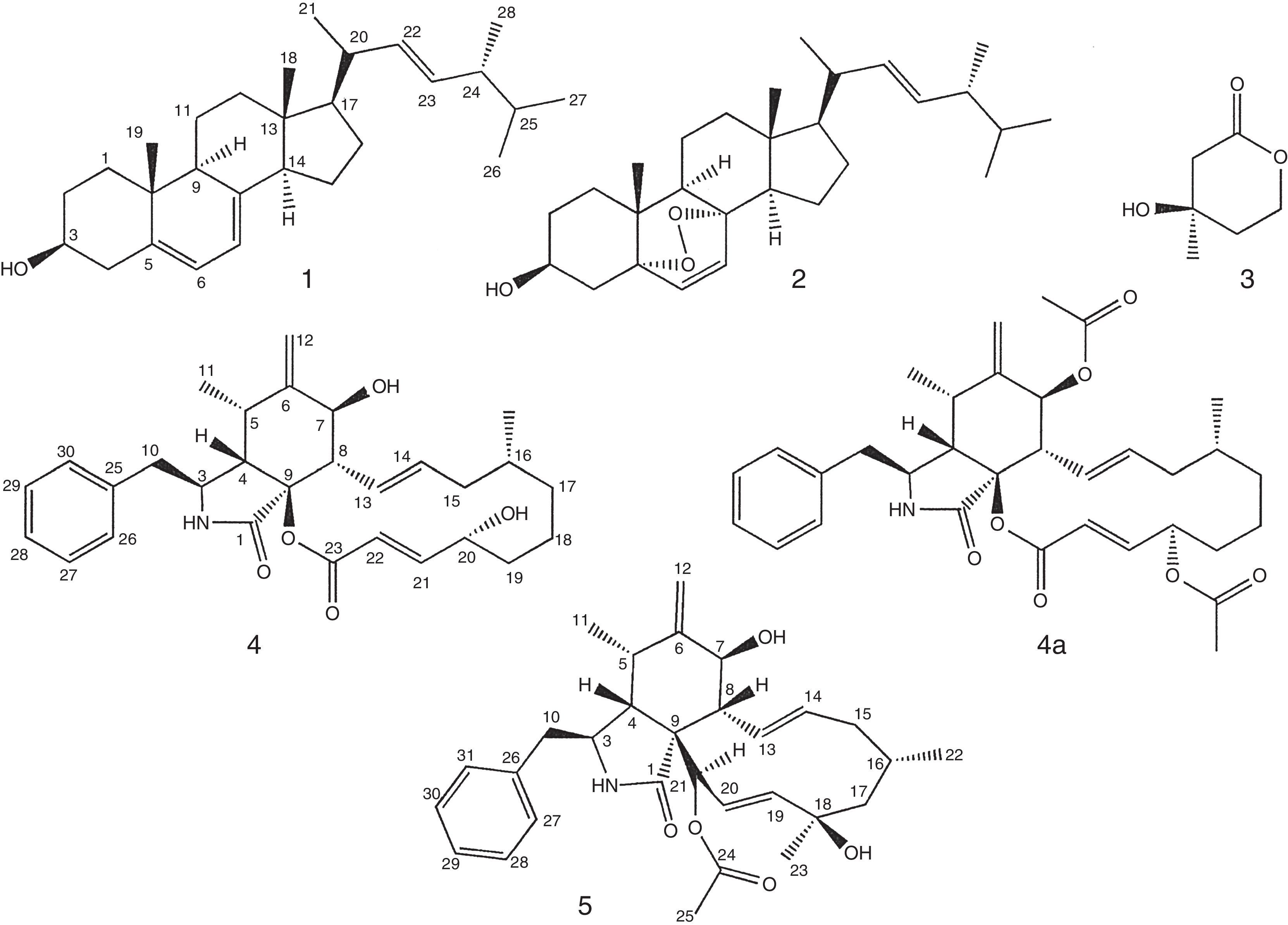
Endophytic fungi are fungi that colonize internal tissues of plants; several biologically active compounds have been isolated from these fungi. There are few studies of compounds isolated from endophytic fungi of Amazon plants. Thus, this study aimed the isolation and structural identification of ergosterol (1), ergosterol peroxide (2), mevalonolactone (3), cytochalasin B (4) and cytochalasin H (5) from Aspergillus sp. EJC 04, an endophytic fungus from Bauhinia guianensis. The cytochalasin B (4) and the diacetate derivative of cytochalasin B (4a) showed high lethality in the brine shrimp assay. This is the first occurrence of cytochalasins in Amazonian endophytic fungi from B. guianensis.
Los hongos endofíticos son hongos que colonizan los tejidos internos de las plantas; varios compuestos biológicamente activos se han aislado a partir de estos hongos. Existen pocos estudios de compuestos aislados de hongos endófitos de plantas amazónicas. Por lo tanto, este estudio tuvo como objetivo el aislamiento y la identificación estructural de ergosterol (1), peróxido de ergosterol (2), mevalonolactona (3), citocalasina B (4) y citocalasina H (5) a partir de Aspergillus spp. EJC 04, un hongo endofítico de Bauhinia guianensis. La citocalasina B (4) y el derivado diacetato de citocalasina B (4a) mostraron una alta letalidad en el ensayo de Artemia salina. Esta es la primera aparición de citocalasinas en hongos endófitos amazónica de B. guianensis.
The Aspergillus genus has more than one hundred species, and belongs to the Ascomycota division, Deuteromycotina subdivision, Hyphomycetes class, Moniliales order, Moniliaceae family. The species are widely found in nature, they can be isolated from plants, soil, air and decaying matter13. Several species have been described as producers of toxic metabolites3. The Aspergillus fungi have been an important source of natural products useful for exploration in medicine, agriculture and industry. Several compounds with cytotoxic activity have been isolated from endophytic fungi1,16,19. Compounds of the xanthones class isolated from Aspergillus sydowii showed immunosuppressive activities17, and the tensyuic A acid isolated from Aspergillus niger showed antimicrobial activity6. There are reports of the isolation of cytochalasin E from an endophytic fungus that showed cytotoxicity18. Petersen et al.12 isolated from the fungus Aspergillus sclerotioniger cytochalasins sclerotionigrin A and B that showed cytotoxic activity in vitro against lymphocytic leukemia cells. The aims of this study were the isolation and structural identification of ergosterol (1), ergosterol peroxide (2), mevalonolactone (3), cytochalasin B (4), cytochalasin H (5), and the derivative 7,20-diacetyl-cytochalasin B (4a) from the endophytic fungus Aspergillus sp. EJC 04 cultures, and testing the lethality of isolated compounds against Artemia salina.
The 1H and 13C NMR experiments were recorded on a NMR spectrometer (Mercury 300, Varian, Oxford, Oxfordshire, UK) with CDCl3 (Cambridge®) as solvent and standard. The MS spectra were carried out in the mass spectrometer using ESI (+) ion mode (Acquity TQD, Waters, Milford, MA, USA). The specific rotation was performed on a specific rotation equipment (Nova Instruments No. 1412, Piracicaba, Brazil).
The fungus Aspergillus sp. was obtained from a collection of “Laboratório de Bioensaios e Química de Micro-organismos – LaBQuiM/UFPA”. This collection contains isolates from Bauhinia guianensis. The fungus was inoculated into a Petri dish containing PDA (Potato Dextrose Agar) culture medium (Himedia®) and incubated at 25°C (BOD, Quimis®) for 8 days to reactivation. One strain is deposited with a code EJC 04. The fungus Aspergillus EJC 04 was identified by observing the morphology and microscopic aspects of the colony in an optical microscope and by DNA sequence through analyses of the ITS5 region.
Six Erlenmeyer flasks (1000ml) containing 200g of rice (Uncle Ben's®) and 75ml of water per flasks were autoclaved for 45min at 121°C (autoclave Prismatec®). Small pieces of PDA containing mycelium of Aspergillus sp. were added to 4 Erlenmeyer flasks under sterile conditions, then, the Erlenmeyer flasks were incubated at 25°C for 23 days, two Erlenmeyer flasks were used as control. Biomass was macerated with ethyl acetate (Tedia®) (3× 500ml). The biomass was separated of the ethyl acetate solution by filtration. Then, the ethyl acetate extracts (10g) were obtained after evaporation of the resulting solution in rotary evaporator at 45°C (Quimis®). Part of the ethyl acetate extract (5.0g) was fractionated on silica gel column using hexane/ethyl acetate (9:1, 4:1, 7:3, 1:1, 3:7), ethyl acetate/methanol (3:7, 1:1) and methanol, resulting in 9 fractions. The hexane/ethyl acetate 9:1 fraction (500mg) was submitted on silica gel column chromatography eluted with hexane/ethyl acetate (9:1, 4:1, 7:3, 1:1, 3:7) and ethyl acetate, resulting in 92 fractions. The fractions were pooled (A1 to A8); the fraction A2 provided a white amorphous solid identified as ergosterol 1 (30mg), and the A4 fraction provided a white amorphous solid identified as ergosterol peroxide 2 (35mg). The hexane/ethyl acetate 8:2 fraction (600mg) was submitted on silica gel column chromatography eluted with hexane/ethyl acetate (9:1, 4:1, 7:3, 1:1, 3:7) and ethyl acetate, resulting in 100 fractions pooled as B1 to B9, the fraction B2 afforded a yellow oil identified as mevalonolactone 3 (6mg). The ethyl acetate fraction (2g) was submitted on silica gel column chromatography, eluted with hexane/ethyl acetate (9:1, 4:1, 7:3, 1:1, 3:7), ethyl acetate, ethyl acetate/methanol (3:7, 1:1) and methanol, resulting in 120 fractions pooled as C1 to C12; fraction C5 afforded a white solid identified as cytochalasin B 4 (100mg) and the fraction C7 gave a white solid identified as cytochalasin H 5 (5mg). All compounds 1–5 were identified by NMR and MS spectrometric data.
The cytochalasin B (4) was acetylated; for this, 10mg of compound 4 were removed and solubilized in 100μl of pyridine (Tedia®), then it was added 250μl of acetic anhydride (Tedia®) and kept at rest for 24h at room temperature. After this period, the material was transferred to a separatory funnel, and it was added 25ml of 5% HCl solution to remove excess pyridine, and extraction was performed with ethyl acetate (3× 15ml). The ethyl acetate phase was further washed with distilled water (3× 25ml) and after separation was added anhydrous sodium sulfate in the ethyl acetate phase, which after filtered was evaporated to obtain the acetylated product 4a.
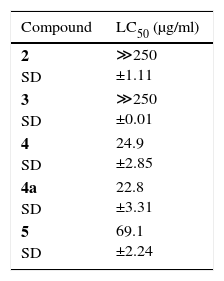
The A. salina lethality test was performed following the Meyer et al.11 method adapted, preparing a solution of sea salt at a concentration of 30g/l. The pH was adjusted between 8.0 and 9.0 using 0.1mol/l NaOH solution. This solution was used for hatching of A. salina and for preparing the other dilutions. The eggs were placed to hatch in saline solution for 48h with constant aeration at 25°C. About ten A. salina larvae were transferred to tubes containing saline solution and samples to be tested, with the following concentrations: 50μg/ml, 25μg/ml, 10μg/ml, 5μg/ml and 1μg/ml, a tube containing only A. salina was used as control. The assay was performed in triplicate, and the counting of the dead and live animals carried out after 24h. This is the first report of isolation of cytochalasins of the endophytic fungi from B. guianensis and of its lethality against A. salina (Table 1, Fig. 1).
The compound 4 was identified through analysis of the spectroscopic data of 1D and 2D NMR and MS-ESI (+), and compared with literature data7. The molecular formula C29H37NO5 was deducted from ESI mass spectrum m/z 480 [M+H]+. The signals observed in the 1H NMR spectrum in 7.17 (m), 7.30 (m) and 7.24 (m) indicate the existence of a monosubstituted aromatic ring in 4, attributed to hydrogens H-27/31, H-28/30 and H-29. The signals at δ 5.94; 5.46; 7.05; and 5.90 were assigned to the hydrogens of the double bonds Δ13,14 and Δ21,22, respectively; the coupling constant (15.5Hz) between H-13/H-14 and H-21/H-22 indicates that both double bonds have the E geometry. Signs at δ 0.89 (d, 6.6) and δ 1.10 (d, 6.6) were assigned for the methyl CH3-11 and CH3-24. The signals of carbinolic hydrogens at δ 3.90 and 4.51 were assigned to H-7 and H-20 hydrogens. In the 13C NMR spectrum were observed 28 signals; 6 in the aromatic region, 6 for olefinic carbon, 7 sp3 CH carbons, 3 carbinolic carbons, 4 CH2 carbons, and 2 carbonyl carbons. In the HSQC spectrum, H-10 is correlated with C-10 (δ 41.7). Following HMBC correlations to H-10, one can find the aromatic ring by correlations with C-25, C-26 and C-30. Yet, HMBC correlations to H-10 and H-4 with the carbonyl carbon C-1 (δ 171.2) allowed proposing a γ-lactam ring. The correlations observed in the COSY spectrum between H-7 and H-8, H-13 with H-8 and H-14, along with the HMBC correlations Me-24 to C-15 (δ 44.2) and C-17 (δ 34.9), and carbinolic hydrogen H-7 with the carbons of the double bond C-21 (δ 152.1) and C-22 (δ 119.3), plus HMBC correlations of H-21 with C-20 (δ 69.0) and carbon carbonyl C-23 (δ 164.5) allowed proposing the macrocyclic ring. Thus, 4 was identified as cytochalasin B and the specific rotation calculated was [α]D: +143 (c 1.0, acetone). The relative configuration to compound 4 was determined by comparison of the chemical shifts values 1H and 13C NMR, coupling constants and comparison with the literature data10.
The molecular formula C30H39NO5 for compound 5 was deduced through ESI mass spectrum m/z 494 [M+H]+. The signal sets shown in 1H and 13C NMR spectra, bi-dimensional NMR data and comparison with the literature data7, led to characterize 5 as cytochalasin H, similar to cytochalasin B (4). The main difference observed in the 1H NMR spectrum of 5 with respect to 4 is the existence of a signal at δ 2.23 (s) assigned to the methyl CH3-25 of the acetate group. The specific rotation calculated to 5 was [α]D: +16 (c 1.0, CH2Cl2). The relative configuration to compound 5 was determined by comparison of the chemical shifts values 1H and 13C NMR, coupling constants and comparison with the literature data10.
Since the compound 4 was obtained in large quantities and showed good lethality with regard to A. saline, it was decided to obtain its acetylated derivative to test the relation structure-activity. The product derivatization of cytochalasin B was identified by 1H and 13C NMR and MS. The 1H NMR spectrum of 7,20-diacetyl-cytochalasin B (4a) showed similarity to 4, where the main differences observed in the 1H NMR spectrum were the presence of two signals singlets at δ 1.96 and δ 2.11 assigned for the methyl of acetate groups CH3-32 and CH3-34. The molecular formula C33H41NO7 was deducted through ESI mass spectrum m/z 564 [M+H]+, in the mass spectra were also observed m/z 504 [M-60] common for the loss of an acetate group and m/z 444 [M-120] compatible with the loss of a second acetate group, which helped to confirm the demethylation in 4a.
The compounds ergosterol (1), ergosterol peroxide (2) and (R)-(−)-mevalonolactone (3) were identified by 1H and 13C NMR, the compounds have been previously isolated from Aspergillus sp. EJC 08, endophyte from B. guianensis, together with the benzophenone monomethylsulochrin. Mevalonolactone and monomethylsulochrin showed good antimicrobial activity14.
The lethal concentration LC50 ranged from 22.8μg/ml to ≪250μg/ml. Considering the Amarante et al.2 criterion, cytochalasins H (5) showed to lethal, while cytochalasin B (4) and 7,20-diacetyl-cytochalasin B (4a) showed high lethality (Table 1). The lethality values were compared with those obtained by Ferraz et al.5 for lapachol (52.5μg/ml), where cytochalasin H (5) showed lethality near to the presented by lapachol, while the compounds cytochalasin B (4) and 7.20-diacetyl-cytochalasin B (4a) were two times more active. However, not was observed significant difference between the activity of cytochalasin B (4) and its derivative 7.20-diacetyl-cytochalasin B (4a) no being possible establish a relation structure-activity to the compounds.
The study of Aspergillus sp. EJC 04 led to the isolation of bioactive compounds, which is in agreement with previous studies with strains of Aspergillus species. For example, from sponge-derived fungus Aspergillus versicolor were isolated xanthones and anthraquinones compounds exhibited significant cytotoxicity against five human solid tumor cell lines and antibacterial activity against several clinically isolated gram-positive strains8. The compounds asperfumoid and asperfumin isolated from Aspergillus fumigatus showed antifungal activity9. From Aspergillus terreus were obtained isocoumarins that exhibited potent antioxidant activity4. The compound benzylazaphilone, aspergilone A, was isolated from the culture broth of a marine-derived fungus Aspergillus sp. exhibited in vitro selective cytotoxicity and showed potent antifouling activity15.
Thus, this work contributed to the chemical study of endophytic fungi from Amazon plants and led to the isolation of substances that present high lethality against A. saline. Furthermore, this is the first report of isolation of the cytochalasins of endophytic fungi from B. guianensis and their lethality against A. saline.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
Authors thank to Fundação Amazônia de Amparo a Estudos e Pesquisa do Pará (FAPESPA), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Vale S/A and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) for financial support.