyme activity showing high sensitivity to Cu2+.
Yeast protein isolationThe pattern of total yeast proteins was determined through SDS-PAGE. Three proteins of 50kDa, 38kDa and 20kDa were present in P. kudriavzevii 2-KLP1. The low molecular weight 20kDa protein is highly expressed in the presence of cellulosic substrates (Fig. 4).
Fermentation analysis and bioethanol productionIn the fermentation medium, enzyme production by yeasts is generally repressed by glucose therefore it is only after glucose exhaustion that the yeast isolate starts producing the xylanase enzyme at high level. In this regard, the present work also demonstrated low level of enzyme production in the presence of 1% glucose; however, the level climbed sharply (more than 100%) in deprivation of glucose when the medium was only supplemented with wheat bran extract as carbon source. Therefore, the wheat bran extract (1%) was the only choice of carbon substrate for the fermentation production medium. The strain released glucose gradually from wheat bran and produced a significantly higher enzyme yield. These observations were in agreement with those made by Akila and Chandra1 and Hrmová et al.12.
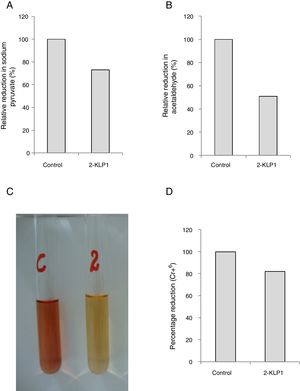
Activities of PDC and ADH were assayed and it was observed that P. kudriavzevii 2-KLP1 was able to produce both enzymes efficiently. However, PDC activity was much more pronounced (73%) (Fig. 5A) when compared to ADH (51%) (Fig. 5B). To confirm the ethanol pathway in yeast, a Cr6+ reduction assay was conducted using the standard diphenylcarbazide method (Fig. 5C and D). Two key enzymes in bioethanol production are PDC and ADH. In the central metabolism of carbohydrates, pyruvate acts as an intermediate and can be converted to acetaldehyde which can ultimately be reduced to ethanol. PDC catalyzes non-oxidative decarboxylation of pyruvate to acetaldehyde, and this acetaldehyde is then reduced by acetaldehyde dehydrogenase to produce ethanol.
(A) Relative reduction of sodium pyruvate (%) by yeast isolate under anaerobic growth conditions. (B) Relative reduction of acetaldehyde (%) by yeast isolate under anaerobic growth conditions. (C) Change in color of Cr6+ by the addition of yeast culture supernatant. (D) Percentage reduction of Cr6+ by ethanol produced during anaerobically grown yeast culture in salt medium after 8 days of incubation at its optimum temperature.
In conclusion, P. kudriavzevii 2-KLP1 was able to produce both enzymes efficiently. Fermentation was carried out both at small (in an anaerobic jar) and large scale (in a fermenter) and the amount of ethanol produced in the fermenter was conspicuously greater than that present in the anaerobic jar system. Improvements in product yields were observed in the fermenter, as compared to the shake flasks in the anaerobic jar, due to better control of process parameters in the former. From these results, it can be suggested that P. kudriavzevii 2-KLP1 would act as a promising candidate for bioconversion of hemicellulosic materials into bioethanol as the yeast strain has the enzymes necessary for its conversion.
FundingThis work was supported by the Research Cell, 10-2012/2013, Quaid-e-Azam Campus, Punjab University, Lahore-54590, Pakistan which is gratefully acknowledged.
Conflict of interestThe authors have declared that no competing interests exist.