Due to the interest in the production and trading of yateí (Tetragonisca angustula) honey in the province of Misiones, Argentina, in this work we assessed microbiological and physicochemical parameters in order to contribute to the elaboration of standards for quality control and promote commercialization. Results showed that yateí honey samples had significantly different microbiological and physicochemical characteristics in comparison to established quality standards for Apis mellifera honey. Thus, we observed that values for pH (3.72), glucose (19.01g/100g) and fructose (23.74g/100g) were lower than A. mellifera quality standards, while acidity (79.42meq/kg), moisture (24%), and mould and yeast count (MY) (3.02 log CFU/g) were higher. The acid content was correlated with glucose (R2=0.75) and fructose (R2=0.68) content, and also with mould and yeast counts (R2=0.45) to a lesser extent. The incidence of microorganisms in yateí honey samples reached 42.85% and 39% for Clostridium sulfite-reducers and Bacillus spp., respectively. No C. botulinum or B. cereus cells were detected. Enterococcus spp. and Staphylococcus spp. incidence was similar (ca. 7.14%), whereas Escherichia coli and Salmonella spp. were not detected. We conclude that the microbiological and physicochemical properties of yateí honey are different from those of A. mellifera honey; hence, different quality standards could be implemented to promote its commercialization.
Debido al interés en la producción y comercialización de la miel de yateí (Tetragonisca angustula) en la provincia de Misiones, Argentina, en este trabajo se evaluaron parámetros microbiológicos y fisicoquímicos con el fin de contribuir con la elaboración de normas para el control de calidad y promover su comercialización. Los resultados demostraron que los parámetros analizados en esta miel (n=28) diferían significativamente de los valores aceptables establecidos para la miel de Apis mellífera. En comparación, se observó que los valores de pH (3,72) y de concentración de glucosa (19,01g/100g) y fructosa (23,74g/100g) eran más bajos, mientras que los valores de acidez (79,42meq/kg) y humedad (24 %), al igual que el recuento de hongos y levaduras (HyL) (3,02 log UFC/g), eran más altos. La acidez mostró una correlación inversamente proporcional con el contenido de glucosa (R2=0,75) y fructosa (R2=0,68), y directamente proporcional con el recuento de HyL, aunque en este caso la correlación fue menor (R2=0,45). En lo que respecta a los parámetros microbiológicos, se observó 42,85 % de Clostridium sulfito-reductores y 39 % de Bacillus spp., y no se detectó presencia de C. botulinum ni de B. cereus. Enterococcus spp. y Staphylococcus spp. se encontraron en una proporción similar (ca. 7,14 %), mientras que Escherichia coli y Salmonella spp. no fueron detectados. Concluimos que las propiedades microbiológicas y fisicoquímicas de la miel de yateí difieren de las de la miel de A. mellifera, por lo cual sería oportuno establecer normas de calidad diferentes para facilitar su comercialización.
The most common stingless bees in the Province of Misiones, Argentina, are the yateí bees (Tetragonisca angustula), from the Meliponini subfamily. Their habitat covers the tropical and subtropical regions of the American continent from Argentina up to Panamá and Mexico21. There are two subspecies of T. angustula identified as T. angustula Latreille and T. angustula Fiebrigi having a black and yellow mesepisternum, respectively. The latter is restricted to southern Brazil, Paraguay and Northeast provinces of Argentina including Misiones, Chaco, Formosa and Corrientes8. They are smaller than the Apis mellifera bees, with shorter flights to a wide variety of floral resources; they construct small nests in tree cavities or branches, and also underground27.
On the whole, the physicochemical and organoleptic characteristics of yateí honey depend on the sugar content, maturity (ripe) and presence of active compounds such as aliphatic and aromatic alcohols, aldehydes, acids and their esters. These active compounds give special properties and define the honey flavour4,7.
Although honey have high osmolarity and low water activity and nutrients, it can hold microorganisms present in pollen, dust, air, soil and nectar which are very difficult sources to control. However, microbial contamination can also be originated from food handlers, equipment and cross-contamination which can be easily controlled by standard sanitation and good manufacturing practices during harvest and honey processing24; otherwise, honey would have high counts of vegetative bacteria11. The microorganisms of concern in honey are some fungi and yeast genera, such as Penicillium, Mucor, Saccharomyces, Schizosaccharomyces and Torula19; which are responsible for fermentation of honey with high moisture content (>21%). Spore-forming bacteria such as Bacillus cereus and Clostridium spp. are also regularly found in honey, which under certain conditions could cause illness in humans, specially bacteria such as Clostridium botulinum which are a high risk in children foods29. Coliforms and yeasts are indicative of sanitary or commercial quality concern24. In this sense, we found no reports in the literature about bacterial contamination, such as Escherichia coli, Staphylococcus, Enterococcus and Salmonella in yateí honey.
Meliponi culture is steadily growing with commercial purposes; for this reason it is very important to study the characteristics and quality of local honey34. The aim of this work was to assess the microbiological and physicochemical properties of yateí honey in order to contribute to the elaboration of standards for quality control for this type of honey, which is not ruled by Mercado Común del Sur (MERCOSUR) Legislation and Código Alimentario Argentino (CAA).
Materials and methodsHoney samplesTwenty eight samples of yateí honey were collected from bee hives located within a variety of handmade boxes designed by producers from different regions of the Province of Misiones, Argentina. All samples were aseptically collected from the shaped combs using sterile syringes. Sampling was carried out during the months of November, December, March and April on sunny days at room temperature (25–35°C). Honey samples in syringes were stored at 5°C and processed within 24–48h after harvest time.
Physicochemical analysisThe moisture content in homogenized undiluted honey samples was determined by the Official Method of Analysis3 (AOAC International). The refractive index of transparent and translucent liquid samples was determined using an Abbe refractometer (Lambda Scientific Systems, Inc., USA) at 20°C and the values recorded were converted to percent moisture using the conversion table9 modified by Wedmore35. Values are expressed as moisture percentages (%).
The pH of honey samples was measured using an Orion 230A pH meter (Orion Research, Inc., USA).
Acidity was determined in a solution containing 2g of honey and 15ml of previously heated and cooled water. This solution was titrated with 0.1 N NaOH until the pH reached 8.5. Values are expressed as milliequivalents of total acid per kilogram of honey (meq/kg).
Sugar (glucose, fructose and sucrose) content was measured in a 10μl aliquot by high pressure liquid chromatography (HPLC) using a chromatographer (Waters & Associates, Inc., USA) connected to an amino column, 250×4.6mm, 5μ (Grace, Inc., USA). For the HPLC analysis of sugar content, samples were prepared as follows: 0.25g of honey were diluted in 25ml of the mobile phase (70:30, acetonitrilo:water).The mix was centrifuged for 2000g and filtered with a 0.45μm filter in a Minisart high flow Syringe Filter (Sartorius AG, Germany). Chromatographic separation was performed under isocratic conditions using the above mobile phase with a flow rate of 1.1ml/min. A refractive index detector, Model 410 (Waters & Associates, Inc., USA), was used for data analysis. The concentration of sugar is expressed as grams per 100 grams of honey (g/100g).
Microbiological analysisCounts of microorganisms were carried out by plating appropriate dilutions of the honey samples. For this procedure, a 10g sample was homogenized in 90ml of 0.1% peptone water (Britania, Argentina) in an Erlenmeyer flask. Subsequent decimal dilutions were prepared in sterile peptone water and analyzed by duplicate by plate count or by the most probable number (MPN) method, depending on the kind of microorganism. The following microbial analyses were carried out:
- •
Total aerobic mesophilic bacteria (TAMB):1ml aliquots from each decimal dilution were plated onto count plate agar (PCA; Britania) and incubated at 30°C for 48h16. Microbial counts are expressed as logarithm of colony-forming units per gram of honey (log CFU/g).
- •
Moulds and yeasts (MY): 0.1ml aliquots from each decimal dilution were spread on a mould and yeast medium (MY; Britania) and incubated at 25°C for 5 days16. Microbial counts are expressed as logarithm of colony-forming units per gram of honey (log CFU/g).
- •
Total coliforms: a series of 3 sequential MPN tubes were inoculated from 10−1, 10−2 and 10−3 dilutions. Lauryl sulphate broth (LSB; Britania) was used as enrichment medium and brilliant green bile (2%) broth (Brilla; Britania) for confirmation. In both cases, samples were incubated at 35°C for 48h, positive samples (growth and gas production) in Brilla medium were used to calculate total coliforms6. Results are expressed as log MPN/g.
- •
Clostridium spp.: 5ml aliquots from 10−1 dilution were treated at 80°C for 5 min. Then, 1ml aliquot was added to a tube (RCM; Britania) molten and cooled at 46°C, and the top covered with Vaseline for anaerobic conditions. The tubes were incubated at 45°C for 5 days for growth and gas production. Cell morphology was observed by Gram stain and malaquita green stain using a CX optical microscope (Olympus, Inc., Japan). Further traditional assays including catalase, gelatine, lecithinase, nitrate and lactose assimilation were carried out to discard Clostridium botulinum presence. Results are expressed as presence or absence of Clostridium spp.
- •
Bacillus spp.: 0.1ml aliquots from appropriate dilution were spread on Bacillus cereus selective agar (BCS; Britania) and incubated at 35°C for 24h. Suspected colonies were identified by Gram stain and biochemical reactions: catalase, modified Voges-Proskauer (VP), gelatine and growth in brain-heart infusion broth (BHI; Britania) plus 6.5% NaCl6. Results are expressed as presence or absence of Bacillus spp.
- •
Escherichia coli: positive tubes from Brilla broth were tested by growth in EC broth (Britania) and EMB agar (Britania). Typical colony growth on EMB agar was confirmed by traditional assays6 including indole, methyl red, VP and citrate. Results are expressed as presence or absence of E. coli.
- •
Salmonella spp.: these bacteria were investigated according to a modification of the standard method suggested by the FDA-BAM6. For the pre-enrichment, 1g of honey was added to 9ml of lactose broth (LB, Merck, Germany) and cultures were incubated at 35°C for 24h. The enrichment step was performed onto selenite cystine broth (SCB; Merck) incubated at 35°C for 24h. Isolations were examined onto bismuth sulfite agar (BSA; Merck) and Hektoen enteric agar (HEA; Britania), after incubation at 35°C for 48 and 24h, respectively. Suspected colonies of Salmonella were tested into triple sugar iron (TSI, Britania) and lysine iron (LIA, Britania) agar. Colonies exhibiting typical reactions on TSI and LIA were purified and further characterized by traditional assays: urease, oxidase, phenylalanine descarboxilase, VP, indole, citrate and gelatine. Results are expressed as presence or absence of Salmonella spp.
- •
Enterococcus spp.: a series of 3 sequential MPN tubes with glucose-azide broth (Merck) were inoculated from 10−1, 10−2 and 10−3 dilutions and incubated at 35°C for 48h. The positive tubes were inoculated onto KF agar (Merck) and typical colonies were analyzed by Gram stain, catalase, growth on BHI broth plus 6.5% NaCl, at 45°C for 48h and 10°C for 5 days16. Results are expressed as log MPN/g.
- •
Staphylococcus spp.: 0.1ml aliquots from 10−1 dilution were spread on Baird Parker agar (BPA, Britania) and incubated at 35°C for 48h. The presence of coagulase positive staphylococci (CPS) was determined by picking colonies from BPA and testing them by Gram stain, TSI agar reactions and coagulation of rabbit plasma6 (BD, USA). Results are expressed as presence or absence of Staphylococcus spp. and CPS.
Mean values and significant differences between parameters were evaluated by one way Analysis of Variance and by the Fisher-test in Minitab-15 software (Minitab Inc., USA). Histogram analyses were also performed by Minitab-15, whereas correlation analyses were performed by using Sigma Plot 10 (Systat Software, Inc., USA).
Results and discussionPhysicochemical propertiesThe pH value of honey samples from T. angustula (Yatei) showed a mean of 3.72±0.02. This value is similar to that obtained by Anacleto et al.2 and Vit et al.33 for yateí honey, but lower than that obtained by Iurlina and Fritz17 for A. mellífera honey (pH means value 4.6).
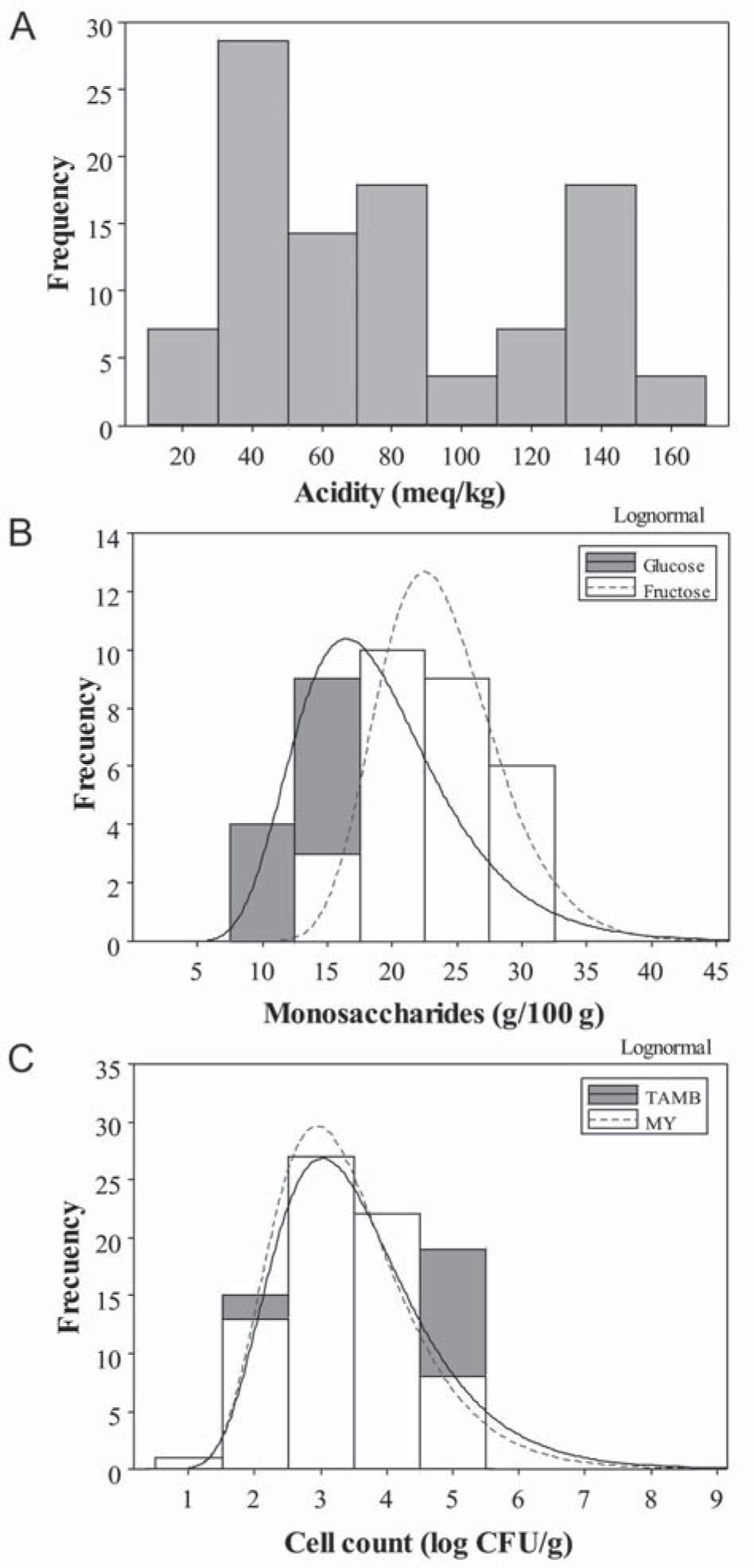
The acidity of yateí honey ranged from 25 to 160meq/kg with a mean value of 79±43 (Fig. 1A). A similar range (7.7–109meq/kg) was previously indicated by Souza et al.25 for yateí honey from Brazil, Mexico and Venezuela. Sgariglia et al.23 also reported a wide range of acidity (45.4–98.4meq/kg) for yateí honey from the northwest of Argentina. However, no previous reports showed acidity values as high as those obtained in this work (130–160meq/kg). This fact may be related to the harvest time, i.e. the maturity of honey, and/or climatic factors, which would favor chemical, enzymatic and microbiological reactions able to release acidic compounds in honey (see below). In contrast, acidity values of A. mellífera honey are significantly lower, between 15 and 38meq/kg. Therefore, the CAA establishes that the acidity value should not exceed 40meq/kg for edible honey.
The amount of water contained in yateí honey was 24±1.5%, similar to values previously reported by several authors for honey from different stingless bees1,17,28,32. The water content in yateí honey is higher than the average reported for A. mellífera honey10,13,25 (ca. 18%).
The concentration of glucose and fructose varied from 11.5 to 31.6g/100g but had significantly different means with a confidence interval of 95% for Fisher test. In Figure 1B we observed that data have a log normal distribution, being the average of 19±6 and 24±4g/100g for glucose and fructose, respectively. These mean values are lower than those reported by other authors for yateí honey, where values reached 22–27 and 31–40g/100g for glucose and fructose, respectively23,27. These differences may be associated with the high levels of acidity that were observed in this work (see above). In comparison, A. mellífera honey has higher concentration of glucose (32g/100g) and fructose (39g/100g) than the yateí honey analyzed in this work10.
We also observed that the sum of water content plus monosaccharides measured in yateí honey represented only 65.5g/100g of the total honey mass, which suggests that other substances, including polysaccharides, would be present in high concentration in this honey. The findings reported by Vit et al.31 support this hypothesis since they showed that the genus Trigona, such as T. angustula, produces honey with high maltose content (24–56g/100g), whereas the genus Melipona, such as Melipona paraensis, produces honey with low maltose content (1–1.23g/100g). The HPLC methodology we used in this study was suitable to detect sucrose but not maltose. As sucrose was not found in the analyzed samples, we suspect that maltose could be another main compound in yatei honey composition.
Absence of sucrose in yateí honey was previously reported by Torres et al.27, who related this fact to the activity of invertase enzymes present in this honey. In contrast, other authors reported the presence of sucrose in yateí honey but in low concentrations2,25 (0.13 and 6.00g/100g).
Microbial countsHoney microorganisms can originate from primary sources (at harvest) such as pollen, the digestive tracts of honey bees, dust, nectar, and others, which are very difficult to control. Other sources (after-harvest) include air, food handlers, cross-contamination, equipment and buildings, which can be controlled by good manufacturing practices24. In this work, we try to avoid these secondary sources of contamination by using sterile syringes and gloves for honey sampling. However, yateí honey showed a wide range of MY counts (1.2–4.7 log CFU/g), with an average of 3.02±0.99 log CFU/g (Fig. 1C), which came from a primary source of contamination. The subsistence of MY in honey is due to the ability of fungi, mainly yeasts, to grow under high sugar concentrations even with limited available water24. However, it is worth highlighting that the mean values for MY in yateí honey were higher than those reported for A. mellífera honey17. This fact was related to the higher water content observed in the former.
About 90% out of MY in yateí honey were yeast strains. Although we found no reports about the total “mould and yeast” count in this honey, we found that a yeast count of ca. 4 Log CFU/g was reported by Texeira et al.26. This author indicated that this count is due to the species Starmerella meliponinorum, which would be metabolically active and able to grow at the expense of sugars present in this food.
The number of TAMB was similar to MY, with an average of 3.13±1.05 Log CFU/g. This value is similar to data reported by several authors for A. mellifera honey17,22,28 and falls within the legislative limits ruled by MERCOSUR and CAA. Only in 10% of samples counts were higher than the established maximum limit (1×104 CFU/g).
Bacterial characterizationOn the whole, honey is a hostile environment for the growth of food-borne pathogenic bacteria. However, spore and vegetative latent forms may be present due to primary and/or secondary pollution. In this work, we evaluated six bacterial genera that can be present in honey and can produce food-borne diseases. These genera included: Bacillus, Clostridium, Staphylococcus, Enterococcus, E. coli and Salmonella.
Clostridium spp. (64%) and Bacillus spp. (39%) were the most prevalent genera in analyzed honey samples (Fig. 2). These spore-forming bacteria are common in air, soil and dust becoming a primary source of contamination; thus, they are commonly found in honey24. The detection of Clostridium spp. in honey is very important to determine its quality mainly taking into account the severity of C. botulinum foodborne disease. Although we detected a high percentage of Clostridium spores in honey from T. angustula, we did not characterize any of the isolates as C. botulinum by the biochemical test performed. The presence of Clostridium spp. in A. mellífera honey is also high. Finola et al.11 found sulfite-reducing spores in about 70% of samples from Córdoba, Argentina. Kokubo et al.18 showed that 79% of the samples analyzed contained sulfite-reducing spores, with six samples containing C. perfringens but none C. botulinum. In stingless bee honey, a few reports indicated absence of C. botulinum in honey samples12,37.
The presence of Bacillus spp. would be expected in honey, since a symbiotic relationship between this microorganism with insects, including honeybees, solitary bees and stingless bees had been reported20. Specifically, Bacillus species were found in abdominal tissues, larval food and honey of several species of tropical stingless bees14.
Since the native habitat of E. coli is the enteric tract of animals, its presence in foods generally indicates direct or indirect contamination of fecal origin22. Only one out of twenty eight samples had total coliforms, in which the count was 1.45 log NMP/g. Fecal coliforms, E. coli, Salmomella spp. were not detected (Fig. 2). These results reflect good manufacturing practices during harvest and laboratory store analysis.
Two samples were positive for Enterococcus spp., with counts of 1.63 and 3.04 log MPN/g (Fig. 2). Cultures were characterized as lactic acid bacteria (LAB), which can originate from bee microbiota, since associations between LAB and invertebrates have been shown. Symbiotic LAB has been found inside the honey crop in A. mellifera, from where it can be transferred to the honey comb30; a similar event could happen in T. angustula honey.
Two samples of T. angustula honey showed the presence of Staphylococcus spp. but none of the isolated strains produced coagulase. We have not found any reports of Staphylococcus in honey from stingless bees. In A. mellifera honey this genus is rarely found, it appears to be unable to survive during processing and is not likely to grow in honey. In that respect, some authors24 have emphasized the need to improve the procedures of honey harvesting and processing to reduce the introduction of microbes like Staphylococcus, and have also indicated that the antibacterial properties of honey should be carefully considered24.
Correlation between acidity, sugars and microorganismsThe high acidity of yateí honey is linked to its high water content, which favors the development of some chemical and enzymatic reactions able to release acidic compounds. As examples of these reactions, we can quote the decomposition of fructose into levulinic and/or methanoic acids5,15 and the conversion of glucose into gluconic acid by the enzyme glucose oxidase36. On the other hand, the high water content in yateí honey can also decrease its osmotic pressure and allow the development of some fermentative microorganisms able to release acidic compounds34. In order to know which of these reactions would be the main cause for acidity in yateí honey, we evaluated the correlation between the acid content, the sugar concentration (glucose and fructose), and the microorganism count (TAMB and MY). We observed that acid content was associated with the sugar concentration with a correlation coefficient (R2) of 0.75 for glucose and 0.68 for fructose (Fig. 3A). On the other hand, the correlation between the acid content and the MY count was significantly lower (R2=0.45) (Fig. 3B). No correlation (R2≤0.28) was observed between the acid content and the TAMB count (Fig. 3B), neither between the sugar concentration and the microorganism count (Figs. 3C and 3D). These results indicate that the acidity of yateí honey is related to chemical and enzymatic reactions of honey itself rather than with fermentative processes carried out by microorganisms.
ConclusionThe results obtained for yateí honey were quite different compared to the quality standards established in the CAA for A. mellifera honey; therefore, we recommend that some quality parameters, such as acidity, moisture content and mould and yeast counts, should be adapted to yateí honey in order to promote its commercialization.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
FundingWe thank the Comité Ejecutivo de Desarrollo e Innovación Tecnológica (CEDIT) of the Province of Misiones and Consejo Federal de Ciencia y Tecnología (COFECYT) for financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We also thank Natasha Schvezov for her critical English revision of the text and all the beekeepers from Misiones (Argentina), Sergio Feversani, Noelia Ramírez, Myriam Garcia, and Paula Novak for their great collaboration.