We describe here the first case of feline sporotrichosis caused by Sporothrix globosa, occurring outside the epizootic area of Buenos Aires province, Argentina. Unlike cases reported with Sporothrix brasiliensis, on this occasion there was no clinical or serological evidence of zoonotic transmission through scratches or bites from the sick cat to the attending veterinarian or the person responsible for its care. This report aimed to improve the knowledge about the pathogenic profile of S. globosa.
En este trabajo se describe el primer caso de esporotricosis felina causada por Sporothrix globosa fuera del área epizoótica de Buenos Aires. A diferencia de los casos documentados de Sporothrix brasiliensis, en este no hubo evidencia clínica ni serológica de transmisión zoonótica por arañazos o mordeduras del gato enfermo a su dueño ni al veterinario que lo atendió. Con este reporte se espera contribuir a un mejor conocimiento sobre el perfil patogénico de S. globosa.
Sporotrichosis is the most frequent implantation mycoses in Central and South America. It is caused by a thermo dimorphic fungus that belongs to the genus Sporothrix, which includes Sporothrix schenckii sensu stricto, Sporothrix globosa and Sporothrix brasiliensis, all of which affect humans and animals13. Historically sapronotic transmission (from an environmental source, such as traumatic inoculation of plant material into the cutaneous and subcutaneous tissues of individuals) was the most common source of human sporotrichosis; however, zoonotic infections have become increasingly common with the emergence of S. brasiliensis7.
In this regard, sporotrichosis due to S. brasiliensis has emerged in Brazil as a zoonotic disease transmitted from infected cats to humans through bites, scratches or contact with exudates from the cutaneous lesions of sick cats7. The sporotrichosis epidemic is still active and a cause of concern in the Southeastern states of Brazil, where a progressive increase in the incidence and prevalence of this infectious disease was observed with over 3510 human cases reported from January 2015 to May 2018 (68.82 cases per month)7. Noteworthy, this zoonotic outbreak is expanding in Brazil and other neighboring countries, such as Argentina, where the frequency of reported cases is on the rise, particularly in the northern area of Buenos Aires province, where a four-fold increase in S. brasiliensis infections was diagnosed in humans and cats from 2011 (0.16 new cases per month) to 2019 (0.75 cases per month)4. Most of these cases occurred in different urban localities bordering the Cuenca del Rio Reconquista (i.e., Paso del Rey, San Miguel, Moreno, Malvinas Argentinas, Los Polvorines and Tigre) indicating that this region is the hotspot for this emerging fungal disease4.
In this study we describe the first case of feline sporotrichosis caused by S. globosa, occurring outside the epizootic area of Buenos Aires province, Argentina, in which there were no clinical or serological evidence of zoonotic transmission through scratches and bites from the sick cat to the attending veterinarian or the person responsible for its care.
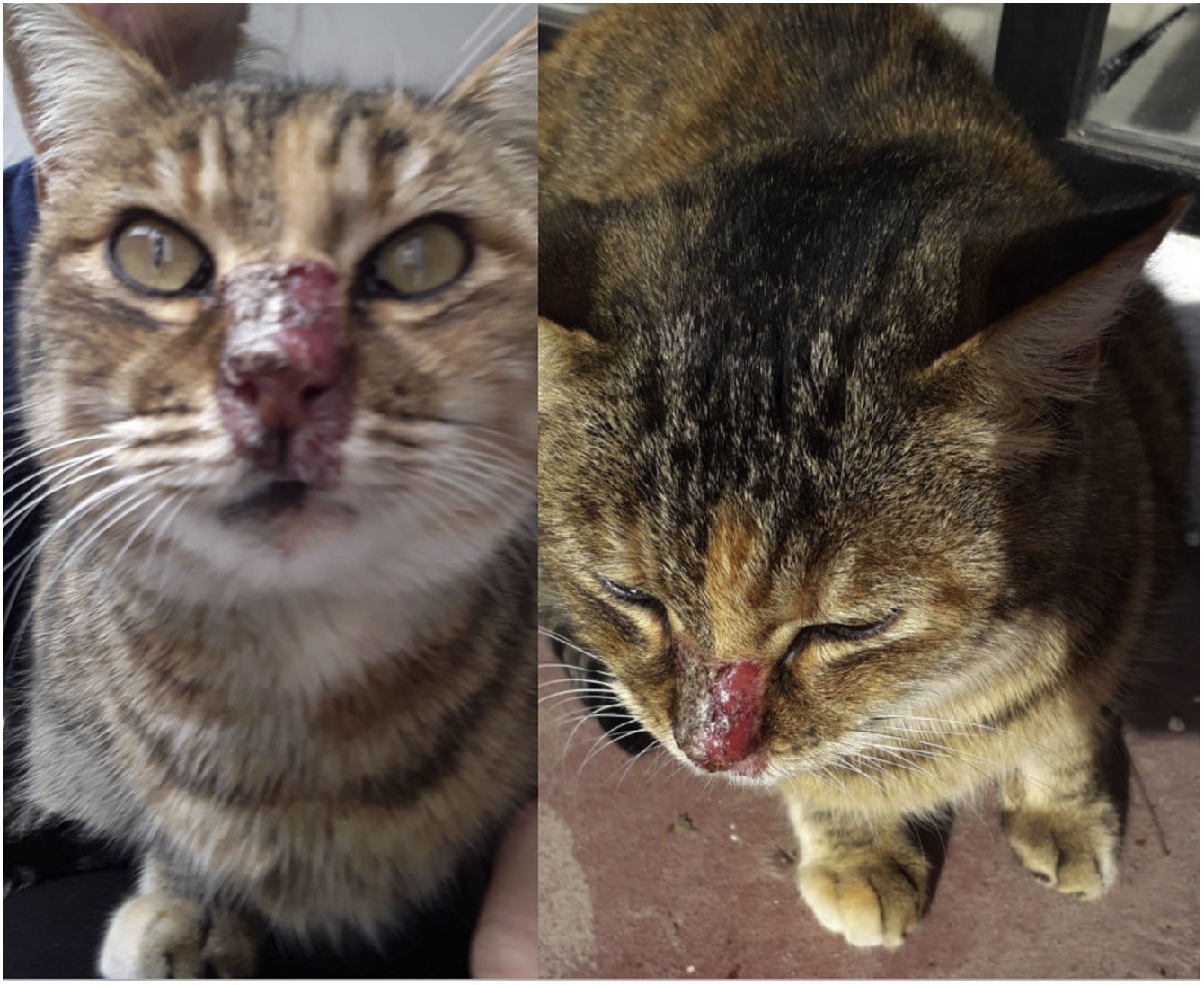
In July 2022, a 1-year-old female unneutered mongrel stray cat was brought to a veterinary consultation due to cutaneous lesions in the rostral part of the nose that had become crusted and ulcerated (Fig. 1). The cat also showed mild signs of upper respiratory infection including sneezing with mucous discharge and rales. The cat lived in an urban area of the Saavedra district in Ciudad Autónoma de Buenos Aires, Argentina, where there are numerous parks with different types of plants and trees.
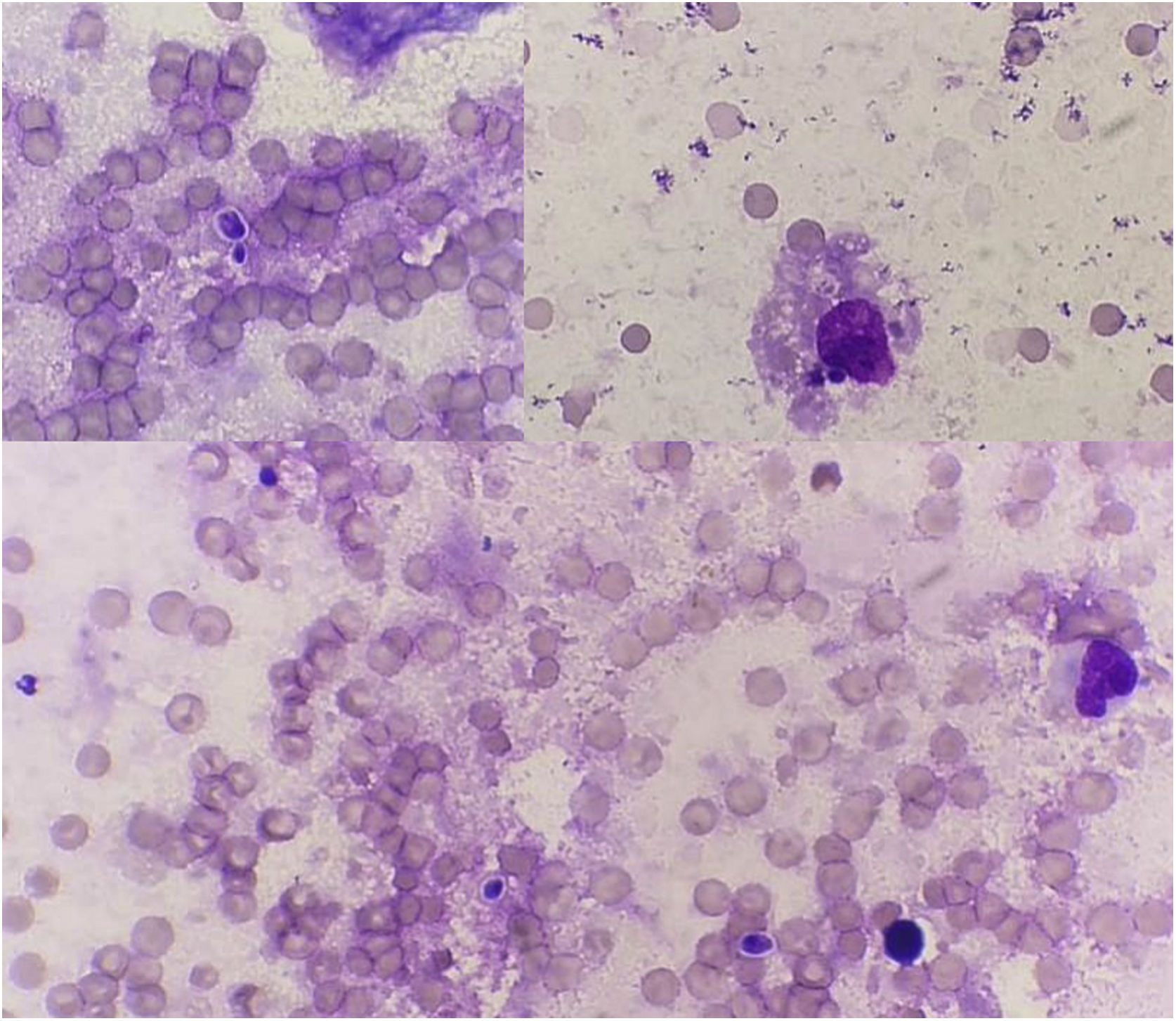
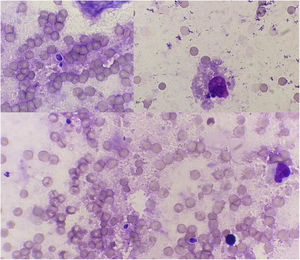
Cytology and histopathology were performed on the ulcerated area, revealing scarce intracellular and extracellular thick-walled, refractile, spherical yeasts measuring 2–3μm, which were periodic acid Schiff (PAS)-positive and exhibited broad-based single budding (Fig. 2). Abundant macrophages and neutrophils were also observed. Moreover, the presence of a suppurative inflammatory lesion forming superficial pustules with extension of the inflammatory process in the dermis with microabscesses was also identified.
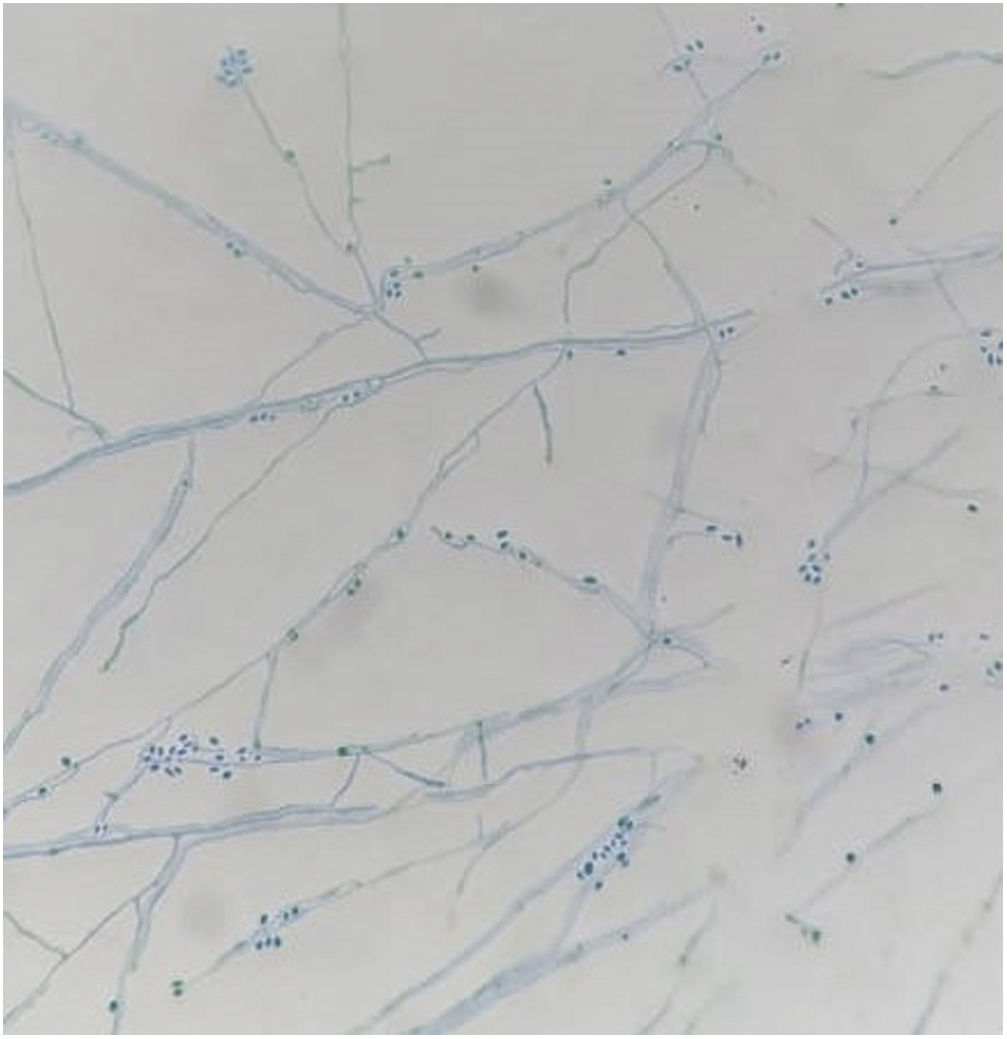
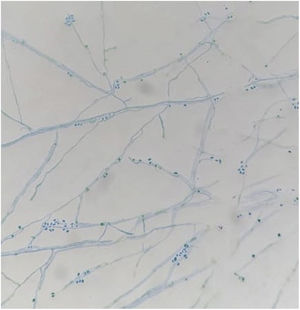
Samples of the cutaneous exudates collected through sterile swabs from the ulcerative lesions of the cat were also obtained for fungal culture and identification. For this purpose, fungal isolation was performed by spreading the specimens on Sabouraud dextrose agar supplemented with chloramphenicol (SDA) at 25°C and on brain heart infusion agar (BHI) at 37°C with daily observation. Microscopic morphology obtained from 25°C cultures showed the presence of septate hyphae and hyaline or slightly pigmented conidia that grouped into rosettes at the tips of the conidiophores. At 37°C, round, oval and fusiform budding cells measuring 1–3μm×2–10μm were observed (Fig. 3).
To identify the fungal isolates at the species level, molecular studies were performed by PCR amplification and sequencing of a fragment of the calmodulin and β-tubulin genes after DNA extraction from the yeast phase11. Phylogenetic analysis using the Neighbor Joining and Maximum likelihood methods were conducted for determining genetic relationships, confirming with high bootstrap support that the fungal isolate was S. globosa.
The minimal inhibitory concentration (MIC) was determined following the microdilution reference method for yeast in accordance with the guidelines proposed by the EUCAST document EDef.7.3.1. Voriconazole, itraconazole, posaconazole and amphotericin B were tested. Candida parapsilosis ATCC 220119 and Candida krusei ATCC 6258 were included in each antifungal susceptibility assay as quality control strains. In all cases, the MIC values were 1μg/ml. The cat was treated with oral itraconazole at a dosage of 10mg/kg/day for three months with favorable evolution.
It is worth mentioning that during the sampling and examination process, the sick cat scratched the index and middle fingers of the left hand of the person responsible for its care and bit the dorsum of the right hand of the attending veterinarian. Due to the zoonotic potential of the disease, samples of the lesions were taken for culture and serum samples were analyzed for anti-S. schenckii-specific IgG antibodies by immunodiffusion. For this latter purpose, to assess seroconversion, paired serum samples obtained after an interval of 21 days were evaluated. In both individuals, SDA and BHI cultures were negative. In addition, they were also seronegative for anti-S. schenckii-specific antibodies.
S. globosa is prevalent in Asia, Europe and North America, particularly in geographic areas with cold climate. However, a recent molecular epidemiological study of human sporotrichosis carried out in Venezuela, revealed a high frequency of S. globosa (30%), being the second species after Sporothrix schenckii sensu stricto in that country, and the highest reported so far in the Americas for this species2. In Argentina, at least three species of the genus Sporotrhrix were described to be circulating: S. schenckii sensu stricto (56.5%), S. brasiliensis (34.7%) and S. globosa (8.7%)3. According to Córdoba et al., the species identified as S. globosa were isolated only in 2004 and 2011 from rural workers residing in the province of Tucuman, in the northern region of Argentina, where the climate is hot and dry. These individuals acquired the infection from the environment, through traumatic injuries caused by splinters and wood stalks3.
Despite the clinical importance and the confirmed circulation of these three pathogenic species in the region, there is lack of available data in Argentina regarding either incidence or prevalence of sporotrichosis. Only few isolated clinical cases have been reported3,4. Nonetheless, in the last few years, in the northern area of Buenos Aires province, cat-transmitted sporotrichosis caused by S. brasiliensis has become a matter of health concern reaching unprecedented levels4.
In veterinary medicine, S. brasiliensis, S. schenckii sensu stricto, S. globosa and Sporothrix pallida have been documented to cause disease in cats, while only S. brasiliensis has been identified as a causative agent of clinical sporotrichosis in dogs8,9,14. However, dog-transmitted sporotrichosis due to S. globosa was described in Colombia, after a dog scratched its owner, although the animal was clinically healthy6.
Cats infected with the S. schenckii complex are known to be an important zoonotic source of human sporotrichosis since the 1980s7. After a cat bite, the S. schenckii complex can be inoculated into the wound, causing ulcerated, verrucous, and nodular skin infections11. Human sporotrichosis due to cat bites/scratches has been reported in several countries (Argentina, Australia, Brazil, Germany, India, Japan, Malaysia, Mexico, Panama, Spain, and the United States of America), but most cases were seen in Brazil, where sporotrichosis was mainly caused by S. brasiliensis13. Outside Brazil, human sporotrichosis due to cat bites is rare and often goes underrecognized and misdiagnosed. However, as in Brazil, human sporotrichosis in Malaysia is transmitted primarily through cat scratches, but with S. schenckii sensu stricto as the prevailing source of feline sporotrichosis in this Asian country9.
Although in Asia S. globosa is the prevalent Sporothrix species (99.3%)12, nearly all cat-associated cases were documented to be caused by S. schenckii in this region9. However, reports from China and Japan reported that S. globosa can also cause cat bite/scratch wound infections to humans10,15. Therefore, S. schenckii isolates recovered from clinical samples may need to be subjected to precise species identification, to recognize the real zoonotic potential of the species belonging to the pathogenic clade of the genus Sporothrix.
In this study we report for the first time in Argentina, a case of feline sporotrichosis due to S. globosa, which occurred outside the epizootic region where feline sporotrichosis due to S. brasiliensis prevails and zoonotic transmission to humans was reported. In the case described herein, the absence of zoonotic transmission of S. globosa through bites and scratches from the sick cat is documented, in contrast to the cases reported by Liu et al.10 and Watanabe et al.15 in China and Japan, respectively. This difference might be linked to the low pathogenicity of this fungal species, compared to the highly virulent species S. brasiliensis, which is associated with high degrees of pathogenicity in both humans and cats1,5.
As zoonotic transmission from cats to humans has been reported in increasing frequency with other Sporothrix species in endemic areas, the understanding of the immunopathogenesis of the different species in cats and its potential transmission to humans, as well as the management of the cat population in an urban environment are essential issues to limit and prevent the further spread of this disease.
Informed consentInformed consent was read and signed by the cat owner and the veterinarian that assisted the sick cat.
Authors’ contributionsAll authors contributed to material preparation, data collection and analysis. The first draft of the manuscript was written by A.N.E. and M.L.C., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingThis work was supported by grants from CONICET (Grant PIP2020). MLC is staff member of CONICET. ANE and KHA thank CONICET for their respective postdoctoral and doctoral scholarships.
Conflicts of interestThe authors declare that they do not anything to disclose regarding funding or conflict of interest with respect to this manuscript.