Chromatin remodeling enzymes are important “writers”, “readers” and “erasers” of the epigenetic code. These proteins are responsible for the placement, recognition, and removal of molecular marks in histone tails that trigger structural and functional changes in chromatin. This is also the case for histone deacetylases (HDACs), i.e., enzymes that remove acetyl groups from histone tails, signaling heterochromatin formation. Chromatin remodeling is necessary for cell differentiation processes in eukaryotes, and fungal pathogenesis in plants includes many adaptations to cause disease. Macrophomina phaseolina (Tassi) Goid. is a nonspecific, necrotrophic ascomycete phytopathogen that causes charcoal root disease. M. phaseolina is a frequent and highly destructive pathogen in crops such as common beans (Phaseolus vulgaris L.), particularly under both water and high temperature stresses. Here, we evaluated the effects of the classical HDAC inhibitor trichostatin A (TSA) on M. phaseolinain vitro growth and virulence. During inhibition assays, the growth of M. phaseolina in solid media, as well as the size of the microsclerotia, were reduced (p<0.05), and the colony morphology was remarkably affected. Under greenhouse experiments, treatment with TSA reduced (p<0.05) fungal virulence in common bean cv. BAT 477. Tests of LIPK, MAC1 and PMK1 gene expression during the interaction of fungi with BAT 477 revealed noticeable deregulation. Our results provide additional evidence about the role of HATs and HDACs in important biological processes of M. phaseolina.
Las enzimas remodeladoras de la cromatina son «escritores», «lectores» y «borradores» importantes del código epigenético. Estas proteínas son responsables de la localización, el reconocimiento y la remoción de las marcas moleculares sobre las terminaciones de las histonas que desencadenan cambios funcionales y estructurales en la cromatina. Es el caso de las desacetilasas de histonas (HDAC), enzimas que remueven grupos acetilo de las «colas» de las histonas, señalizando la formación de heterocromatina. La anterior es una actividad necesaria en los procesos de diferenciación celular de los eucariotas, y se conoce que la patogénesis fúngica en las plantas requiere de adaptaciones diversas para ocasionar enfermedad. Macrophomina phaseolina (Tassi) Goid. es un ascomiceto fitopatógeno, necrótrofo e inespecífico, causante de la pudrición carbonosa. Este es un hongo frecuente y altamente destructivo en cultivos como fríjol común (Phaseolus vulgaris L.), particularmente bajo estrés hídrico y térmico. En este trabajo evaluamos los efectos del inhibidor de HDAC clásicas tricostatina A (TSA) sobre el crecimiento in vitro y la virulencia de M. phaseolina. El TSA redujo el crecimiento de M. phaseolina en medio sólido y el tamaño de los microesclerocios (p<0,05), lo que afectó la morfología colonial. En invernadero, el tratamiento con TSA disminuyó (p<0,05) la gravedad de la infección en la variedad de frijol BAT 477. La expresión de los genes de patogenicidad LIPK, MAC1 y PMK1 durante la interacción del hongo con la planta reveló una desregulación importante. Estos resultados proporcionan evidencia adicional del papel que cumplen las HDAC en la regulación de procesos biológicos fundamentales de M. phaseolina.
In eukaryotic organisms, epigenetic mechanisms such as DNA methylation and histone modifications control gene expression and cell specialization. The post-translational modification of histones is a dynamic biochemical process occurring in histone tails by the action of chromatin remodeling enzymes. A variety of reversible covalent post-translational modifications have been reported to be involved in the activation or repression of gene transcription as part of the language that has been called the epigenetic code. One of these is histone acetylation, which is the transfer of an acetyl group from acetyl-coenzyme A to the ɛ-amino group of lysine residues through the action of histone acetyltransferases (HATs). Histone acetylation relaxes chromatin and allows transcription; however, this epigenetic mark can be removed by histone deacetylases (HDACs), favoring gene silencing and the formation of heterochromatin1,2.
HDACs belong to an ancient superfamily of proteins distributed among animals, plants, fungi, eubacteria and archaebacteria. Currently, HDACs are classified into two families: classical HDACs and sirtuins. Classical HDACs are proteins that require Zn2+ for deacetylase activity and, unlike sirtuins, are independent of NAD+. Classical HDACs are divided into classes I, II and IV3. Members of HATs and HDACs have been identified in Saccharomyces cerevisiae and other fungi. In these models, they have proven to be critical for transcriptional regulation processes4,5. In recent years, many HDAC inhibitors have been discovered and developed as a potential therapeutic strategy for the treatment of some human diseases. Nevertheless, these inhibitors can also be a useful tool for studying the biological effects of chromatin modifications in eukaryotes. Trichostatin A (TSA) is a hydroxamate produced by Streptomyces hygroscopicus that acts as a noncompetitive inhibitor of classical HDACs by mimicking lysine and chelating the zinc atom required for the deacetylation reaction6. TSA has been useful for studying histone acetylation/deacetylation in different models, including fungi7–9.
Many plant pathogenic fungi adopt different cellular forms at different stages of their life cycle, and these changes allow them to survive and cause disease. The morphogenetic, physiological, and biochemical modifications underlying these changes constitute cell differentiation. Macrophomina phaseolina (Tassi.) Goid. is a highly aggressive soil- and seed-borne fungus that infects more than 500 plant species, including economically important food crops such as sorghum (Sorghum bicolor), soybean (Glycine max), sesame (Sesamum indicum), corn (Zea mays), oats (Avena sativa) and common bean (Phaseolus vulgaris), among others. M. phaseolina is a cosmopolitan anamorphic ascomycete of the Botryosphaeriaceae family known to be a vascular pathogen and the etiologic agent of charcoal root disease. M. phaseolina causes high pre- and postemergence plant mortality, particularly under drought stress and high temperature conditions10,11. This phytopathogen forms distinct gray to black colonies in culture media, and its hyphae are septate and pigmented and produce microsclerotia, conidia and pycnidia. In soils, microsclerotia germinate and infect the seeds and roots of host plants, invading and destroying tissues and even causing host death. The main pathogenicity mechanisms of the fungus are cell wall degrading enzymes (CWDE) and toxin production12,13. Although the M. phaseolina genome has already been sequenced14, this fungus has not yet been well characterized at the molecular level.
Previously, five classical HDACs were identified in the M. phaseolina genome: MpHOS2, MpRPD3a, MpRPD3b, MpHDA1 and MpHOS3. Furthermore, it was demonstrated that the HDAC inhibitors valproic acid and sodium butyrate affect the growth, morphology, and virulence of this fungus15. In this work, we incorporated TSA into the set of inhibitors previously tested, and additionally, we evaluated its effect on the expression of some genes belonging to signal transduction pathways involved in the pathogenicity of other fungi.
Materials and methodsCulture conditionsThe fungus was primarily cultured in minimal medium (MM) (Holliday 1974): glucose (10g/l), KNO3 (3g/l), salt solution (62.5ml/l) and agar–agar 2%. The salt solution: KH2PO4 (16g/l), Na2SO4 (4g/l), KCl (8g/l), MgSO4 (2g/l), CaCl2 (1g/l), H3BO3 (0.06g/l), MnCl2·4H2O (0.14g/l), ZnCl2 (0.4g/l), NaMoO4·H2O (0.04g/l), FeCl3·6H2O (0.1g/l) and CuSO4·5H2O (0.4g/l)16. The alternative carbon sources tested were sucrose, fructose, arabinose, raffinose, cellulose, xylose, and pectin17. Plates were incubated at 30°C. Additionally, M. phaseolina was also cultured in agar V8:V8 juice (200ml/l), CaCO3 (2g/l) and agar–agar 1.5%18 at 30°C and 37°C (V8-30 and V8-37). For inhibitory conditions, the culture media were prepared with TSA (1μg/ml) (Sigma©, T8552-5MG).
Preparation of fungal inoculumThe inoculum of M. phaseolina was prepared from 5-day-old cultures at 30°C, obtained by a central stab on plates with fungal mycelia. The culture surface was shaved with a sterile razor, and the pieces of the colony were macerated in a mortar with 0.85% saline solution (SS). The suspension was washed with 0.85% SS twice by centrifugation (3000×g, 30min), and finally, the pellet was resuspended in 15ml of 0.85% SS. For quantification, the mean number of microsclerotia counted in three independent drops of 20μl of inoculum by microscopy was calculated. Standard plate counts in solid MM were also performed using serial dilutions. For inhibitory conditions, TSA (1μg/ml) was added to the final volume. This concentration was selected from previous reports on other fungal models9,19.
Growth kinetics of M. phaseolina in the presence of epigenetic inhibitorsThe growth of M. phaseolina strain HMP05 isolated from P. vulgaris L. (common beans) from Cotaxtla, Veracruz (Mexico) (Laboratorio de Biotecnología Vegetal, CBG, IPN) was evaluated by plate growth kinetics in solid MM, solid MM with dimethyl sulfoxide (MM+DMSO), solid MM with 1μg/ml TSA (MM+TSA) (10 replicates) and solid MM supplemented with several carbon sources, with and without TSA added (5 replicates each)9. A volume of 20μl of fungal inoculum (1.7×103CFU/ml) was pipetted onto the center of the agar surface, and the plates were incubated at 30°C. Growth was registered as the mean of the diameter of colonies measured in two opposite directions every 12h for five days using a transparent ruler. The growth rate was calculated during the time of greatest growth (exponential phase)20.
Microscopic measurements of microsclerotia diameterDisks of 8mm diameter were taken from the border of the plates used for growth kinetics. Each disk was placed on a microscope slide, the agar was removed with a blade, each colony was cut into small pieces, the fragments were covered with lactophenol blue, and a coverslip was placed for microscopic observation (OM, 40×). Microsclerotia diameters were calculated as the mean of two perpendicular measurements for each structure (Olympus BX41TF, Infinity 1, Olympus U-TV 0.35 XC-2, Tokyo, Japan; and Leica DM750, Leica ICC50HD, Heerbrugg, Switzerland).
Virulence assaysVirulence assays were carried out under greenhouse conditions using the strain M. phaseolina HMP05 and common bean cv. Pinto UI-114 and BAT 477 (Pinto UI-114 was classified as susceptible and BAT 477 as resistant to M. phaseolina21). Fungal inoculum obtained from solid MM, solid MM+DMSO and solid MM+TSA (1.0 E×103CFU/ml) were used for the infection of seeds of both common bean cultivars. Inoculum pellets from MM+DMSO and MM+TSA were resuspended in 0.85% SS with DMSO or TSA (1μg/ml), respectively. For no inoculum controls, water with DMSO and water with inhibitors added were used. The wells of the seed trays were filled with a sterile substrate for plant growth (Premier Sphagnum Peat Moss, Sphaigne©). In each well, a central orifice of approximately 5cm was created to place previously cleaned bean seeds (20% NaClO3 for 2min, two washes with deionized water for 1min in constant agitation). Seeds were inoculated with 1ml of fungal suspension or control water according to the treatments mentioned above and covered with peat moss. The treatments and the locations of the seeds were assigned randomly. The wells were watered every day between day 2 and day 15, when the seeds or plants were collected. The degree of effect was evaluated according to the 1–9 scale, where 1=no detectable symptoms (healthy plant) and 9=death of the plant (more than 75% of plant tissues damaged). Scores between 2 and 8 represent an increase in the severity of symptoms22. All statistical analyses were performed with IBM SPSS Statistics 23 (IBM, USA).
In vitro establishment of P. vulgaris seedlings for interaction assaysCommon bean seeds from cv. BAT 477 were cleaned with soap and water under constant agitation for 5min, and all soap was then completely removed. In a laminar flow cabinet, the seeds were placed in 70% ethanol for 1min and washed with sterile distilled water. The seeds were placed in a solution of NaClO (0.5%, v/v) for 10min and washed again with sterile distilled water. Finally, a solution of citric acid (150mg/l) and ascorbic acid (100mg/l) was used to cover the seeds for 5min to avoid their oxidation. The seeds were germinated in polypropylene flasks in Murashige and Skoog agar23 at 25°C with a photoperiod of 16h light/8h darkness and 2000lux of intensity until the plants reached the V3 state and developed abundant roots.
Interaction assays between M. phaseolina and P. vulgaris L.Interaction assays were performed with M. phaseolina strain HMP05 and P. vulgaris L. of BAT 477. M. phaseolina cultures were prepared in solid MM and solid MM+TSA (1μg/ml). Inside a laminar flow cabinet, plants in the V3 state were placed inside Petri dishes on the fungal mycelium, ensuring that the root surface was in direct contact with the mycelium. Each experiment evaluated three interaction times (24, 48, and 72h), with three replications for each point, in the presence or absence of the inhibitor. Controls for fungal growth in MM and MM+TSA (1μg/ml) without any contact with plants were also observed at 0, 24, 48, and 72h. Cultures and interactions were incubated in a bioclimatic camera at 25°C with a photoperiod of 16h light/8h darkness, 2000lux of intensity. During the interactions at 24, 48, and 72h, the roots of the plants were cut with a sterile blade and placed in Falcon tubes with liquid nitrogen. Mycelia from the controls were also collected. All samples were kept at −80°C until RNA extraction.
RNA extraction from fungal mycelia and interaction assaysRNA extraction was performed following the TRI REAGENT® method (Molecular Research Center, Inc.). Preserved samples were macerated in liquid nitrogen, producing a fine white powder that was used for RNA extraction with TRIzol® according to the manufacturer's instructions. RNA was cleaned with DNase I (Invitrogen®). RNA quality and concentration were verified by electrophoresis and spectrophotometry (NanoDrop 2000 Spectrophotometer, Thermo Fisher Scientific©, USA).
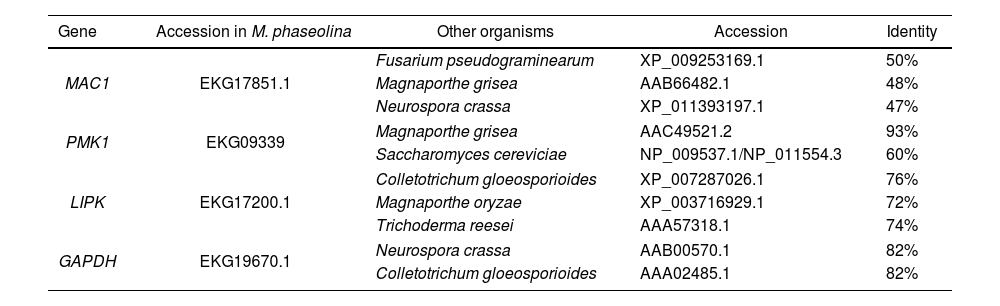
Design of specific primers for RNA amplification from the MAC1, PMK1 and LIPK1 genes in expression assaysLIPK1 (lipid-induced protein kinase), MAC1 (adenylate cyclase) and PMK1 (pathogenicity MAP kinase 1) expression was measured in M. phaseolina during the interaction assays. These genes participate in the signal transduction pathways PKC, cAMP-PKA and MAPKs, respectively, and mutations of these genes cause loss of virulence. The genes were identified in the fungus from protein sequences reported in other fungi using the BLAST program from NCBI. The constitutive gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was selected as a control for the expression assays and identified in the same way (Table 1). Primers designed for the amplification of fragments of less than 500bp were located flanking an intron in each of the genes. MAC1-F, 5′-GAGAGGAGTTCAATGGTGTCG-3′; MAC1-R, 5′-CCCTTTGGCTGATCTTATTGA-3′; PMK1-F, 5′-GCCGATCCCATTCCCGAAG-3′; PMK1-R, 5′-CGCATAATCTCCTGGTAGATG-3′; LIPK-F, 5′-CCCGCTGGCGAACACCTT-3′; LIPK-R, 5′-GCATCGAACCGGCAACTGAG-3′; GAPDH-F, 5′-GAGCACGGCGACGTTGA-3′; GAPDH-R, 5′-AAGGACCTCAATGTCAGACTTG-3′. Conditions for amplification by PCR with these primers were optimized using DNA of M. phaseolina HMP05 extracted by the method proposed by Raeder and Broda24. The same conditions were also applied for the DNA of cv. Pinto UI114 and BAT 477 that were extracted using a Wizard® Genomic DNA Purification kit (Promega) to verify the specificity of the primers.
Identificacion of homologous for MAC1, PMK1, LIPK and GAPDH in M. phaseolina.
| Gene | Accession in M. phaseolina | Other organisms | Accession | Identity |
|---|---|---|---|---|
| MAC1 | EKG17851.1 | Fusarium pseudograminearum | XP_009253169.1 | 50% |
| Magnaporthe grisea | AAB66482.1 | 48% | ||
| Neurospora crassa | XP_011393197.1 | 47% | ||
| PMK1 | EKG09339 | Magnaporthe grisea | AAC49521.2 | 93% |
| Saccharomyces cereviciae | NP_009537.1/NP_011554.3 | 60% | ||
| LIPK | EKG17200.1 | Colletotrichum gloeosporioides | XP_007287026.1 | 76% |
| Magnaporthe oryzae | XP_003716929.1 | 72% | ||
| Trichoderma reesei | AAA57318.1 | 74% | ||
| GAPDH | EKG19670.1 | Neurospora crassa | AAB00570.1 | 82% |
| Colletotrichum gloeosporioides | AAA02485.1 | 82% | ||
RT-PCR assays were performed using a QIAGEN OneStep RT-PCR kit according to the manufacturer's instructions. A control without RNA was included for each treatment. The intensity of the PCR products was measured using Quantity One™ 4.6.7 (Bio-Rad, Gel Doc™ XR System, CA, USA).
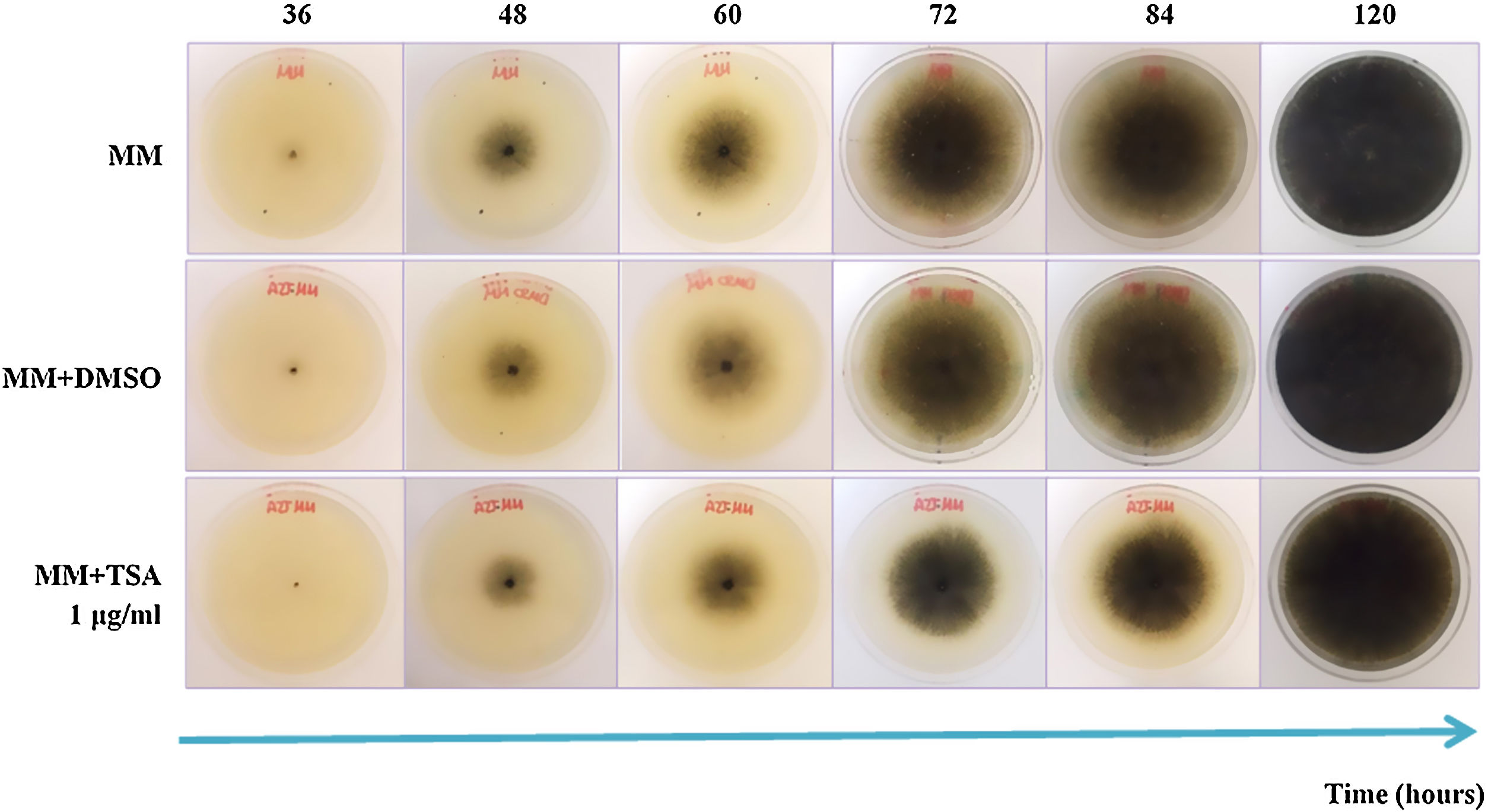
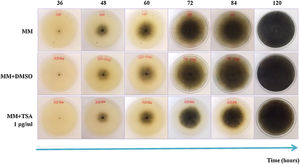
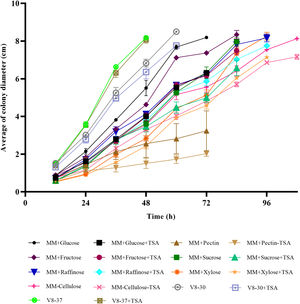
ResultsEffect of epigenetic inhibitors on in vitro growth and synthesis of reproductive structuresIn MM+TSA, there was delayed fungal growth, and after five days of incubation, the mycelium neither reached the border of the Petri dish nor showed a fully mature appearance. At 72h of incubation, the reduction in the colony diameter in MM+TSA with respect to MM was 13.1% (Fig. 1). At 24h, the diameter of the colonies in MM+TSA was significantly lower than those in MM and MM+DMSO (p=0.000). A Tukey's test confirmed the formation of the two subgroups, MM+TSA (p=1.000) and MM and MM+DMSO (p=0.83).
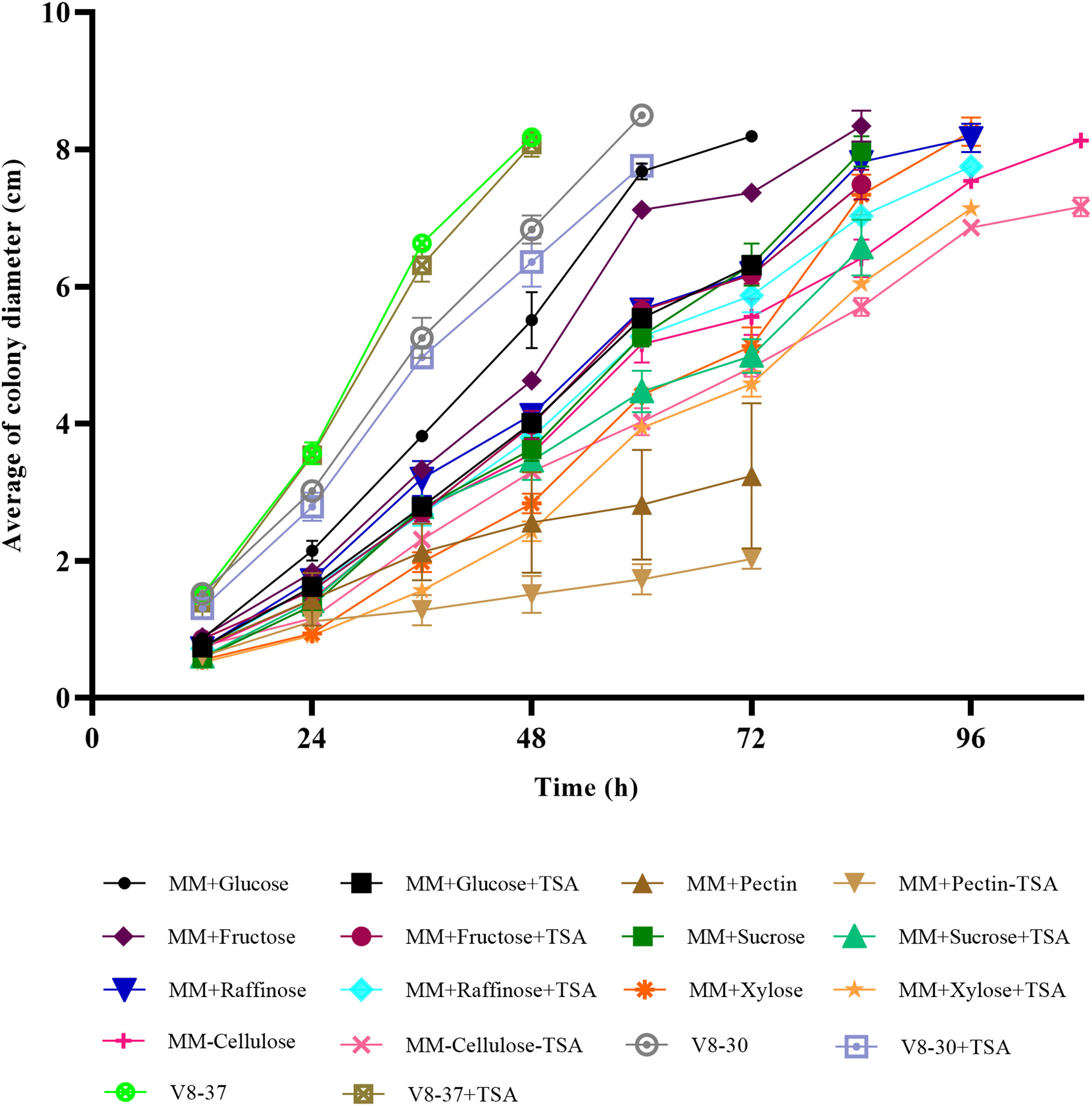
In the medium containing pectin, fructose and cellulose, there were significant differences in colony diameter at 24h of incubation (p=0.000, p=0.006 and p=0.004, respectively); in arabinose, raffinose and xylose at 36h (p=0.038, p=0.005, and p=0.000, respectively); and in V8-30 from 48h (p=0.036) and sucrose from 60h (p=0.001) (Figs. 2a and b). There were no differences in M. phaseolina growth in V8-37 with and without inhibitor. The fungus showed faster growth at 37°C. Except for sucrose, the growth rate during the exponential phase was lower in the treatments with TSA; however, the differences were only significant for the comparisons between MM+glucose and MM+glucose+TSA, MM+fructose and MM+fructose+TSA, and MM+cellulose and MM+cellulose+TSA. In some cases, the start of the exponential phase was delayed (Fig. 2c).
Growth of M. phaseolina on different carbon sources in the presence and absence of TSA (1μg/ml) (error bars: 95% CI). A reduction of growth caused by TSA action was observed in treatments with fructose, raffinose, cellulose, arabinose, pectin, sucrose, xylose and V8-30. (a) Simple carbon sources. (b) Complex carbon sources and V8 medium. (c) Growth rate at exponential phase for each treatment (MM+Glucose, 48–60h; MM+Glucose+TSA, 48–60h; MM+Sucrose, 72–84h; MM+Sucrose+TSA, 72–84h; MM+Fructose, 48–60h; MM+Fructose+TSA, 48–60h; MM+Raffinose, 72–84h; MM+Raffinose+TSA, 48–60h; MM+Arabinose, 72–84h; MM+Arabinose+TSA, 84–96h; MM+Cellulose, 48–60h; MM+Cellulose+TSA, 84–96h; MM+Pectin, 12–24h; MM+Pectin+TSA, 12–24h; MM+Xylose, 48–60h, MM+Xylose+TSA, 48–60h; V8-30, 24–36h; V8-30+TSA, 24–36h; V8-37, 24–36h; V8-37+TSA, 24–36h).
The size of microsclerotia in MM+TSA (x¯=92.10±16.91 μm) was smaller than that in MM (x¯=101.64±19.38 μm) and MM+DMSO (x¯=104.96±18.79 μm) (p<0.001). Tukey's test showed the formation of two subgroups: MM+TSA (p=1.000) and MM and MM+DMSO (p=0.160) (Fig. S1). The diameter of the microsclerotia was also affected in carbon sources such as arabinose (p=0.009), raffinose (p=0.001), cellulose (p=0.016), xylose (p=0.001), sucrose (p=0.001), V8-37°C (p=0.022), and V8-30°C (p=0.001), where TSA induced a reduction in microsclerotia size (Figs. 1 and 2).
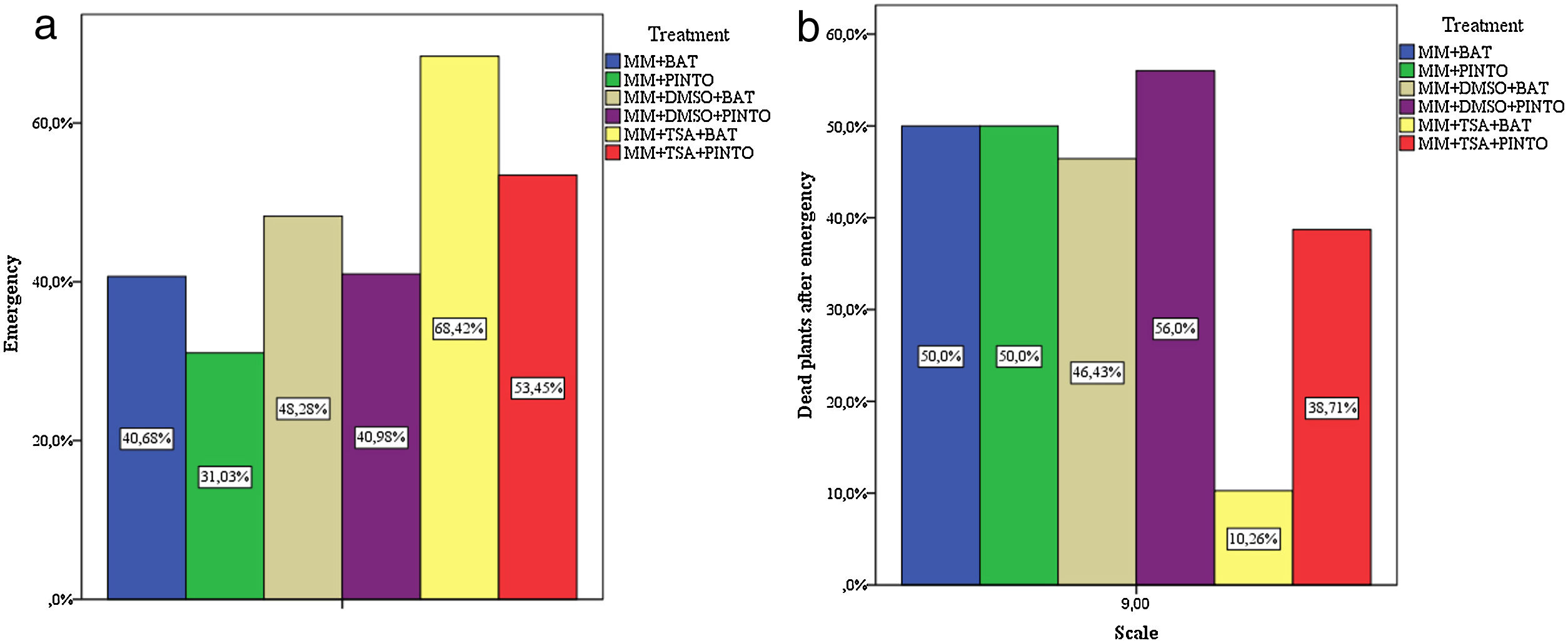
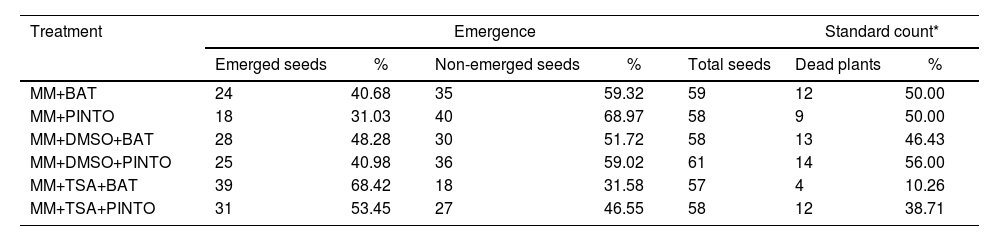
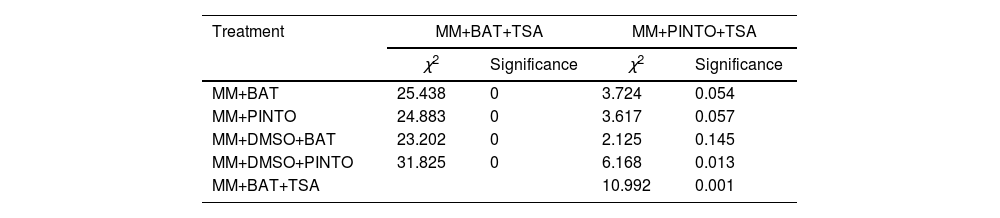
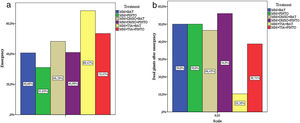
Effect of the inhibitors on the virulence of M. phaseolinaThe percentages of plant emergence and the standard count (which includes only the number of dead plants after emergence) showed that, under these parameters, the less severe treatments were MM+TSA+BAT and MM+TSA+PINTO, and the most severe ones were MM+PINTO and MM+DMSO+PINTO (Table 2). For MM+TSA+BAT and MM+TSA+PINTO, a lower occurrence of dead plants was evident as well as an increase in the number of plants with mild or no symptoms (Fig. 3 and Table S1); disease severity was different among treatments (χ2=52.639, p=0.000). MM+TSA+BAT was less severe than MM+BAT; however, the differences between MM+TSA+PINTO and MM+TSA were not significant (Table 3).
Results from the emergence and standard count performed during virulence assays.
| Treatment | Emergence | Standard count* | |||||
|---|---|---|---|---|---|---|---|
| Emerged seeds | % | Non-emerged seeds | % | Total seeds | Dead plants | % | |
| MM+BAT | 24 | 40.68 | 35 | 59.32 | 59 | 12 | 50.00 |
| MM+PINTO | 18 | 31.03 | 40 | 68.97 | 58 | 9 | 50.00 |
| MM+DMSO+BAT | 28 | 48.28 | 30 | 51.72 | 58 | 13 | 46.43 |
| MM+DMSO+PINTO | 25 | 40.98 | 36 | 59.02 | 61 | 14 | 56.00 |
| MM+TSA+BAT | 39 | 68.42 | 18 | 31.58 | 57 | 4 | 10.26 |
| MM+TSA+PINTO | 31 | 53.45 | 27 | 46.55 | 58 | 12 | 38.71 |
Results of the Kruskal–Wallis test in the comparisons of treatments for disease severity score.
| Treatment | MM+BAT+TSA | MM+PINTO+TSA | ||
|---|---|---|---|---|
| χ2 | Significance | χ2 | Significance | |
| MM+BAT | 25.438 | 0 | 3.724 | 0.054 |
| MM+PINTO | 24.883 | 0 | 3.617 | 0.057 |
| MM+DMSO+BAT | 23.202 | 0 | 2.125 | 0.145 |
| MM+DMSO+PINTO | 31.825 | 0 | 6.168 | 0.013 |
| MM+BAT+TSA | 10.992 | 0.001 | ||
In the interaction on MM at 48h of incubation, the mycelium covered practically all roots, which showed color changes in red and black tones, indicating necrosis. After 72h of incubation, the necrosis was extensive, and withering and death of the plants occurred. This process was similar in MM+TSA, but with a slight delay in the colonization at 48h of incubation.
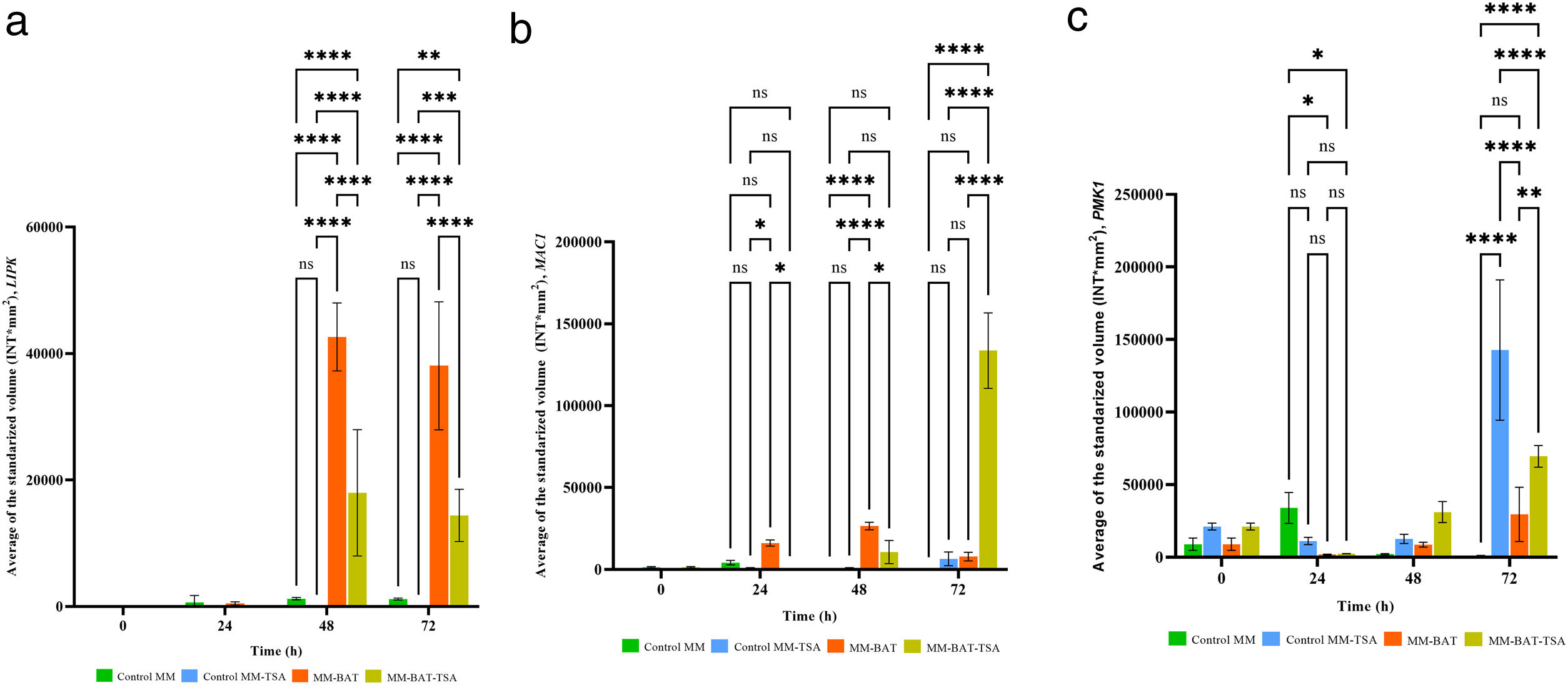
The levels of LIPK1 and MAC1 expression in the Control MM and Control MM+TSA were low, and at some time points, no expression was detected. The expression level of PMK1 in the Control MM was low, but TSA enhanced its expression in the fungus above basal levels even in the absence of the host. The expression levels of LIPK were notoriously higher in the Control MM and MM+BAT when interacting with plants at 48h and 72h (p<0.0001). In MM+TSA+BAT, gene activation was also present but at lower levels than in MM+BAT (p<0.0001); activation was delayed or diminished by TSA action.
The expression of MAC1 in MM+BAT was higher than in both controls, reaching its maximum peak at 48h. The expression in MM+BAT and MM+TSA+BAT was different (p<0.001) at 24 and 48h, being greater in the case of MM+BAT; however, in MM+TSA+BAT, the expression increased dramatically at 72h (p≤0.0001). Although TSA delays the expression of MAC1, this delay is triggered. The expression of PMK1 in MM+BAT and MM+TSA+BAT was increased and reached its maximum level at 72h, and the expression in MM+TSA+BAT was higher than that in MM+BAT (p<0.0001). The expression of PMK1 in MM+TSA reached its highest levels at 72h (p<0.0001), and TSA increased its expression. Our results showed that the genes analyzed are involved in M. phaseolina pathogenicity and that their expression is dysregulated by TSA (Fig. 4).
DiscussionMany plant pathogenic fungi adopt different cellular forms at different stages of their life cycle, and these changes allow them to survive and cause disease. The morphogenetic, physiological, and biochemical modifications underlying these changes constitute cell differentiation. The acetylation/deacetylation of histones is one of the most studied epigenetic modifications in recent decades due to its fundamental role in the regulation of gene expression in eukaryotic organisms. However, little attention has been given to the role of these modifications in plant fungal pathogenesis. Previously, the effect of two HDAC inhibitors, valproic acid and sodium butyrate, was evaluated in the polyphagous fungus M. phaseolina with interesting results15. In the present study, we wanted to continue with the exploration of the relationship between the function of classical HDACs and the development and virulence of the necrotrophic fungus M. phaseolina. We decided to use the classical HDAC inhibitor TSA and evaluate its effect on this fungus. The use of inhibitors in other studies has demonstrated very similar phenotypes to those observed in equivalent mutant organisms7,9,25,26, and they have been widely used in the characterization of the role of histone modifications in eukaryote development and function27,28. We consider that the results presented here provide additional evidence about the role of histone acetylation/deacetylation in the regulation of fundamental elements involved in processes related to vegetative growth, synthesis of reproductive and resistance structures, and the regulation of virulence genes of the fungus.
Previously, the molecular targets for TSA were identified in the M. phaseolina genome15. After exposing the fungus to the inhibitors, it was found that, in general, fungal growth was slower, and the diameter of the colonies was significantly decreased compared to the controls under almost all conditions evaluated. The size of the microsclerotia produced by the fungus was also reduced in several carbon sources. Under inhibitory conditions, the colony morphology was affected, and the mycelium had a less pigmented appearance. The latter fact makes us consider that melanin synthesis could be slightly compromised. Melanin is an important pigment for virulence and the resistance of the fungus to hostile environments29. Similar abnormalities were registered after the inhibition of the fungus with valproic acid and sodium butyrate15. These results confirmed that the TSA concentration used was sufficient to observe a phenotypic effect without an alteration of fungal viability, which was necessary for subsequent evaluations.
Comparable phenotypic alterations have been observed in other fungi with deleted HDACs. This is the case with the HOS2 mutant of Cochliobolus carbonum, in which a reduction in conidial size and septum number was observed, although the germination rate was not altered17. In the HdaA (class II HDAC) mutant of Aspergillus fumigatus, the growth rate was not affected, but the germination rate was decreased; the morphology and pigmentation of colonies were different from those of the wild-type strains30. In Fusarium graminearum, the deletion of hdf1 (hos2 homologous) caused reduced conidiation, fewer pigmented colonies and a slight reduction in vegetative growth31. In S. cerevisiae and S. pombe, Rpd3 is necessary for sporulation32,33. Equal defects have also been found in HAT mutants. In Trichoderma reesei, the gcn5 mutant strain showed a strongly decreased growth rate and misshapen hyphal cells and abolished conidiation34. In Candida albicans, it was demonstrated that Esa1 and Gcn5 are important for filamentous growth35,36. Mutation of gcn5 in A. nidulans affects normal conidiophore development and conidiation37; in Ustilago maydis, it causes a constitutive mycelial phenotype38. In Magnaporthe oryzae, radial growth and asexual reproduction were reduced after the deletion of Rtt10939. The rtt109 knockout strain of A. flavus also displayed defects in growth, sclerotia development, and conidiation40. All of these findings emphasize the major role of histone acetylation/deacetylation processes in the development of phenotypes in fungi.
With regard to the virulence of M. phaseolina, it was significantly reduced in P. vulgaris BAT 477 by the influence of TSA. We also performed virulence assays in P. vulgaris Pinto UI 114 as a host, but the same result was not obtained (a p value slightly above 0.05). Despite this fact, fungal treatment with TSA resulted in an evident decrease in the severity of the disease in 114 Pinto UI plants. This is consistent with previous results for valproic acid and sodium butyrate15. BAT 477 is a drought-tolerant, high nitrogen-fixing advanced line from CIAT41; on the other hand, Pinto UI 114 is a cultivar introduced by the University of Idaho with resistance to the curly top virus and some strains of bean-mosaic virus42. As mentioned above, BAT 477 is considered resistant to charcoal rot disease, while Pinto UI 114 was classified as susceptible. The biological and molecular bases of resistance/susceptibility have not been fully described; however, some authors indicate that the differences are related to the transpiration rate, turgor potentials, stomata resistance and relative water content of the plants, as well as the anatomical or chemical characteristics of the cell walls and the synthesis of antimicrobial compounds in each cultivar21. In the histopathological observations, Mayek-Pérez et al. found that disease development was faster in Pinto UI 114 than BAT 477, both under water stress and irrigated conditions, and the damage caused in Pinto UI 114 extended to a greater amount of plant tissue compared to BAT 47721. Based on this evidence, HDACs are necessary for the complete virulence of the fungus in BAT 477; however, in susceptible genotypes such as Pinto UI 114, the appropriate function of these enzymes could be less critical. In agreement with our results, the loss of genes encoding HDACs or their complexes in plant pathogenic fungal models, such as M. oryzae, C. carbonum, F. graminearum, Fusarium pseudograminearum, Fusarium fujikuroi, U. maydis and Botrytis cinerea, has been associated with decreased virulence9,17,31,43–48.
In recent years, the sequencing of the genome of M. phaseolina made evident that its main mechanism of pathogenicity is its high capacity for the synthesis of enzymes that degrade the cell wall and cuticle of plants, in accordance with its necrotrophic behavior14. In C. carbonum, a necrotrophic fungus that infects maize, the deletion of HOS2 causes a strong reduction in its virulence associated with a decreased efficacy in the penetration of the plant. This mutant strain exhibits diminished growth in complex carbon sources and a reduction in the expression of extracellular depolymerases, which explains its deficiency in the degradation of the host tissues17. Additionally, the phenotype of the C. carbonumHOS2 mutant is highly similar to that of the SNF1 mutant in the same organism. SNF1 is required for the expression of glucose-repressed genes and, therefore, for the expression of CWDEs and the use of alternative carbohydrates as carbon sources49. In Verticillium dahliae, the deletion of SNF1 affected the expression of genes encoding CWDEs, its growth in alternative carbon sources and disease severity in tomato and eggplant50. A similar phenotype was also found in the SNF1 mutant in F. oxysporum51. CWDEs are fundamental for fungal pathogenesis. There is evidence that Snf1 activation can directly affect the functions of HATs (Gcn5) and HDACs or stimulate the synthesis of acetyl-CoA and NAD+, participating in this way in the chromatin remodeling process and genetic regulation52,53. As previously presented, the development of M. phaseolina is affected in media with glucose and alternative carbon sources in the presence of TSA. The above findings suggest that HDACs could be involved in the regulation of the expression of CWDEs or at some step of the Snf1 pathway in the fungus. This is likely one of the reasons for the decreased virulence by TSA observed in the pathogenicity assays. Both its fermentative and oxidative metabolism are affected.
The expression of three pathogenicity genes, LIPK, MAC1, and PMK1, was also tested during interactions with BAT 477. These three genes and possibly also the signaling pathways associated with them were dysregulated in the presence of TSA. This finding can also explain the observed virulence loss. The expression of LIPK in M. phaseolina during the interaction was delayed by the action of TSA. LIPK is a homologous gene to mammalian PKC genes, described in Colletotrichum trifolii as fundamental for appressoria synthesis and complete virulence. LIPK is specifically induced by cutin or long-chain fatty acids54. In S. cerevisiae, Pkc1 has been related to nutrient signaling and energy metabolism through the cell wall integrity (CWI) pathway, in which Pkc1 participates by triggering a MAPK module55,56. The expression of MAC1 during the interaction was potentiated by the action of TSA. MAC1 encodes adenylate cyclase, a protein responsible for the conversion of ATP to cyclic AMP (cAMP). The concentrations of cAMP determine the activation of PKA, which is responsible for the phosphorylation of transcription factors and the induction of the expression of specific genes57. In fungi, the cAMP/PKA transduction pathway controls morphogenesis (filamentous growth, dimorphism), cell differentiation (appressorium formation), sexual development (conjugation, sporulation), monitoring of nutritional status and stress (nutrient starvation) and virulence58. The reduction in virulence associated with alterations in the cAMP/PKA pathway in phytopathogenic fungi has been reported in the literature59–64.
Snf1 and PKA are members of antagonistic signaling pathways that share several downstream molecular targets. PKA is activated in the presence of high concentrations of glucose and Snf1 in the presence of low concentrations of glucose or alternative carbon sources when available. In yeast, crosstalk between the Snf1 and cAMP/PKA pathways occurs at various points, including the phosphorylation of Snf1-activating kinase Sak1, β-subunit Sip1 and transcriptional activator Adr1 by PKA. Additionally, Snf1 phosphorylates adenylate cyclase and controls the cAMP concentration and therefore negatively regulates PKA34,41. The high levels of MAC1 found in the interaction resulting from TSA action could suggest high PKA activity inside the fungal cells and very low Snf1 pathway activity, which is reflected in the diminished ability of M. phaseolina to grow in alternative carbon sources and in its reduced virulence in P. vulgaris BAT 477. Hnisz et al. found in C. albicans that the Set3/Hos2 histone deacetylase complex negatively controls the cAMP/PKA pathway, regulating morphogenesis and virulence26. Something similar might occur in M. phaseolina; HDACs could negatively regulate the activity of MAC1, which is the reason for the overexpression of MAC1 in the inhibitory state.
We also found that PMK1 is overexpressed in the interaction under inhibitory conditions. PMK1 is a homolog of FUS3 and KSS1 from S. cerevisiae. In M. oryzae and M. grisea, PMK1 is responsible for appressorium development and mycelial invasive growth after penetration65,66. Homologs of PMK1 are also fundamental for pathogenesis in other fungal models67,68. In M. phaseolina, appressorium formation is presumed to be necessary for penetration of the host, but experimental evidence has not been reported32,39. We found that the expression of PMK1 in vegetative mycelia of the fungus was low. However, TSA activates its expression in both vegetative and infective hyphae. This observation indicates that HDACs negatively control PMK1 expression or the expression of elements upstream of Pmk1 in mycelium. The Pmk1 pathway also crosstalks with the CWI and cAMP/PKA pathways. Pmk1 is activated in the presence of stress stimuli such as glucose depletion, but this activation requires an operational cAMP/PKA pathway69–72.
Our results indicate that histone acetylation/deacetylation processes, as epigenetic mechanisms of gene expression regulation, are involved in the control of vegetative development and M. phaseolina virulence. We expect that the reliable implementation of a genetic transformation system should allow more detailed studies about the biology and pathogenicity of this interesting and aggressive fungus.
Conflict of interestThe authors declare no competing interests.
The authors thank Á. Salazar-Bravo, J.C. Flores-Hernández, and K.M. Vargas-Guzmán for their important support in the laboratory. NAVS and VHRG thank CONACYT (395818, 435333), PIFI-BEIFI-IPN and COFAA-IPN for scholarships provided.