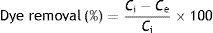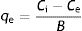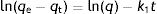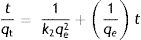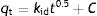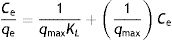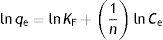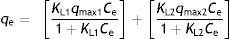Many industries generate a considerable amount of wastewater containing toxic and recalcitrant dyes. The main objective of this research was to examine the biosorption capacity of Reactive Blue 19 and Reactive Red 141 by the Antarctic yeast Debaryomyces hansenii F39A biomass. Some variables, including pH, dye concentration, amount of adsorbent and contact time, were studied. The equilibrium sorption capacity of the biomass increased with increasing initial dye concentration up to 350mg/l. Experimental isotherms fit the Langmuir model and the maximum uptake capacity (qmax) for the selected dyes was in the range of 0.0676–0.169mmol/g biomass. At an initial dye concentration of 100mg/l, 2g/l biomass loading and 20±1°C, D. hansenii F39A adsorbed around 90% of Reactive Red 141 and 50% of Reactive Blue 19 at pH 6.0. When biomass loading was increased (6g/l), the uptake reached up to 90% for Reactive Blue 19. The dye uptake process followed a pseudo-second-order kinetics for each dye system. As seen throughout this research study, D. hansenii has the potential to efficiently and effectively remove dyes in a biosorption process and may be an alternative to other costly materials.
Muchas industrias generan un gran volumen de aguas residuales que contienen colorantes, los cuales son compuestos tóxicos y recalcitrantes. El objetivo principal de este estudio fue examinar la capacidad bioadsortiva de la biomasa de la levadura antártica Debaryomyces hansenii F39A, en presencia de los colorantes azul reactivo 19 y rojo reactivo 141. Se estudiaron algunas variables del proceso, incluyendo el pH, la concentración de colorante y de adsorbente utilizada y el tiempo de contacto. La capacidad de adsorción se incrementó al aumentar la concentración del adsorbato hasta 350mg/L. Los datos de las isotermas obtenidas experimentalmente se ajustaron con el modelo de Langmuir, donde la capacidad máxima de adsorción (Qmáx) para ambos colorantes se encuentra dentro del rango 0,0676-0,169mmol/g de biomasa. A una concentración inicial de 100mg/L de adsorbato en presencia de 2g/L de adsorbente a 20?1?C y un valor de pH=6, D. hansenii F39A fue capaz de adsorber aproximadamente un 90% del rojo reactivo 141 y un 50% del azul reactivo 19. Cuando la concentración de biomasa se incrementó (6g/L), la remoción del azul reactivo 19 alcanzó el 90%. El proceso de adsorción para cada colorante sigue una cinética de pseudo segundo orden. D. hansenii tiene el potencial de remover eficientemente los colorantes estudiados, a través de un proceso de bioadsorción y puede considerarse una alternativa a otros materiales adsorbentes de mayor costo.
The textile industry uses large amounts of water and it is one of the major consumers in the dyestuff market. Almost 15–20% of the initial dye concentration applied in the dyeing process ends up in the textile wastewater and its direct discharge into water bodies causes environmental problems1,14,21,25. These coloured compounds reduce the penetration of sunlight, with a consequent reduction of photosynthetic activity. Furthermore, many of the dyes used and their by-products could have toxicity, mutagenic or/and carcinogenic effects on some aquatic organisms38.
Dyes have different and stable chemical structures, making it possible a wide range of bright colouration to the industry and fastening tendency. They are designed to resist fading upon exposure to light, water, chemical oxidizing agents, and microbial attacks. Due to their chemical structure, they are not degraded or removed easily from the textile wastewater32,34. To treat the wastewater discharged, several physical, chemical and biological methods have been used over the years, not only for the elimination of colour contained in the wastewater, but also for the complete mineralization of the dyes and to reduce the toxicity levels38. Biological processes are economic, produce a small amount of sludge, and are environmentally friendly22.
The removal of many dyes seems to be efficient using the biosorption method, which has high selectivity, is easily applicable in conditions of large volumes and low concentrations as is the case of textile effluents. It is low cost effective because the materials to be used as adsorbents are generally cheap and abundant, and they can be useful to effluents containing a mixture of dyes and have an easy subsequent separation1,30. Biosorbents from carbonaceous materials (wood, rice), raw agricultural solid wastes (apple pomace and wheat straw, sugarcane bagasse), natural materials (zeolites, siliceous), industrial solid waste (fly ash) and biological materials (fungi, yeast, bacteria, algae) have been used to decolorize textile effluent1,13,14. Using non-growing or non-living microbial biomass as biosorbents to concentrate and remove dyes from textile effluents offers a potential alternative to the existing decolorization methods. The use of dead or pretreated biomass is a metabolism-independent mechanism that involves binding of the dyes to the surface of cell membranes or walls through physical adsorption, chemical precipitation, ion exchange, chelation and electrostatic interaction2,26,28,30.
The extent of dye biosorption depends on the interaction of the textile dyes with microorganisms. This interaction depends on the dye molecule properties such as their structure and the type and position of groups that are part of it, and the specific chemistry and microbial biomass.
Yeast cell walls mainly contain polysaccharides, proteins and lipids with many functional groups that differ in their affinity and specificity for dye molecule binding. The adsorption process increases with the presence of hydroxyl, azo and nitro groups but decreases with sulfonic acid groups. The selectivity and efficiency of the adsorption process are due to the interaction between molecules of dye from solution with the adsorbent by physical and chemical adsorption15. The effectiveness depends also on environmental conditions and other variables, such as: pH, ionic strength, temperature, existence of competing organic or inorganic ligands in solution, contact time and adsorbent concentration36.
Many studies related to Antarctic yeasts have been focused on their potential use in different biotechnological industries because of their ability to produce different types of enzymes with particular characteristics6,24. The potential of these yeasts as biological agents for wastewater treatment has been little studied. As far as we know, the ability of the Antarctic yeasts to decolorize dyes was only studied by Rovati et al.,33 in solid media. The aims of this study were to investigate the potential use of an Antarctic yeast, Debaryomyces hansenii F39A, as a biosorbent for textile dye adsorption, Reactive Red 141 (RR-141) and Reactive Blue 19 (RB-19). The mechanism of adsorption, the effects of initial pH, dye, and adsorbent concentration in the biosorption process, and the kinetics and isotherm of dye adsorption were also studied.
Materials and methodsBiosorbents and adsorbentYeast selection and preparation of the biosorbentDye-decolorization screening was performed using a collection of sixty-one yeasts isolated by Martinez et al.23 during the Antarctic expedition to King George Island. The screening was carried out using the Normal Decolorization Media (NDM) proposed by Ramalho et al.32 (g/l): 10 NaCl, 5 KH2PO4, 0.5 MgSO4, 0.13 CaCl2, 40 glucose, 2.5 yeast extract, 1.25 urea, 1.5 agar and 0.1 of dye. The dyes used were Reactive Black 5, Reactive Blue 19, Acid Blue 74 and Reactive Orange 16. Plates were inoculated with actively growing yeast from Potato Dextrose Agar media (PDA, Britania SA, Buenos Aires, Argentina), incubated at 20°C for 72h. The plate without dye was also inoculated as control. The plates were observed daily until a decolorization halo was visible surrounding the colony, as well as the colouration that the colony acquired29.
Debaryomyces hansenii F39A was used as biosorbent in this study, and was deposited in the Microbiological Culture Collection of CINDEFI-CONICET Institute and in the Cátedra de Microbiología, Facultad de Química, UdeLar Culture Collection (Montevideo, Uruguay). The strain was cultured in liquid Yeast Extract Peptone Dextrose (YPD) medium. The growth medium consisted of 20g/l glucose, 20g/l agar, 5g/l yeast extract, 5g/l bactopeptone. The medium was sterilized by autoclaving at 121°C for 30min. In order to obtain the biosorbent (biomass) culture batch in an agitated stirred tank bioreactor LH series 210 (Iceltech, Toulose, France) at 20°C, aeration of 1 VVM (volume of air per volume of culture medium per minute) and 400rpm with 1l of culture medium for 72h was performed.
At the end of the culture, yeast biomass was separated from the solution by centrifugation at 4000 x g for 15min (Sorvall RC5C, Connecticut, USA). The pellet obtained was washed twice with distilled water; the end wet basis biomass was homogenized and then stored at −20±2°C until used.
Dye solutionsThe dyes used in this study were chosen because they represent different dye groups including double azo, anthraquinone and reactive dyes. Reactive Red 141 (RR-141) and Reactive Blue 19 (RB-19), were kindly provided by ALCONIC SRL (Buenos Aires, Argentina). Both are reactive and anionic. RR-141 has a double azo bound and has a molecular weight of 1774.19g/mol, while RB-19 is an anthraquinone dye with a molecular weight of 626.55g/mol. Dyes stock solutions in a range of 1–5g/l were prepared.
Batch biosorption studiesThe experiments were conducted in 100ml Erlenmeyer flasks containing 50ml of solution of 100mg/l of dye, with 2g/l of D. hansenii F39A yeast biomass as the adsorbent, agitated on a rotary shaker (Dlab SK-L180-Pro, Beijing, China) at 150rpm, at room temperature (20±2°C), until equilibrium was reached. At pre-determined periods of time, samples (1ml) were taken and the yeast biomass was separated from the suspension by centrifugation at 13000 x g for 10min. The residual dye concentration in the treated medium was analyzed by UV–visible spectrophotometer (Beckman Coulter Inc., Indianapolis, Indiana, United States) at λmax of the dyes. The maximum wavelength absorptions for RR-141 and RB-19 were 550 and 595nm, respectively. Dye removal efficiencies by adsorption process (R%) were calculated according to the equation (Eq. (1)):
where Ci is the initial adsorbate concentration and Ce is the concentration at equilibrium (mmol/l)21.Dye biosorption capacity (qe) of the yeast biomass at equilibrium was calculated using the following equation (Eq. (2)):
where B is the bioadsorbent concentration (g/l)41.This experiment was performed twice as an independent experiment for each dye analysis.
The presence of dye metabolites was analyzed before and after the adsorption step by high performance liquid chromatograph (Waters HPLC pump model 510, Waters, Milford, Massachusetts, United States). Twenty μl were injected into an XBridge C18 (particle size 5μm, 5.6mm×250mm I.D., at room temperature), column using phosphoric acid (pH=2.1) and methanol as the eluent at 1.0ml/min constant flux. Samples were analyzed with a Waters 2996 PAD detector at 260nm using Empore Pro software.
Study of process variablesThe adsorption process is greatly affected by physicochemical variables, and, for this reason, several experimental conditions were studied:
- -
The influence of initial pH of the solution on dye adsorption in the range of 2.0–10.0, with 2g/l of D. hansenii F39A biomass and dye concentration of 100mg/l.
- -
The influence of initial dye concentration between 25 and 350mg/l on the adsorption process, with 2g/l of the yeast biomass, pH 7.0.
- -
The influence of the biomass dosage. For the removal of 100mg/l of the dye RR-141 the range of biomass studied was 1–3g/l and for 100mg/l of RB-19 was 1–6g/l, respectively, pH 7.0.
In every experience, the samples were withdrawn, and biomass separated by centrifugation and absorbance of the dye in the supernatant was measured at λmax of each dye. All the experiments were performed in duplicate.
Kinetics of biosorption studiesThe study of kinetics serves to determine the rate-controlling steps involved in the adsorption process of dyes RR-141 and RB-19, such as the transfer of the dye to the surface of the adsorbent, the adsorption on the yeast biomass and the internal diffusion and retention on the active sites via a chemical or physical reaction process. To corroborate the above, three different kinetic models were analyzed, the Lagergren pseudo-first order model (Eq. (3))35, a pseudo-second order model (Eq. (4))20 and the intraparticle diffusion model (Eq. (5))42. Dye biosorption kinetics was determined using the data obtained from the equilibrium of the batch studies.
The pseudo-first order kinetic model is based on the sorption capacity of the solid phase. From the integral of Lagergren model, the following equation is obtained:
where qe (mmol/g) is the amount of dye adsorbed on the yeast biomass at equilibrium and qt (mmol/g) is the amount adsorbed at a time t, and k1 (1/min) is the Lagergren adsorption constant. This model assumes that the limiting step in the adsorption process is the transfer mass of the adsorbate within the solution towards the adsorbent surface.The pseudo-second order kinetic model proposed by Ho and McKay assumes that the adsorption process involves the sorption capacity of the biomass surface and also the chemisorption mechanism; the linear equation is expressed as:
where k2 is the second-order biosorption constant (g/mmolmin).The intraparticle diffusion model that permits to recognize the diffusion mechanisms through the internal structure of the adsorbent pores, is expressed by:
where C is the boundary layer thickness and kid is the model rate constant (mmol/gmin0.5). The kinetic parameters kid and C can be determined by plotting qt versus t0.5. If C is zero, it means that the intraparticle diffusion is the controlling step of bioadsorption dynamics.Biosorption isotherm studiesThe results obtained in a batch system for biosorption of different concentrations of RR-141 and RB-19 dyes onto D. hansenii F39A biomass were analyzed by the adsorption isotherms proposed by Langmuir (Eq. (6)) and Freundlich (Eq. (7)), the double Langmuir model was also analyzed (Eq. (8)), to interpret how adsorbates interact with adsorbents.
The Langmuir model assumes a complete monolayer sorption of adsorbate onto the biomass surface with a finite number of identical sites, and refers to homogeneous adsorption. Graphically, it is characterized by a plateau, an equilibrium saturation point where once a molecule occupies a site, no further adsorption can take place16. The linear form of the mathematical expression of Langmuir isotherm is:
where qe (mmol/g) is how much dye was removed from the solution per unit weight of yeast biomass and Ce (mmol/l) is the unadsorbed dye concentration at equilibrium, respectively. The maximum adsorption capacity, qmax (mmol/g), is the maximum amount of the dye removed per unit weight of adsorbent corresponding to a monolayer adsorption on the surface35. The Langmuir adsorption constant, KL (l/mmol), is higher when the adsorption energy is higher, which is related to the affinity between the adsorbent and the adsorbate7,14.The dimensionless parameter, RL, is used to check if the fit to the Langmuir model is favourable, is defined as RL=1/(1+KLCi), where Ci is the initial concentration of the dye studied (mmol/l). RL can be used to define the type of isotherm that is obtained as follows: favourable (0<RL<1), unfavourable (RL>1), linear (RL=1) and irreversible (RL=0)7.
The Freundlich equation describes the sorption on different adsorption sites that have different affinities over a heterogeneous surface, it is not restricted to the formation of monolayer adsorption16; the linearized isotherm is given as:
where KF and n are the model constants, which are indicators of adsorption capacity and intensity, respectively14. The constant n refers to the affinity between the adsorbent and adsorbate, where a value greater than 1 represents a good adsorption intensity15.In a complex system, it seems unreasonable to assume that all adsorbing sites will have the same bonding energy. If the total surface consists of two or more component surfaces with different bonding energies, the linear Langmuir transformation of the isotherm will produce straights with different slopes, because adsorption occurs simultaneously on all surfaces. In these systems, the double Langmuir model is more suitable for the mathematical modelling. The double Langmuir model assumes that adsorption occurs on two types of surfaces with different bonding energies, and is expresses by:
Results and discussionSixty-one yeast isolates previously retrieved from King George Island were tested for dye accumulation using a widely known technique where yeasts were incubated on solid media supplemented with the selected dye. It could be observed that 81% were able to produce a halo around the colony and 63% of them demonstrated the ability to accumulate the dyes tested. Of this 63%, Debaryomyces hansenii F39A was selected for dye adsorption studies. Debaryomyces hansenii has been reported to be a microorganism of biotechnological importance and potential. It is a non-pathogenic, osmotolerant and oleaginous microorganism31. The main characteristic that this yeast has in relation to textile wastewater treatment is its halotolerance5,18. It is well documented that textile effluents are characterized by high salinity40. Debaryomyces hansenii could be applied in dye bioadsorption experiments where it can grow in simulated media to a textile effluent with high concentration of salts.
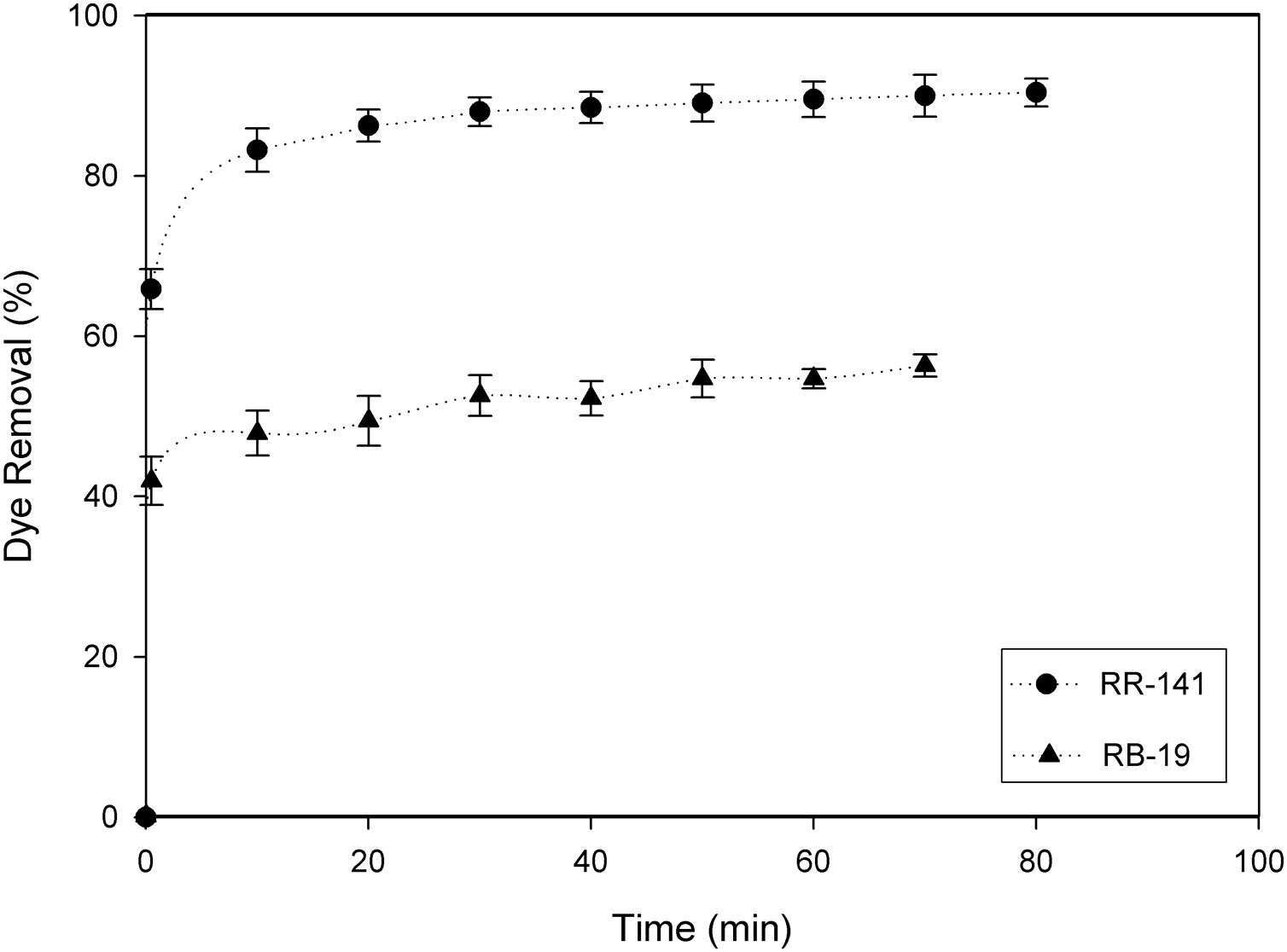
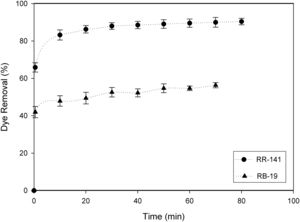
Firstly, the adsorption process was studied using 2g/l of D. hansenii F39A biomass with an initial dye concentration of 100mg/l. After 70min of contact time the equilibrium was reached, and the yeast biomass adsorbed 90% of RR-141 and 56% of RB-19 (Fig. 1). The percentage of dye adsorbed increased based on the contact time. It was observed that decolorization of both dyes was extremely rapid in the first 15min of the process. Similar results were reported by de Castro et al.,7 where after 5min the adsorption of the dyes gradually decrease until reaching equilibrium at 30min for Reactive Red 239 and Reactive Black B and 60min for Reactive Blue 85; attributing the fast adsorption to the number of unoccupied sites in the yeast biomass at the beginning of the process.
HPLC experiments were performed in samples before and after the dye adsorption treatment proposed. After the treatment of 350mg/l of each dye used, the chromatograms obtained showed no presence of sulfanilic acid. These results are better than those reported by García et al.17 In their sorption experiments using Macrocystis pyrifera biomass and zerovalent iron nanoparticles after 6.5h of contact time, HPLC demonstrated the presence of sulfanilic acid, a toxic and carcinogenic compound12. These results suggest that the dyes were not degraded by the yeast biomass, only the bioadsorption of the dye from the solution occurred.
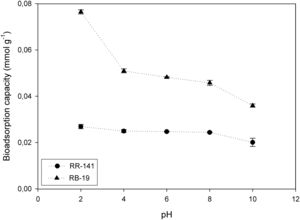
Effect of initial pHThe biosorption process is significantly affected by the pH of the solution, which also affects the dye solubility. The yeast surface contains different functional groups such as amino, carboxyl, hydroxyl, phosphate and other charged groups, which are ionized by the influence of the pH value14.
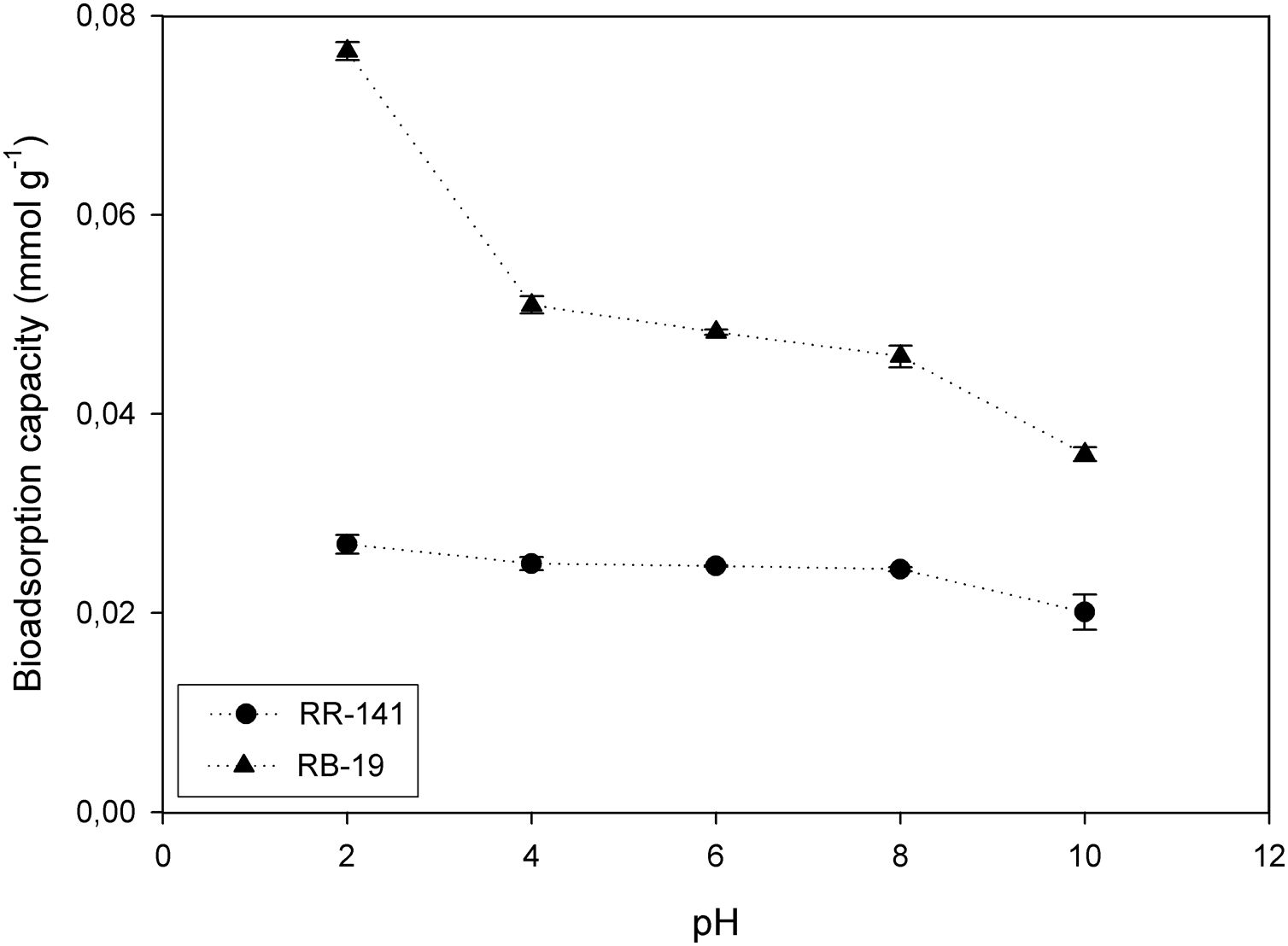
The influence of the initial pH of the medium of RR-141 and RB-19 adsorption was studied in the range of 2.0–10.0 at an initial dye concentration of 100mg/l and 2g/l of yeast biomass; the results are shown in Figure 2. For RB-19, at pH 2.0, the highest amount of biosorption was highlighted (97.15%), the adsorption capacity being (qe) 0.076mmol/g. At pH 4.0 qe decreased at 0.050mmol/g and did not change significantly up to pH 8.0, where it declined with further increase of the pH value. In this case the electrostatic interactions play a dominant role in the adsorption mechanism when the dye molecule interacts with chemical groups present on the biomass surface. The different interactions between anionic dyes and adsorbent is related to the protonation of the yeast biomass42, generating electrostatic interactions. When the pH value of the medium increases, the adsorption capacity decreases due to the electrostatic repulsion between the dye and the negatively charged sites on the biomass surface. Then, electrostatic attractions, dispersive interactions, hydrophobic attraction, hydrogen bonding interactions and physical adsorption should operate between the yeast surface and the dye molecules1,7,14.
The adsorption capacity for Reactive Red 141 seems to be not influenced by the medium pH. At pH 2.0, qe is 0.027mml/g, at pH 8.0 is 0.0244mmol/g and at pH 10.0 decreases slightly up to 0.020mmol/g. In this case the electrostatic interactions have a lesser influence than in the adsorption of Reactive Blue 19. This feature could be due to a steric masking of certain reactive groups present in the dye.
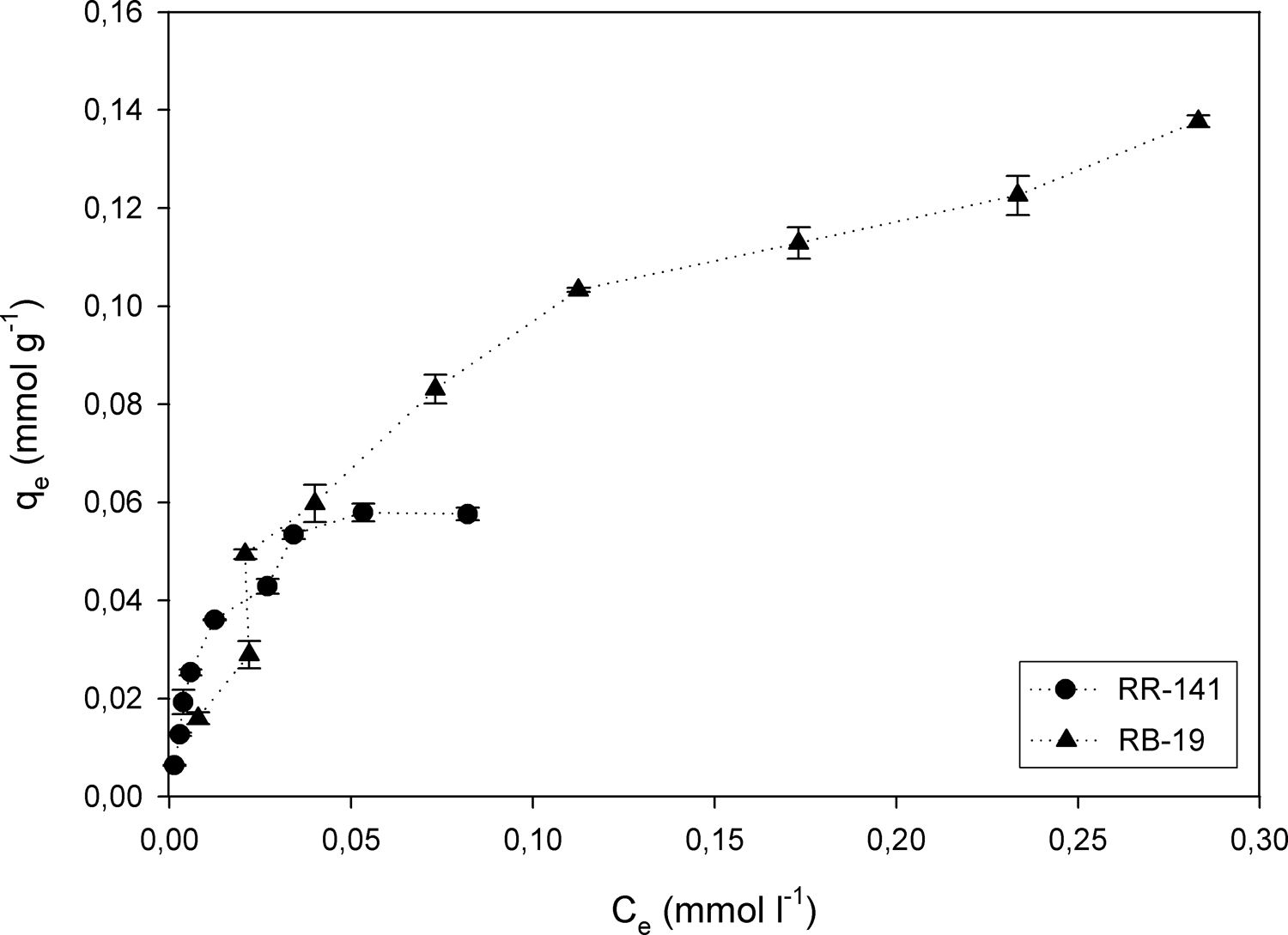
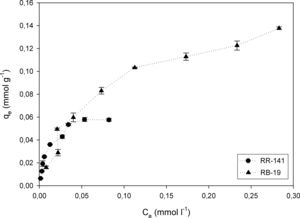
Effect of initial dye concentrationThe adsorption process improves with an increment in the initial dye concentration, due to the increment of the interactions between the higher number of dye molecules and the absorbent1. The amounts of RR-141 and RB-19 adsorbed onto 2g/l of D. hansenii F39A biomass were studied in the range of 25–350mg/l; the results are shown in Figure 3. By increasing the initial dye concentration, the adsorption capacity (qe) increased up to 0.0575mmol/g for RR-141 and 0.137mmol/g for RB-19, respectively. With a further increase in concentration, the dye uptake did not change significantly, due to saturation of the active sites on the adsorbent. From the results obtained the adsorption capacity of D. hansenii appears to be greater with Reactive Blue 19 than with Reactive Red 141 as adsorbate, which could correspond to their chemical structure. Similar results were reported by Ergene et al.,14 where a greater elimination of Remazol Black B and Remazol Red R was obtained with an increase of the initial dye concentration until saturation at 200mg/l.
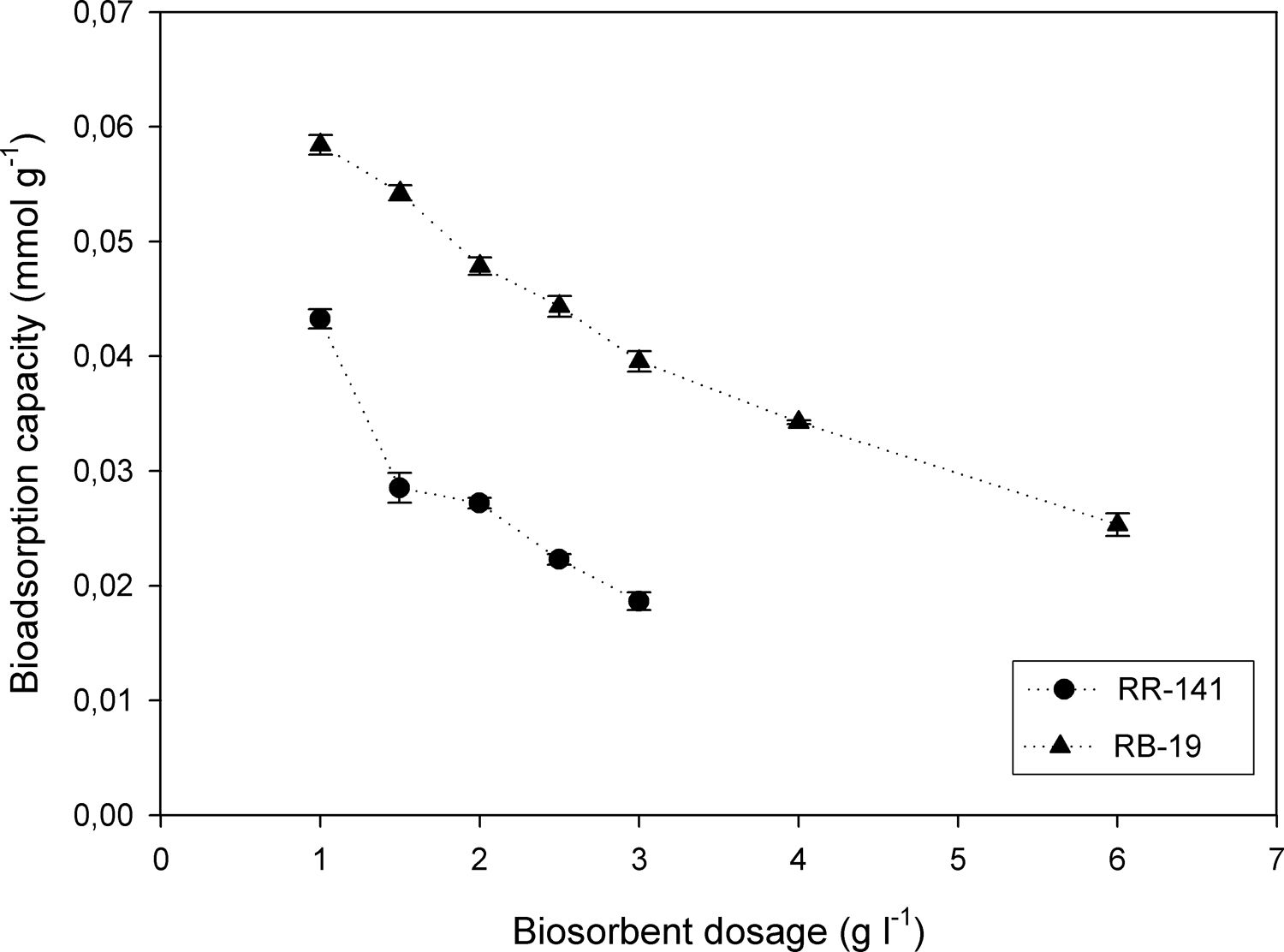
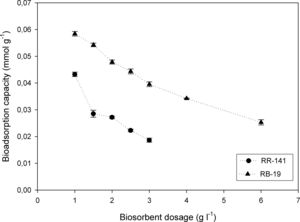
Effect of the adsorbent doseFigure 4 shows the uptake of 100mg/l of dye by different dosages of D. hansenii. For RR-141 the removal efficiency by a bioadsorption mechanism significantly increased from 71.89 to 92.97% when the adsorbent dose increased from 1 to 3g/l. In the case of RB-19 the increment observed was from 34.03 to 84.63% when the adsorbent dose increased from 1 to 6g/l. These maximums (92.97 and 84.63%) can be explained because there is an excess of the biosorption available sites with the increase of the absorbent load, which cannot be occupied by the absorbates2. Moreover, the biosorption capacity decreased from 0.043 to 0.019mmol/g for RR-141 and from 0.058 to 0.025mmol/g for RB-19 in the range of biomass dosage used. At a higher adsorbent dose, the biosorption capacity decreases, one of the reasons may be that the binding sites do not get saturated7. It could be concluded that dye RB-19 has higher bioadsorption capacity than e RR-141 at the absorbent concentrations used, which could be due to the molecule size that probably causes a steric impediment between the dye and the chemical groups in the yeast biomass.
Kinetic modelsKinetic studies are used to explain the adsorption mechanism for the design and optimization of the adsorption system. The kinetic plots for the three models for D. hansenii F39A at different initial dye concentrations with 2g/l biomass loading were obtained.
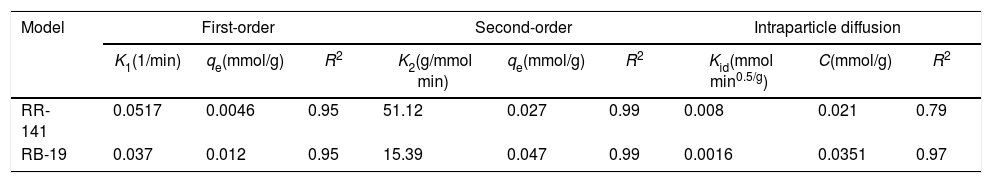
The values of first-order, second-order and intraparticle diffusion constants and correlation coefficient (R2 value) are shown in Table 1. Meanwhile, experimental qe is 0.048mmol/l for Reactive Blue 19, qe estimated by the model is 0.012mmol/l for adsorption in the first-order but it is 0.047mmol/l using the second-order kinetic model. For Reactive Red 141 when experimental qe is 0.027mmol/l, qe calculated by the model is 0.0046mmol/l for adsorption in the first-order but it is 0.027mmol/l for the second-order kinetic model. Therefore, qe values calculated from the plots for the pseudo second-order kinetic model were closer to the qe obtained with experimental data than those for the pseudo first-order kinetic model for both dyes used. The values of the parameter C for the intraparticle diffusion model were 0.021mmol/g for RR-141 and 0.0351mmol/g for RB-19 respectively, suggesting that intraparticle diffusion does not control the adsorption process. This model fits better with RB-19 than RR-141, which could be partially explained because of the large size difference between the dye molecules; in the case of RR-141 some sites could be steric masked, since the functional groups present in both dyes are similar.
Comparison of kinetic parameters estimated by the first-order, second-order and intraparticle diffusion models for initial dye concentration of 0.0564mmol/l for RR-141 and 0.160mmol/l for RB-19 concentration of RR-141 and RB-19 biosorption on the D. hansenii F39A biomass.
| Model | First-order | Second-order | Intraparticle diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1(1/min) | qe(mmol/g) | R2 | K2(g/mmol min) | qe(mmol/g) | R2 | Kid(mmol min0.5/g) | C(mmol/g) | R2 | |
| RR-141 | 0.0517 | 0.0046 | 0.95 | 51.12 | 0.027 | 0.99 | 0.008 | 0.021 | 0.79 |
| RB-19 | 0.037 | 0.012 | 0.95 | 15.39 | 0.047 | 0.99 | 0.0016 | 0.0351 | 0.97 |
As can be observed, the second order kinetic fits to a R2>0.99 for both dye bioadsorption systems studied.
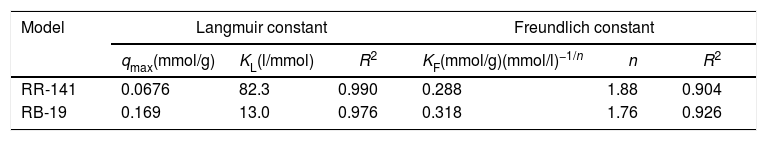
Biosorption isothermThe adsorption isotherm parameters along with the correlation coefficients for fitting the isotherms models are shown in Table 2. Linear relationships were evidenced by the R2 values (0.990 and 0.976 for RR-141 and RB-19, respectively for the Langmuir model, while for the Freundlich model these values were 0.904 and 0.926 for RR-141 and RB-19, respectively) indicating that the experimental data for both dyes fitted the Langmuir model better than the Freundlich isotherm.
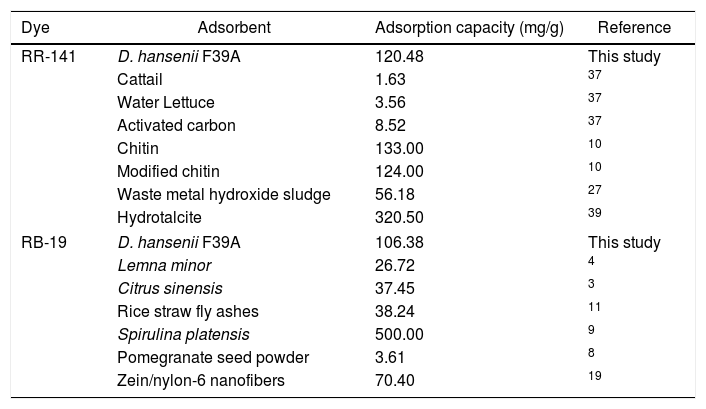
The maximum adsorption capacity (qmax) calculated from the Langmuir isotherm is a parameter that describes the adsorption process. It was found to be 0.0676 and 0.169mmol/g for RR-141 and RB-19, respectively (at the same contact time with a dye concentration of 0.0564mmol/l for RR-141 and 0.160mmol/l for RB-19). The comparison of the monolayer maximum adsorption capacity (qmax) of D. hansenii with various other adsorbents reported in the literature is shown in Table 33,4,8,9,10,11,19,27,37,39. The adsorption capacity of the yeast biomass for both dyes was found to be higher than other adsorbents reported except for chitin-based and hydrotalcite adsorbents for RR-141, and Spirulina plantensis for RB-19. The biomass of D. hansenii as an adsorbent of textile dyes is an easy and economical material to produce and dispose after treatment, and it also has great potential for its use in the proposed adsorption process.
Comparison of maximum adsorption capacities for Reactive Red 141 and Reactive Blue 19 dyes reported in the literature.
| Dye | Adsorbent | Adsorption capacity (mg/g) | Reference |
|---|---|---|---|
| RR-141 | D. hansenii F39A | 120.48 | This study |
| Cattail | 1.63 | 37 | |
| Water Lettuce | 3.56 | 37 | |
| Activated carbon | 8.52 | 37 | |
| Chitin | 133.00 | 10 | |
| Modified chitin | 124.00 | 10 | |
| Waste metal hydroxide sludge | 56.18 | 27 | |
| Hydrotalcite | 320.50 | 39 | |
| RB-19 | D. hansenii F39A | 106.38 | This study |
| Lemna minor | 26.72 | 4 | |
| Citrus sinensis | 37.45 | 3 | |
| Rice straw fly ashes | 38.24 | 11 | |
| Spirulina platensis | 500.00 | 9 | |
| Pomegranate seed powder | 3.61 | 8 | |
| Zein/nylon-6 nanofibers | 70.40 | 19 | |
The RL values found were 0.0476 and 0.121 for RR-141 and RB-19, respectively, indicating that the adsorption process is favourable for both dyes. Additionally, the values of the Freundlich adsorption constant, n, were found to be 1.88 and 1.76 for RR-141 and RB-19, respectively; therefore, the adsorption process is favourable with high affinity between dye molecules and biosorbent2,15.
In Figure 3, both isotherms show marked inflections that suggest a distribution of different reactive groups with different affinities to bind the dyes. It is possible to note a first zone of high affinity with a load capacity, followed by a zone of lower affinity with similar load capacity. Then, there is a zone of low capacity, almost a plateau in case of RR-141 and with a very slight slope for RB-19. The isotherm for RR-141 shows a marked biphasic behaviour with two clear plateaus, showing a double Langmuir behaviour. Fitting the isotherm to this model, two constants of apparent affinity of −5.7 and 90.4 were obtained, which has no physical meaning; therefore, it cannot fit the model. For the RB-19, after a marked initial increase of sigmoidal shape after the inflection, a plateau is not obtained, but capacity is increased in a fairly linear manner. This behaviour also suggests different adsorption sites, although with a less marked tendency that indicates a more continuous distribution than for RR-141. Furthermore, non-saturation may be due to the intraparticle diffusion of the dye into the yeast biomass. The double Langmuir model shows in this case two constants of 13.8 and 14.4, the model is not applicable to this adsorption. In this study, the single Langmuir model, fits better.
ConclusionsThe aim of the present research was to examine the potential use of a non-growing biomass of an Antarctic yeast D. hansenii F39A as a biosorbent for textile dye adsorption. The dyes used were Reactive Red 141 and Reactive Blue 19. The results of this investigation have shown an extremely rapid decrease in dye concentration in the first 15min of the process. A pure adsorption mechanism occurred, that is, without the release of any toxic compound or element into the dye-wastewater.
It can be suggested that a biosorption process with D. hansenii F39A could be an economical and effective alternative for the treatment of dye-containing effluents. Finally, it can be concluded that the biomass of the selected yeast as biosorbent may be an option for other costly materials such as activated carbon. To the best of our knowledge, this research is one of the first attempts to thoroughly study the adsorption potential of dyes by Antarctic yeasts in liquid media.
Conflicts of interestNone declared.
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT 2014-1655 and 2015-3699).
ALCONIC SRL: for contributing the dyes used in this study.