Bovine coronavirus (BCoV) is a viral enteric pathogen associated with calf diarrhea worldwide being, in Argentina, mostly detected in dairy husbandry systems. The aim of the present work was to study if maternal IgG1 antibodies (Abs) to BCoV acquired by colostrum intake modulate the development of BCoV infection in calves reared in a dairy farm in Argentina. Thirty Holstein calves were monitored during their first 60 days of age. Animals were classified into two groups depending on their initial BCoV IgG1 Ab titers. The “failure of passive transfer” (FPT) group had significantly lower IgG1 Abs to BCoV than the “acceptable passive transfer” (APT) group of calves (log10 1.98 vs. 3.38 respectively) (p<0.0001). These differences were also observed when the total protein levels in both groups were compared (p=0.0081). Moreover, 71% (5/7) of calves from the FPT group showed IgG1 seroconversion to BCoV compared to 29.4% (5/17) of animals from the APT group. Regarding viral circulation, BCoV was detected in 10% (3/30) of all calves and BCoV IgG1 Ab seroconversion was detected in 42% of the total animals showing that almost half of the calves were infected with BCoV. In conclusion, calves with high titers of specific BCoV IgG1 (≥1024) were mostly protected against viral infection, while animals with low titers of IgG1 (<1024) were mostly infected with BCoV. IgG1 Abs from colostrum origin are critical for prevention of BCoV infection.
El coronavirus bovino (Bovine coronavirus, BCoV) es un enteropatógeno viral asociado a la diarrea neonatal del ternero. El objetivo del presente trabajo fue estudiar si los anticuerpos IgG1 anti-BCoV adquiridos pasivamente mediante el calostro modulan la infección por BCoV en terneros de un rodeo lechero de Argentina. Se monitorearon 30 terneros raza Holstein durante los primeros 60 días de vida. Estos animales fueron clasificados en dos grupos según sus niveles de IgG1 anti-BCoV maternales: grupo con transferencia de inmunidad pasiva aceptable (APT) y grupo con fallas en la transferencia pasiva (FPT). Este último grupo tenía un título de IgG1 significativamente menor comparado con el primer grupo (log10 1,98 vs. 3,38, respectivamente; p<0,0001). La misma diferencia se observó cuando se compararon los niveles de proteínas séricas totales (p=0,0081). Además, el 71% (5/7) de los terneros del grupo FPT mostró seroconversión de IgG1, mientras que el 29,4% (5/17) de los terneros del grupo APT la mostró. Con respecto a la circulación viral, se detectó BCoV en el 10% (3/30) de los terneros así como también seroconversión de IgG1 en el 42% del total de los animales, lo que evidencia que aproximadamente la mitad de los terneros se infectaron con BCoV. Este estudio mostró que los terneros con altos títulos de IgG1 específica (≥1.024) estuvieron mayormente protegidos contra la infección con BCoV, mientras que los animales con títulos bajos de IgG1 (<1.024) estuvieron predispuestos a la infección. Esto confirma que los anticuerpos IgG1 calostrales son críticos para la prevención de la infección por este agente viral.
Bovine coronavirus (BCoV) is a major viral pathogen associated with neonatal calf diarrhea (NCD)30, winter dysentery in adult cattle35, and respiratory tract disorders in cattle of all ages9,15. In addition, it causes important economic losses to the beef and dairy industry worldwide5,38.
BCoV is comprised of a single stranded, non-segmented positive sense genomic RNA, 32kb long, which associates to the nucleoprotein (N) forming a nucleocapsid having helical symmetry24. It belongs to the Betacoronavirus genus cluster within the Coronavirinae subfamily, Coronaviridae family, and the order Nidovirales (http://ictvonline.org/virusTaxonomy.asp). BCoV has several structural proteins which have different functions in the viral cycle. Among them, the S protein is responsible for the interaction between the virus and the cellular receptor, also eliciting neutralizing antibodies (Abs).
BCoV is an enteric/respiratory virus that replicates in enterocytes from the gastrointestinal tract as well as in the epithelium of the upper respiratory tract5. Although it causes severe hemorrhagic diarrhea which is sometimes fatal in young animals, the spiral colon is the hot spot for viral replication in the gastrointestinal epithelium leading to osmotic diarrhea17. Moreover, BCoV is shed both through respiratory and enteric secretions in high amounts (1 billion virus particles per ml of feces) for up to 14 days23. Consequently, BCoV infection is transmitted by fecal-oral or respiratory route and generally occurs by horizontal transmission from the mother to the offspring or between calves14,18.
The incidence of BCoV varies between 15% and 70% in naturally occurring outbreaks worldwide25,27,31. In the Southern hemisphere, Al Mawly et al.1 detected a BCoV prevalence of 14% during 2011 calving season in dairy farms in New Zealand. Additionally, Stipp et al.26 and Lorenzetti37 reported 15.6% and 33.3% of BCoV PCR-detection rates in diarrheic calves from dairy and beef farms in Brazil in 2009 and 2013 respectively. In Argentina, the BCoV detection rate by ELISA was 1.71% in calves with diarrhea, corresponding to 5.95% of the herds analyzed from 1994 to 2010. Additionally, those Argentina-specific strains were distantly related to the Mebus reference strain in a phylogenetic analysis6,30.
As previously suggested, BCoV outbreaks may occur in calves from beef and dairy herds28,37. However, in a previous study conducted in Argentina, BCoV infection was mostly associated with diarrhea in dairy husbandry systems6. This difference may be due to the close interaction between calves in dairy farms, since these animals were reared under intensive management systems and fed milk replacers lacking Abs, in stark contrast with beef cattle farms, where herds were reared under extensive management systems and calves were fed directly from the dams’ milk until they reached 6 months of age4.
Regarding the prevalence of BCoV, serological surveys indicate that approximately 90% of the worldwide cattle population has Abs against BCoV8. However, Ohlson et al.31 observed that BCoV Ab-positive herds remained persistently high (75–100%) in Swedish Southern regions compared with Northern regions where the percentage of positive herds were lower (38–80%). In Argentina, 100% of the adult cattle population is estimated to be seropositive for Abs to CoVB (Dr. Parreño, personal communication).
Colostrum intake is the natural and most useful method to control BCoV calf diarrhea12. Because BCoV-associated diarrhea is an early age disease, the continuous presence of neutralizing Abs in the intestinal lumen, mostly IgG1 Abs, seems to be essential for prevention of BCoV diarrhea9. Protective levels of BCoV Abs in calves could be achieved by vaccination of the pregnant cows during the last three months of pregnancy. Three commercial vaccines are available in Argentina, all of them containing the inactivated BCoV Mebus strain, which confers cross-protection with local circulating strains6. However, Ab transfer from the colostrum to the calf bloodstream may fail due to deficiencies in the quality and quantity of colostrum produced by the dam, failure of colostrum intake by the calf, or the newborn physical condition29.
There are few studies reporting the transference of passive maternal Abs from the dams to their calves via colostrum intake under field conditions, and its role in the protection against BCoV infection21. Thus, the aim of the present study was to determine if IgG1 passive maternal Abs to BCoV acquired by colostrum intake modulate the development of BCoV natural infection and disease in calves reared in a dairy farm in Argentina.
Materials and methodsExperimental designThirty Holstein calves reared under an educative, intensive dairy management system were monitored during their first 60 days of age. Calves were reared tied to individual stakes with no contact of one calf with the other. Calves were initially acquired from different farms with varying levels of sanitary status. Thus, vaccination and colostrum administration differed depending on the standard operating procedure of each farm. The age of the calves at the beginning of the study (upon arrival to the herd) ranged from one to ten days. All calves were fed with two liters of milk replacer twice a day and progressive amounts of calf starter (AproAgro S.A., Argentina). Neither preventive antibiotics nor anti-parasitic drugs were administered to the calves. In order to evaluate colostrum intake, total serum protein levels for each calf were measured by refractometry at the beginning of the study (0 days). Additionally, serum samples were collected from each calf every seven days to measure IgG1 Ab titers to BCoV by ELISA. Animals were classified into two groups depending on their initial BCoV IgG1 Ab titers. Calves with BCoV IgG1 Ab titer≤256 (n=7) were assigned to the failure of passive transfer (FPT) group and animals with BCoV IgG1 Ab titers≥1024 (n=17) were assigned to the acceptable passive transfer (APT) group.
In order to evaluate the presence of neonatal diarrhea in calves with varying titers of colostrum-derived Abs, and to understand the BCoV infection dynamics under field conditions, feces were collected daily from each calf and a diarrhea score was recorded (0=normal, 1=pasty, 2=semi liquid, 3=liquid, 4=hemorrhagic; a fecal score of 2 or higher was considered diarrhea). Additionally, we analyzed several parameters associated with the clinical infection of the calves. Those parameters were diarrhea onset (days after the beginning of the experiment), age of calves at diarrhea onset, diarrhea duration (in days) and diarrhea severity (measured as the average of the area under the curve of fecal scores of each calve during the period analyzed).
Furthermore, mortality and BCoV infection in calves with different levels of passive immunity was recorded.
Finally, all calves’ weight was registered at 21 days of age and at the end of the study, at 60 days of age.
IgG1 to BCoV Ab ELISAIgG1 Ab titers to BCoV were measured using a double sandwich ELISA. Briefly, 96-well Maxisorp NUNC plates (Thermo Scientific®, USA) were coated with 100μl of guinea pig hyperimmune serum to BCoV (1:5000 dilution in carbonate/bicarbonate buffer, pH 9.6) and incubated overnight (ON) at 4°C. Plates were then washed and blocked with 10% non-fat milk solution diluted in PBS-0.05% Tween 20. After incubation during 1hour at 37°C, BCoV Mebus strain-infected (107FFU/ml) and mock-infected HRT-18 supernatants were added as positive and negative antigens, respectively. Serial 4-fold dilutions of the serum samples were tested in duplicate followed by incubation with an HRP-conjugated commercial Ab to bovine IgG1 (Bethyl Laboratories, Inc., USA). The reaction was developed using hydrogen peroxide and ABTS as substrate/chromogen system (Sigma Aldrich, USA) and read at a wavelength of 405nm (Multiskan Ex, Labsystems Inc.). The titer of each sample was expressed as the reciprocal of the highest serum dilution with a corrected optical density (OD405C; OD405 values in the BCoV-coated wells minus OD405 values in the HRT-18-coated wells) greater than the cut-off value of the assay. The cut-off value was established as the average of OD405C values of four blank wells (PBS-0.05% Tween 20) plus three standard deviations (SD).
BCoV detectionFecal samples from calves were collected daily and stored at -20°C until viral detection. The presence of BCoV antigens in the calves’ feces was detected by an indirect antigen-capture ELISA that uses monoclonal Abs directed to the viral proteins HE, N, and S as previously described36. Virus shedding was confirmed by a CCIF assay which detects Fluorescent Focus forming Units of BCoV in HRT-18 cell monolayers inoculated with the fecal samples, and developed using a fluorescent-labeled anti-BCoV hyperimmune serum.
Differential diagnosisCalves’ fecal samples were also tested for Group A Rotavirus (RVA), which is the most common causative agent of neonatal gastroenteritis. The diagnosis was performed using an indirect antigen-capture ELISA as previously reported2,3,11,16. No bacterial or parasite diagnosis was performed.
Statistical analysisAb titers to BCoV determined by ELISA were log10-transformed prior to the statistical analysis. Negative samples at a dilution of 1:4 were assigned an arbitrary Ab titer of 2 for the calculation of geometric mean titers (GMTs). Statistical significance was assessed at p<0.05 for all comparisons. Statistical analyses were conducted using Infostat® statistical software22.
Group effects on the IgG1 Ab titers to BCoV were analyzed by a general linear mixed statistical model (GLMM). The model included two main fixed factors: group (APT and FPT, as between subjects’ factor) and time (with six levels, as within subjects’ factor). Animals were included in the model as a random factor. The Akaike Information Criterion (AIC) was used for choosing the best-fitting model as a minimal adequate one. Thus, the model with the lowest AIC value was selected. The GLMM analysis was conducted by using the glmer function (lme4 package, R Development Core Team, 2014). The analysis was performed with R 3.0.3 (R Development Core Team, 2014). Statistical significance was assessed at p<0.05 for all comparisons.
ResultsThirty Holstein calves reared under an educative intensive dairy management system were monitored for BCoV infection during their first 60 days of age. Six out of thirty (6/30; 20%) calves died because of additional causes other than BCoV diarrhea.
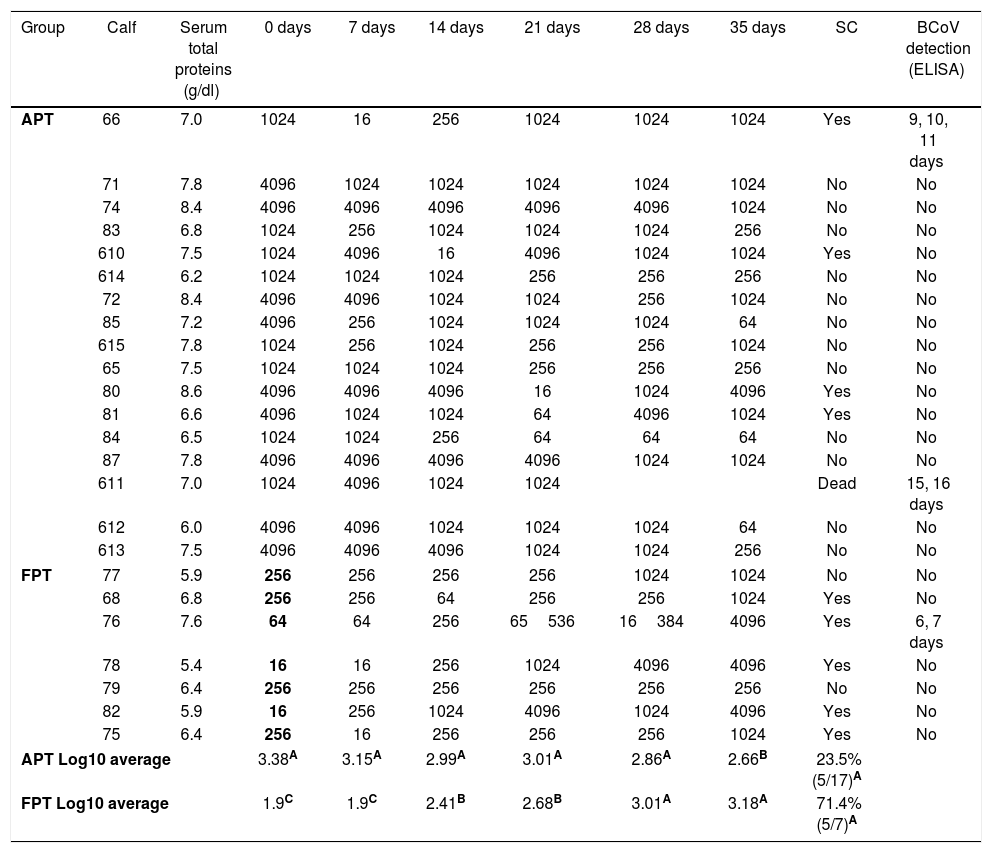
In order to evaluate colostrum intake, total serum protein levels for each calf were measured at the beginning of the study. The mean (±SD) value of total serum proteins in sera was 7.1±0.9g/dl. When the kinetics of the IgG1 Ab levels to BCoV was evaluated by ELISA for each individual calf, we observed that the IgG1 Ab titers to BCoV in the calves’ sera (representing colostrum intake) were highly heterogeneous on arrival to the farm (IgG1 Ab titers for each calf are shown in Table 1).
IgG1 antibody titers to BCoV
| Group | Calf | Serum total proteins (g/dl) | 0 days | 7 days | 14 days | 21 days | 28 days | 35 days | SC | BCoV detection (ELISA) |
|---|---|---|---|---|---|---|---|---|---|---|
| APT | 66 | 7.0 | 1024 | 16 | 256 | 1024 | 1024 | 1024 | Yes | 9, 10, 11 days |
| 71 | 7.8 | 4096 | 1024 | 1024 | 1024 | 1024 | 1024 | No | No | |
| 74 | 8.4 | 4096 | 4096 | 4096 | 4096 | 4096 | 1024 | No | No | |
| 83 | 6.8 | 1024 | 256 | 1024 | 1024 | 1024 | 256 | No | No | |
| 610 | 7.5 | 1024 | 4096 | 16 | 4096 | 1024 | 1024 | Yes | No | |
| 614 | 6.2 | 1024 | 1024 | 1024 | 256 | 256 | 256 | No | No | |
| 72 | 8.4 | 4096 | 4096 | 1024 | 1024 | 256 | 1024 | No | No | |
| 85 | 7.2 | 4096 | 256 | 1024 | 1024 | 1024 | 64 | No | No | |
| 615 | 7.8 | 1024 | 256 | 1024 | 256 | 256 | 1024 | No | No | |
| 65 | 7.5 | 1024 | 1024 | 1024 | 256 | 256 | 256 | No | No | |
| 80 | 8.6 | 4096 | 4096 | 4096 | 16 | 1024 | 4096 | Yes | No | |
| 81 | 6.6 | 4096 | 1024 | 1024 | 64 | 4096 | 1024 | Yes | No | |
| 84 | 6.5 | 1024 | 1024 | 256 | 64 | 64 | 64 | No | No | |
| 87 | 7.8 | 4096 | 4096 | 4096 | 4096 | 1024 | 1024 | No | No | |
| 611 | 7.0 | 1024 | 4096 | 1024 | 1024 | Dead | 15, 16 days | |||
| 612 | 6.0 | 4096 | 4096 | 1024 | 1024 | 1024 | 64 | No | No | |
| 613 | 7.5 | 4096 | 4096 | 4096 | 1024 | 1024 | 256 | No | No | |
| FPT | 77 | 5.9 | 256 | 256 | 256 | 256 | 1024 | 1024 | No | No |
| 68 | 6.8 | 256 | 256 | 64 | 256 | 256 | 1024 | Yes | No | |
| 76 | 7.6 | 64 | 64 | 256 | 65536 | 16384 | 4096 | Yes | 6, 7 days | |
| 78 | 5.4 | 16 | 16 | 256 | 1024 | 4096 | 4096 | Yes | No | |
| 79 | 6.4 | 256 | 256 | 256 | 256 | 256 | 256 | No | No | |
| 82 | 5.9 | 16 | 256 | 1024 | 4096 | 1024 | 4096 | Yes | No | |
| 75 | 6.4 | 256 | 16 | 256 | 256 | 256 | 1024 | Yes | No | |
| APT Log10 average | 3.38A | 3.15A | 2.99A | 3.01A | 2.86A | 2.66B | 23.5% (5/17)A | |||
| FPT Log10 average | 1.9C | 1.9C | 2.41B | 2.68B | 3.01A | 3.18A | 71.4% (5/7)A | |||
BCoV IgG1 Ab titers in serum of each calf measured every seven days. APT: acceptable passive transfer; FPT: failure of passive transfer; SC: seroconversion; BCoV detection is shown in days of experiment. Means in the same column with different superscript upper case letters differed significantly (repeated measurements over time ANOVA Test, p<0.05).
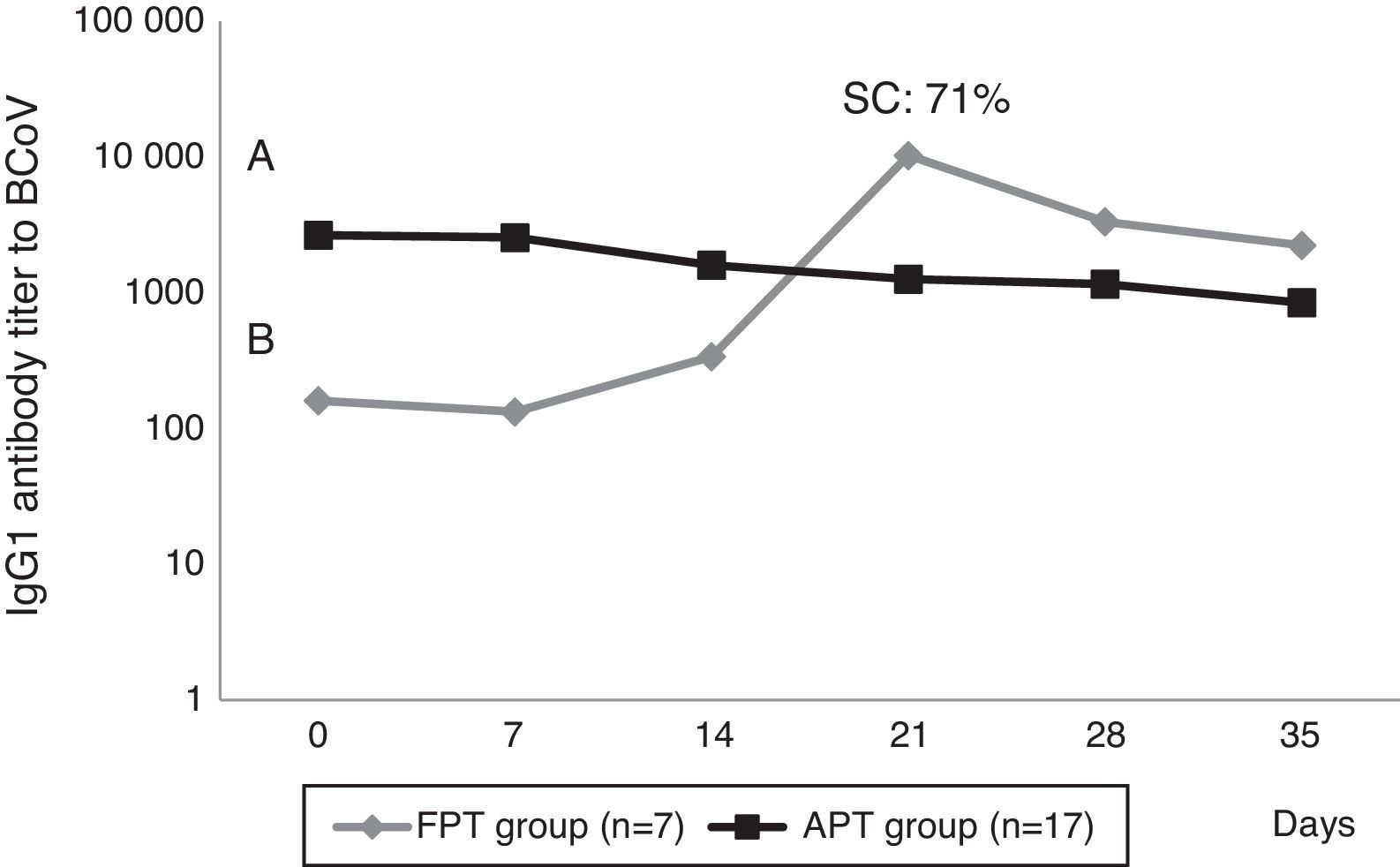
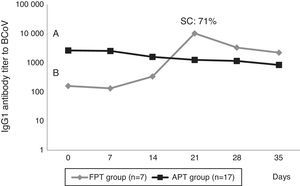
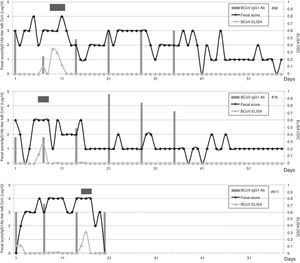
Calves’ passive immunity to BCoV was also analyzed in order to understand BCoV pathogenesis. Animals were classified into the FPT (titers≤256) and APT (titers≥1024) groups depending on their initial BCoV IgG1 ELISA Ab titers (Fig. 1). The IgG1 Abs to BCoV significantly differed between groups depending on the time (GLMM Group-Time F(5,120)=12.17, p<0.0001). The FPT group had significantly lower IgG1 Ab titers to BCoV than the APT calves for up to seven days after the beginning of the experiment. These significant differences were also observed when measuring total protein levels by refractometry (Kruskal–Wallis rank sum test, p=0.0081) which is the preferred method used to measure colostrum Abs transfer under field conditions. As expected, the higher the initial level of IgG1 Abs in the calves’ sera, the longest it took for them to decrease to a susceptible level. When BCoV IgG1 Ab titers decreased to 16 or 64, BCoV infection was evidenced by either virus detection in feces, or by a minimum 16-fold increase of anti-BCoV IgG1 ELISA Ab titers for around two weeks (defined as seroconversion for the purpose of this study) (Table 1, Fig. 2). Moreover, 71% (5/7) of calves from the FPT group showed BCoV IgG1 seroconversion between 14 and 21 days of the experiment while only 29.4% (5/17) of the animals from the APT group presented sequential seroconversion during the same period (Fisher's exact test, p=0.085) (Fig. 1 and Table 1). However, the moments of seroconversion in the different groups of calves are not necessarily correlated with the days of age because, as we mentioned before, the calves’ age was between 1 and 10 days at the beginning of the experience. The APT group average age at day 0 of the experience was 2.8 days while the FPT group average age was 5.4 days (data not shown).
BCoV IgG1 Ab profiles from calves with acceptable passive transfer (APT) compared with calves with failure of passive transfer (FPT) during the first 35 days of life. Letters shows statistically significant differences in IgG1 Ab titers to BCoV after colostrum intake (p<0.0001). SC: seroconversion shows the percentage of animals infected with BCoV in the FPT group.
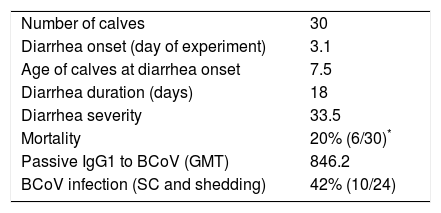
Considering the presence of neonatal diarrhea in calves with varying titers of colostrum Abs, it was observed that all calves were affected by one, two or even three events of severe diarrhea with an average duration of 18 days (Table 2); BCoV shedding of short time duration (2–3 days) was detected in 10% (3/30) of calves (Tables 1 and 2) and the highest BCoV infectious titer was 105FFU/ml of stool. Although viral shedding was detected at a low rate in the calves’ stool, BCoV IgG1 seroconversion was observed in 42% of animals, suggesting that almost half of the calves were exposed to BCoV (Table 1).
Clinical parameters and BCoV infection
| Number of calves | 30 |
| Diarrhea onset (day of experiment) | 3.1 |
| Age of calves at diarrhea onset | 7.5 |
| Diarrhea duration (days) | 18 |
| Diarrhea severity | 33.5 |
| Mortality | 20% (6/30)* |
| Passive IgG1 to BCoV (GMT) | 846.2 |
| BCoV infection (SC and shedding) | 42% (10/24) |
Average of clinical parameters from the calves under study.
Calves shedding BCoV in stools were further analyzed. Calf #66 had a first 14 day-diarrhea episode in which BCoV was detected from 9 to 11 days of the study (9, 10 and 11 days of age). This calf had additional diarrhea episodes that were not associated with BCoV infection. Calf #76 had diarrhea since the experiment started (ten days of life for this calf) and during 15 days, in which BCoV was detected at 6 and 7 days of the experiment (15 and 16 days of age). Then, this calf had other diarrhea episodes without etiologic diagnosis. Finally, calf #611 had 16 days of severe diarrhea beginning at day two of the study (six days of life), while BCoV was detected at 15 and 16 days of the experiment (20 and 21 days of age). In these three cases the presence of BCoV was associated with severe diarrhea (fecal score≥3). Two out of three calves showed BCoV IgG1 seroconversion at the same time of viral detection (Fig. 2). The third BCoV-positive calf died with severe diarrhea.
Calves’ fecal samples were also tested for RVA as another common causing agent of neonatal gastroenteritis. RVA shedding was detected in 50% (15/30) of the calves, between three to ten days of age. No bacterial or parasite diagnosis was performed; however, taking into consideration that neither antibiotics nor anti-parasitic drugs were administered to the calves, diarrhea caused by either of these groups of agents may not be discarded.
As a final important parameter, all the calves’ weight was registered. Even though all the calves presented severe diarrhea at some point during the study, once the clinical signs of gastroenteritis disappeared, all animals gained weight reaching an average of 90kg by the end of the experiment. All calves duplicated their weight during the study period (60 days) with no significant differences between the groups.
DiscussionIn order to better understand BCoV infection and prevention in calves reared under artificial management systems, 30 calves were followed up during their first 60 days of life at an educative dairy farm. Both, the immunologic status of the calves at birth and the colostrum quality of the dams are the most important factors influencing prevention of neonatal calf diarrhea34. The outcome of BCoV infection was clearly affected by passively acquired Abs from colostrum. IgG1 subtype is the main IgG Ab isotype actively concentrated during colostrogenesis19, and was the isotype analyzed in this study.
Refractometry is the commonly used method for total protein measurement to evaluate colostrum intake in calves’ serum. However, the exact titer of BCoV-specific IgG1 transferred to calves from maternal colostrum required to prevent BCoV diarrhea in the field remains unclear. Total protein level≥5.2g/dl (corresponding with IgG≥1000mg/dl) in serum from a healthy calf is considered to be an optimal level of protein transfer39. In this study, we observed a mean protein level of 7.1g/dl which is optimal; however, the FPT group had a significantly lower level of total protein in serum than the APT group at day 0 of the experiment (Kruskal–Wallis non-parametric rank sum Test, p=0.0081). This correlates with the higher detection of BCoV IgG1 Ab seroconversion in the FPT group compared with the APT animals.
This study also aimed to further define BCoV pathogenesis, and to better understand BCoV-specific Ab kinetics. In Argentina, we previously reported a significantly higher rate of BCoV diarrhea in dairy herds compared to beef herds6. We detected viral shedding in a small number of animals but high viral circulation manifested through BCoV seroconversion. Low viral detection could be due to a lower sensitivity of ELISA compared with the RT-PCR assay to detect BCoV in feces7. However, another country from the southern hemisphere shows a low detection rate using RT-PCR1. BCoV shedding was detected at high titers during only two or three days differing with other experiments in which BCoV shedding was long lasting23,32. This supports the idea of a lower sensitivity of the ELISA assay for BCoV detection used. Moreover, we showed that calves with high BCoV IgG1 Ab titers were often protected against infection, while animals with low BCoV IgG1 Ab titers showed significantly higher BCoV infection rates (p<0.0001). On the one hand, BCoV infection dynamics was drastically affected by these Ab titers since 71% of FPT animals showed BCoV IgG1 seroconversion during the experiment. The significant difference in Ab titers at the beginning of the study was also associated with a significant difference in total protein levels. Furthermore, when IgG1 titers decreased over time and reached non-protective levels (BCoV IgG1 Ab titers of 16 or 64), calves seroconverted confirming BCoV infection, even when the antigen was not detected in the feces by the methodology used. On the other hand, 70.6% of animals from the APT group maintained elevated IgG1 Ab titers over time and only five calves eventually seroconverted. Moreover, calves with maternal BCoV IgG1 Ab titers of 4096 or higher seemed to be protected against BCoV infection over the course of this study, suggesting that specific IgG1 Abs from colostrum are critical for the prevention of BCoV infection20. Although there was a tendency to observe more BCoV IgG1 seroconversion in calves from the FPT group compared with calves from the APT group, this difference was not significant (Fisher's Exact Test, p=0.085). This could be due to a low number of experimental units in the FPT group.
Weight gain was evaluated in all calves at the end of the study (60 days of age), compared to baseline measurements obtained at the beginning of the study. No significant weight differences between groups were observed. Even though we do not have a negative control group – animals with no signs of diarrhea – it has been reported that average daily weight gain does not correlate with the occurrence of NCD33. However, other studies demonstrated the influence of NCD on weight gain in beef calves10. In addition, it has also been established that when BCoV is present in diarrhea outbreaks, it causes more aggressive clinical signs compared to other gastrointestinal viruses, which can compromise the animal survival5. Indeed, one of the animals shedding BCoV died due to the severity of the diarrhea during the course of this study.
Vaccination is the most effective strategy used to prevent NCD. However, vaccination success can be affected by several factors including deficiencies in herd management and varying vaccine quality29. As was mentioned previously, we have no information either about the vaccination of dams against neonatal diarrhea or about the quality of commercial CoVB vaccines. Therefore, Abs transferred to calves through colostrum intake might be due to previous exposure to BCoV or to an active immunization of the dam with this agent. The lack of a good passive immune transference in addition to a diet based on milk replacer might only have contributed to the occurrence of diarrhea in 100% of the studied animals. All calves suffered a severe and long lasting diarrhea (17 to 19 days). The etiology of diarrhea could be diverse since other infection agents were not discarded, and neither antibiotic nor anti-parasitic drugs were used as preventive strategies. BCoV infection was evidenced by antigen shedding or seroconversion in 42% of the calves, while RVA infection was evidenced by shedding in 50% of calves. Thus, RVA is a major agent associated with NCD, as has been reported elsewhere1,2,13.
The present study highlights the critical role of maternal colostrum-derived Abs in the development of BCoV infection. The systematic immunization of cows with a vaccine of proved efficacy, the monitoring of colostrum quality, and the efficient passive transfer to the calf are key factors to consider when attempting to control BCoV-related diarrhea. The results displayed in this study point to useful end-points to measure when evaluating the potency and efficacy of a BCoV vaccine under field conditions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
We are very grateful for the technical assistance of Dr. Osvaldo Zábal, for the input from the professors at the Milk Production Department of Litoral National University in Argentina, Dr. Federico Guzman Coraita, Dr. Mario Cabellier, Dr. Julieta Bianchini, Dr. Carolina Repetto, Dr. Pilar Bustamante, Dr. Alberto Cabrera, Leonela Riera, Paula Patricelli, Corina Pautasso, and their students. We are also grateful to Dr. Linda Saif for her assistance in setting up the diagnostic assay, to Dr. Karin Bok for English edition and to Dr. Karina Hodara for statistical support. This study was supported by funds from FONARSEC N° 003 INTABIO AF, MinCyT, Argentina. MB and Dr. VP are members of CONICET.