The use of lactic acid bacteria (LAB) in foods as biocontrol agents against foodborne pathogens has become increasingly known. Under the premise that controlling the adhesion of microorganisms to food contact surfaces is an essential step for meeting the goals of food processing, the aim of this work was to investigate the inhibitory and anti-biofilm effectiveness of Lactobacillus rhamnosus GG (ATCC 53103) and Lactobacillus casei (ATCC 393) against Escherichia coli O157:H7, Salmonella enterica and Listeria monocytogenes. Lactobacillus strains (108CFU/ml) and pathogens (104CFU/ml) were evaluated to monitor LAB anti-adhesive and antibiofilm effect, in two main scenarios: (i) co-adhesion and (ii) pathogen incorporation to stainless steel surfaces with a protective biofilm of Lactobacillus cells. In (i) the predominant effect was observed in L. rhamnosus against S. enterica and L. monocytogenes, whereas in (ii) both LAB significantly reduced the number of pathogenic adherent cells. The effect of pre-established LAB biofilms was more successful in displacing the three pathogens than when they were evaluated under co-adhesion. These findings show that both LAB can be considered good candidates to prevent or inhibit the adhesion and colonization of L. monocytogenes, S. enterica and E. coli O157:H7 on surfaces and conditions of relevance for juice processing industries, offering alternatives for improving the safety and quality of fruit-based products.
Existe un creciente interés en el uso de bacterias ácido lácticas (BAL) como agentes de biocontrol frente a patógenos de transmisión alimentaria. Bajo la premisa de que el control de la adhesión de microorganismos a superficies de contacto con alimentos es el paso esencial para evitar su contaminación, el objetivo de este trabajo fue investigar la efectividad inhibitoria y antibiofilm de Lactobacillus rhamnosus GG (ATCC 53103) y Lactobacillus casei (ATCC 393) frente a Escherichia coli O157:H7, Salmonella enterica y Listeria monocytogenes. A fin de cumplir con el objetivo propuesto, las cepas de Lactobacillus (108UFC/ml) y los patógenos (104UFC/ml) se ensayaron en 2 escenarios: (1) coadhesión, y (2) incorporación de los patógenos a las superficies de acero inoxidable con un biofilm preformado de Lactobacillus. En (1), el efecto predominante se observó con L. rhamnosus frente a S. enterica y L. monocytogenes, mientras que en (2), ambas BAL redujeron significativamente el número de células patógenas adheridas. En función de estos resultados, concluimos que el efecto de un biofilm preformado de ambas BAL fue más exitoso en el desplazamiento de los 3 patógenos que en coadhesión. Ambas BAL pueden considerarse buenas candidatas para mitigar la adhesión y colonización de L. monocytogenes, S. enterica y E. coli O157:H7 en superficies en condiciones de relevancia para la industria procesadora de jugos, y, de esta manera, ofrecer alternativas para mejorar la seguridad y calidad de los alimentos a base de frutas.
Fruits and fruit juices have been traditionally assumed to be low risk foods due to their acidic pH (∼2.0 and 4.5); nonetheless, they have been recognized as “emerging vehicles” for foodborne illnesses caused by human pathogens such as Escherichia coli O157:H7, Salmonella spp., and Listeriamonocytogenes14. Salmonella enterica and E. coli O157:H7 outbreaks have been previously associated with the consumption of apple and citrus juices15, whereas other fruits such as mango, papaya and cantaloupe were responsible for foodborne outbreaks caused by Salmonella and L.monocytogenes9. Concentrated and reconstituted juices together with frozen puree or pulps have also been associated with foodborne pathogens such as E. coli O157:H7, S. enterica and L.monocytogenes30. They represent one of the major causes of foodborne outbreaks from food itself or, as result of biofilm formation, by cross-contamination from colonized food-related environments16. Indeed, Bridier et al.6, reported, in a survey carried out in France, that ca. 60% of food-borne infections occurred as a result of microbial transfer from equipment and surfaces to processed foods, reinforcing the need for control.
With mechanical and process automation, the surfaces are in repeated contact with raw juice, thus increasing the opportunities for pathogenic microorganisms to transfer and attach, leading to biofilm formation. In this regard, L. monocytogenes is commonly found in food-processing environments, residing in so-called refuge sites such as cracks, worn equipment and in hard to reach places such as complex machinery that may be subjected to suboptimal disinfection concentrations, allowing them to survive and possibly adapt to cleaning and sanitation treatments7. Mendonça et al.28, also demonstrated that E. coli O157:H7 has the potential to form biofilms on different surfaces (e.g. polystyrene, polytetrafluoroethylene, glass and stainless-steel) commonly used in food-processing plants, and in the case of Salmonella spp., common sites for their presence are, for example, filling or packaging equipment, floor drains, walls, cooling pipes and conveyors34. The persistence and survival of these pathogens on food-contact surfaces represent a potential chronic source of microbial contamination, threatening their safety and quality besides hurting the commercial aspects of production5,21,37.
Food companies traditionally rely on cost-effective easy to use chemical methods, such as sodium hydroxide or sodium hypochlorite solutions for the elimination or prevention of biofilm development along processing plant pipes and surfaces. However, the development of biofilms on some food industry structures cannot be controlled with these cost-effective methods47,48. It is interesting to note that decades of using sanitizers in the food industry may be one of the main driving forces regarding the development of antibiotic resistance in bacteria and its dissemination to pathogens16. Biofilms in the food industry have been suggested as hot-spots for plasmid transfers, including antibiotic multi-resistant plasmids. Moreover, there is a growing demand and awareness of consumers of food quality and safety, as well as environmental concerns, which have led to the search of potential alternative hurdle technologies to control biofilms independently of the traditional chemical approach that regulates the protocols implemented nowadays.
A promising strategy toward biofilm prevention and control is the use of lactic acid bacteria (LAB) which, through the colonization of hard surfaces and based on the competitive exclusion principle, prevent the proliferation of other bacterial species13. This concept, in which the potential of LAB as bioprotective agents against pathogenic microorganisms is highlighted, has been designated as biocontrol44. For many centuries, microbial antagonism has been used in food processing to improve food safety and, in the last decade, several studies have shown that anti-adhesive and anti-biofilm attributes for some LAB represent a potential counteracting approach to prevent pathogen implantation, without conferring any risks to consumers31,38,39,42. In this regard, food biocontrol with natural and microbiological compounds may be a satisfactory approach to solve economic losses not only due to microbial spoilage of raw materials and food products, but also to reduce the incidence of foodborne illness12. However, one of the main challenges that need to be contemplated when using LAB as a means of biocontrol in food or food-contact surfaces is to ensure their growth and survival in the food environment where they must exert their effect. Most of the research performed up to now, has been carried out under in vitro optimal conditions; however, to develop a relevant biocontrol approach, it is mandatory to take into account the in situ prevailing conditions that influence the performance of antagonistic bacteria and their effectiveness, such as target microorganism, growing state and nature of the food matrix.
Since the application of LAB as biocontrol agents in food-contact surfaces of fruit juices processing industries has not yet been fully developed, the main objective pursued here was to test the ability of Lactobacillus casei ATCC 393 and Lactobacillus rhamnosus ATCC 53103 to mitigate E. coli O157:H7, S. enterica and L. monocytogenes during colonization and biofilm formation. A key factor when testing situations related to food industry, is to use procedures that are relevant to the environment in which the microorganisms will be exposed, and, therefore, in the present study the following factors were taken into account to approximate to a real model: (1) apple and grape juice as culture medium; (2) incubation of cultures at 25°C, which is usual in juice processing plants and (3) stainless steel AISI 304 used as food contact material.
Materials and methodsFood matrixApple (Ap, 12°Brix) and grape (G, 23°Brix) juice without pulp, additives, or preservatives, produced by local producers of the Alto Valle de Río Negro (Patagonia, Argentina) were used as food matrices. The 12°Brix clarified Ap juice used was prepared from 72°Brix concentrated Ap juice, maintaining the specifications of the original matrix. Before use, each juice was sterilized by microfiltration with 0.45μm pore size membranes (Metricel®Grid, Gelman-Sciences, Ann Arbor, MI, USA) and Brix levels were determined with a digital WYA Abbe refractometer.
Microorganisms and culture conditionsThree bacterial pathogens were used: E. coli O157:H7 EDL 933 (ATCC 700927) Stx1 and Stx2 producer; L. monocytogenes (ATCC 7644) serovar 1/2c and S. enterica serotype Enteriditis isolated from poultry at the Laboratorio de Microbiología Industrial y de los Alimentos of the Universidad Nacional del Sur (Buenos Aires, Argentina). The identification of Salmonella spp. was confirmed by using conventional standard biochemical and serological tests at the reference laboratory Instituto Dr. Carlos G. Malbrán (Buenos Aires, Argentina). LAB used included L. casei (ATCC 393) and L. rhamnosus (ATCC 53103), both purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA).
Stock cultures of L. rhamnosus and L. casei stored at −70°C in 20% (v/v) glycerol were suspended in De Man, Rogosa and Sharpe broth (MRS, Biokar Diagnostics, Beauvais, France) and incubated for 24h at 37°C, while pathogens were grown in Tryptic Soy Broth (TSB, Difco, Detroit, MI, USA) for 24h at 37°C. After incubation, suspensions were harvested by centrifugation at 5000×g for 10min (Labofuge 200, Kendro, Hamburg, Germany) and cell pellets were washed twice with sterile Phosphate-Buffered Saline (PBS: 0.15mol/l NaCl, 0.05mol/l KH2PO4, 0.05mol/l K2HPO4, pH 7.2) before re-suspension in the corresponding food matrix: Ap and G juice. For LAB, suspensions were adjusted by optical density (OD) at 600nm to 0.250 (∼108cells/ml) whereas for the pathogens, a working culture suspension of 104CFU/ml was obtained by serially diluting an overnight culture.
Preparation of acid-adapted pathogensTo adhere to surfaces and proliferate, pathogen cells must first survive in the acidic environments of the food systems encountered. Thus, the use of acid-adapted cells better represents the ongoing reality rather than the use of healthy exponential growing cultures; to do so E. coli O157:H7, S. enterica and L. monocytogenes were acid-adapted using the method described in Tarifa et al.41. Briefly, pathogens were pre-adapted by their inoculation in Ap or G juice for 4h at 25°C, harvested by centrifugation at 5000×g for 10min and resuspended in the corresponding juice to prepare the final working inoculum.
Surface pre-condition, adhesion tests and biofilm formationSurface pre-conditionStainless steel coupons (SSC) of 1cm2 area, type AISI-304 were used as abiotic surfaces for attachment assays. The experiments were performed in sterile polyvinyl chloride 24-well microtiter plates (JET BIOFIL, Argentina) with one sterile SSC per well.
Adhesion testsThe potential of LAB to control the adherence of pathogens to SS and mitigate their ability to develop biofilms was evaluated by competition and exclusion. To this purpose, the combinations tested were: (i) L. casei or L. rhamnosus+E. coli O157:H7; (ii) L. casei or L. rhamnosus+S.enterica; (iii) L. casei or L.rhamnosus+L. monocytogenes.
For the competition assays, L. casei and L. rhamnosus were co-cultured with the three pathogens separately. To do so, standardized suspensions of each microbial group (LAB and pathogens) were mixed in a 1:1 ratio, poured into each well containing a SSC and incubated at 25°C for 24h. On the other hand, for the exclusion assays, a 24h preformed biofilm of L. casei and L. rhamnosus was grown on the SSCs, then the supernatant was carefully discarded and surfaces followed two rinsing steps with PBS to remove the loosely attached cells and leave the adherent ones. Afterwards, the adjusted suspensions of each pathogenic bacterium were separately put in contact with the pre-adhered Lactobacillus cells and incubated for another 24h at 25°C. Meanwhile, the attachment of LAB and pathogens in Ap and G juice was tested independently as control. E. coli O157:H7, S. enterica and L. monocytogenes were put in contact with SSC for 24h, while L. casei and L. rhamnosus for 24 and 48h. All the assays described were carried out under static conditions at 25°C.
Quantification of cellsFor each condition, coupons were carefully removed from the wells using sterile forceps and rinsed by immersion for 2min in 5ml of PBS with gentle agitation to remove the loosely attached cells and then properly processed to accomplish count procedures. Before enumeration, SSCs were subjected to two sequential detachment treatments according to Lindsay and Von Holy25, and Trampuz et al.45, with some modifications. First, the surfaces were placed in test tubes containing PBS and glass beads (5mm diameter, up to complete coverage) and sonicated (Digital Ultrasonic Cleaner, PS-10A, Arcano, China) for 2min at 20°C and 40kHz. Next, the coupons were vortexed for 2min followed by plating the proper decimal dilutions in MRS agar (Biokar, France) in the case of LAB, Eosin Metil Blue agar (EMB, Britania, Argentina) for E. coli O157:H7, gelose Oxford agar (Biokar Diagnostics, Beauvais, France) with selective supplementation (Oxford BS00308, Biokar Diagnostics, Beauvais, France) for L. monocytogenes and Bismuth Sulfite Agar (BSA, Merck KGaA, Germany) for S. enterica, followed incubation for 24–48h at 37°C. Results were expressed as log CFU of adhered cells per cm2 (Log10 CFU/cm2).
To ensure complete removal of the bacterial cells, each SSC was stained with sterile fluorescein diacetate (FDA) acetonic solution in 0.1mol/l PBS (0.04% v/v), pH 7.5. After 90min agitation in darkness, the coupons were rinsed twice with sterile distilled water, air-dried, and observed under an epifluorescence microscope (Olympus BX 51, NY, USA) using a 1009 oil-immersion objective, blue excitation U-MWB2.
Scanning electron microscopy (SEM)To determine the adhesion patterns of Lactobacillus cells on SS, samples were fixed with glutaraldehyde (2.5%) in phosphate buffer (0.1mol/l, pH 7.2); washed three times with the same buffer and dehydrated by critical point drying (E3000, Polaron Instruments, Hatfield, PA, USA). Samples were gold-coated (300Å) in a Pelco Model 3 Sputter Coater 91000 metal evaporator and viewed under a scanning electronic microscope (LEO EVO 40, Cambridge, UK) at 7.0kV acceleration voltage.
Physicochemical analysisIn order to test the potential effect that the Lactobacillus strains proposed as biocontrol agents could have on the food matrix, the main physicochemical determinations associated to their impact on juices were measured: pH, °Brix, titratable acidity (TA) and turbidity. To this purpose, the system described in Tarifa et al.40, was used with some modifications. Briefly, SS disks (50mm diameter, 0.5mm thickness; AISI 304, food grade) were placed in beakers and, to allow microbial attachment, were filled with an adjusted suspension of each LAB prepared as described in “Microorganisms and culture conditions” section up to a final working volume of 250ml per beaker system. Initially, Lactobacillus cells were allowed to deposit on the SS disks for 24, 120 and 168h at 25°C, then the surfaces containing the protective LAB biofilm were carefully moved to another sterile beaker, filled with the same working volume of fresh juice and incubated for 48h, time after which the juice was taken and analyzed. pH was determined using a Hanna HI5521 digital pH meter (Hanna Instruments SL, Gipuzkoa, Spain) calibrated with buffers at pH 4.0 (Merck, Darmstadt, Germany) and 7.0 (Cicarelli, Santa Fe, Argentina). TA was performed using the AOAC method No 16.0233 by titration with 0.1N NaOH solutions and expressed as percentage of lactic acid3. Brix levels were determined with a digital WYA Abbe refractometer as previously mentioned and turbidity was measured using a Velp Scientifica TB1 portable turbidimeter and expressed as nephelometric turbidity units (NTU).
Statistical analysisEach measurement was performed in triplicate with independent bacterial cultures in each case. Counts were converted to decimal logarithmic values (log CFU) to nearly match the assumption of a normal distribution and the results were expressed as in mean with standard deviation. In order to verify the difference between samples, a variance analysis (one-way ANOVA) was applied followed by the Tukey test or t-test at a significant level of 5% (p<0.05). All statistical analyses were performed using GraphPad InStat (Software, Inc. 2003).
Results and discussionBacterial adhesion in monocultureInitially, the adhesion potential to SS of each of the species separately (L. casei, L. rhamnosus, E. coli O157:H7, S. enterica and L. monocytogenes) was tested. The results indicated the following: (1) both types of microbial groups (LAB and pathogens) can survive in both types of juice under the conditions tested, (2) they can adhere to SS, with a stronger potential for LAB strains compared to pathogens and (3) the adhesion degree depended on the type of juice being greater in G than in Ap juice.
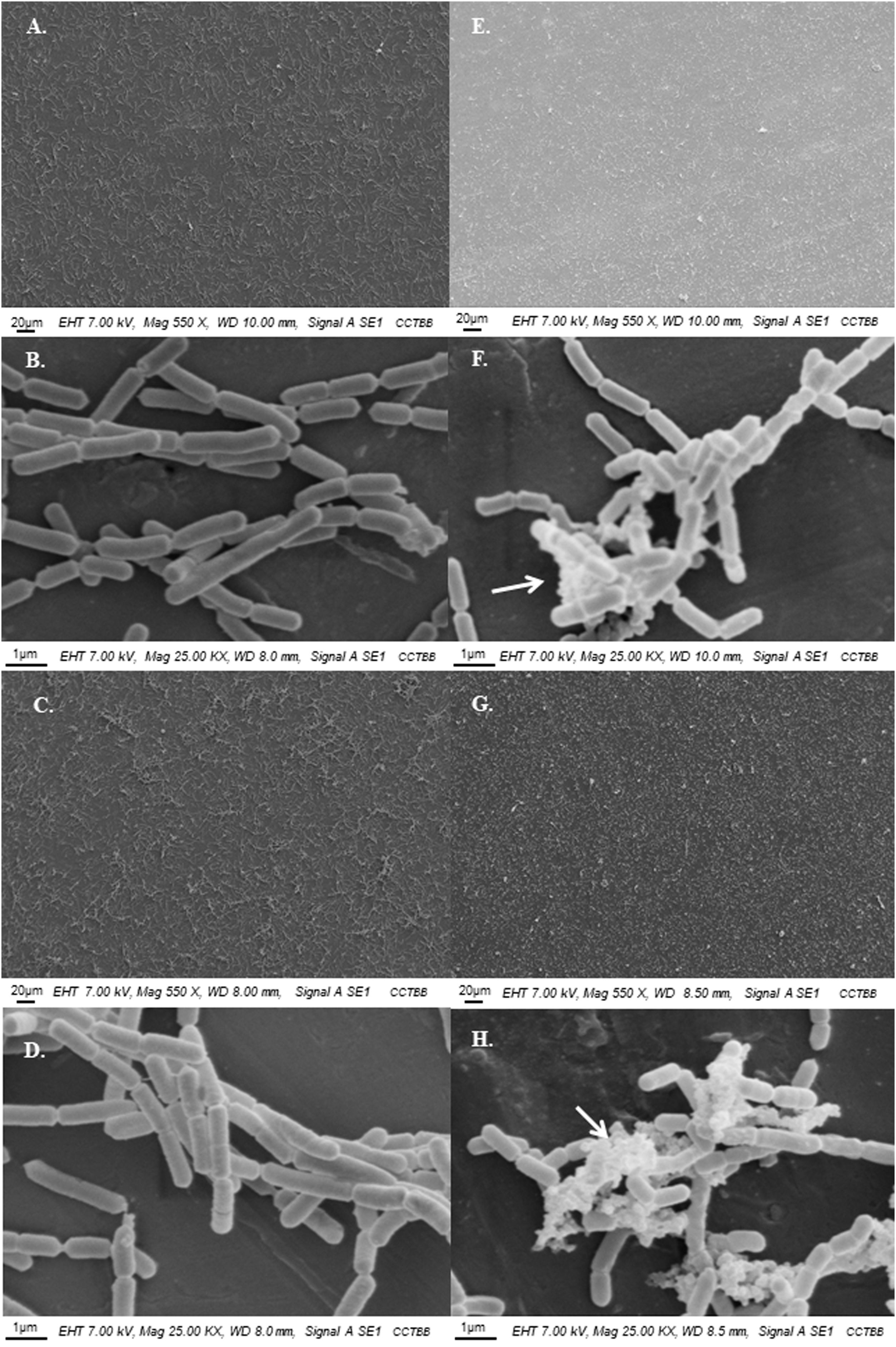
Lactobacillus casei was the species with the highest population of adherent cells with viable counts in Ap juice of 6.69±0.12log CFU/cm2 after 24h adhesion and 6.95±0.31log CFU/cm2 at 48h vs 7.08±0.06log CFU/cm2 and 7.48±0.26log CFU/cm2 in G juice after 24 and 48h adhesion, respectively. In the case of L. rhamnosus, counts ranged between 4.85±0.69log CFU/cm2 and 5.71±0.65log CFU/cm2 for 24 and 48h in Ap juice, and in G juice between 6.51±0.05log CFU/cm2 and 6.61±0.21log CFU/cm2, at 24 and 48h respectively. When analyzing their adhesion pattern by SEM (Fig. 1), the coverage potential could be confirmed with a homogeneous progression from 24 to 48h. This fact, together with the presence of extracellular polymeric substances (EPS) in the case of L. casei (Figs. F and H), is a clear indicator of the good adhesive capacity of both Lactobacillus species, thus suggesting that they could be good candidates to form protective biofilms on SS under juice production environments. Our findings support the hypothesis previously reported by Falagas and Makris13, in which they propose using non-pathogenic microorganisms as part of daily cleaning products to lower the incidence of pathogenic microorganisms, and also that reported by Toushik et al.44, which states the promising anti-biofilm properties of two LAB to control foodborne pathogens in the seafood safety research sector. In fact, the present study focuses on an unattended sector such as juice processing industries, seeking to identify a potential biocontrol strategy capable of reducing juice perishability by inhibiting foodborne pathogens.
SEM observations of the attached cells of lactic acid bacteria on stainless steel AISI 304 incubated at 25°C in 12°Brix apple juice. Left panel: L. rhamnosus ATCC 53103 and right panel: L. casei ATCC 393; (A, B, E, and F) 24h, (C, D, G, and H) 48h. Arrows show the presence of EPS.
In terms of the three pathogens tested, the highest adhesion potential was observed for S. enterica (2.81±0.47log CFU/cm2 [Ap]–4.53±0.45log CFU/cm2 [G]) followed by L. monocytogenes (2.48±0.74log CFU/cm2 [Ap]–4.33±0.19log CFU/cm2 [G]) and E. coli O157:H7 (1.85±0.21log CFU/cm2 [Ap]–3.89±0.04 log CFU/cm2 [G]). SEM analysis of the attachment capacity and adhesion patterns of the three pathogens has been previously described in studies conducted by the group1,41. As evidenced by the results, E. coli, S. enterica and L. monocytogenes can, not only tolerate the adverse conditions imposed, for example, by the acidic food matrix, deploying mechanisms of resistance to stress18,33, but also adhere to SS surfaces, implying a permanent and persistent risk of cross-contamination of products in contact with them. These results reinforce the ones achieved in previous reports1,40,41 of the group, where, combined, they establish the capacity of E. coli O157:H7, S. enterica and L. monocytogenes to adapt after short periods of time to moderate acidic conditions, such as the ones imposed by Ap juice, improving their survival in the food matrix. Even though, Just and Daeschel23, reported survival periods for E. coli O157:H7 and Salmonella spp. of up to 16 d in grape juice, a small amount of information is available concerning their adhesion and behavior on SS under conditions of relevance for juice-producing industries. What is more, to the best of our knowledge this is among the first and latest reports done on the survival and adaptation of these bacterial pathogens in G juice under simulated industrial conditions. The survival, adhesion and resistance reported in this work in Ap and G juice support the possibility that fresh juices without further processing could act as a vector for pathogens and, thus, the urge for their control in processing facilities where they are produced.
According to González-Pérez et al.20, who stated that the adaptation of bacteria to different niches often confers particular characteristics, the experimental results show that the conditions used (food matrix, surface and temperature) influenced the adhesion potential of the studied species, especially for pathogens, which are the target microorganisms. In view of the above, the importance of studying the dynamic interactions between the different groups of bacteria and the surfaces in each specific food processing environment is highlighted in order to provide more effective measures.
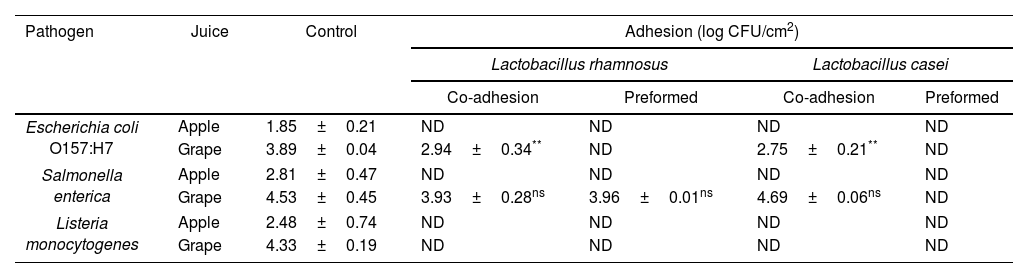
Effect of probiotic biofilms on pathogen sessile growth: competition (co-culture) and exclusion assays (preformed LAB biofilm)In order to evaluate the effect that LAB biofilms could have on the initial attachment steps of pathogens, evidence was provided on the growth of L. monocytogenes, E. coli O157:H7 and S. enterica in their sessile form under different circumstances such as competition (co-adhesion) and exclusion (pre-formed biofilm). As shown in Table 1, L. casei and L. rhamnosus were able to inhibit E. coli O157:H7, S. enterica and L. monocytogenes adhesion to SS in Ap juice to below the detectable limits under competition and exclusion. Similar to our study, Gómez et al.19, used in situ biofilms formed by potential probiotic LAB strains isolated from Brazilian charqui, goat cheese, ripened cheese and salami to inhibit pathogenic growth and found total inhibition of E. coli O157:H7, L. monocytogenes and Salmonella Typhimurium biofilm formation after 24, 48, and 72h of exposure using L. lactis 368, L. curvatus MBSa3 and L. sakei MBSa1 arguing that exclusion mechanisms could be responsible for the final result. Similarly, Woo and Ahn46, have shown that probiotic Lactobacillus strains (L. acidophilus KACC 12419, L. paracasei KACC 12427, L. casei KACC 12413, and L. rhamnosus KACC 11953) can effectively reduce biofilm formation by the foodborne pathogens S. enterica subsp. enterica serovar Typhimurium and L. monocytogenes, through competition, exclusion, and displacement mechanisms; even though, Rendueles and Ghigo36, reported that the production of inhibitory compounds may also act by delaying or inhibiting their growth. Moreover, Pérez-Ibarreche et al.31, using pre-colonized SS and polytetrafluoroethylene surfaces by L. sakei CRL1862 prevented the attachment of L. monocytogenes FBUNT cells, an Argentinian clinical isolate serotype 4b.
L. casei ATCC 393 and L. rhamnosus ATCC 53103 inhibition effect toward E. coli O157:H7, S. enterica, and L. monocytogenes adhesion and biofilm formation on stainless steel after co-adhesion in a 1:1 ratio, and incorporation to pre-adhered Lactobacillus spp. cells using apple and grape juice as food matrix.
| Pathogen | Juice | Control | Adhesion (log CFU/cm2) | |||
|---|---|---|---|---|---|---|
| Lactobacillus rhamnosus | Lactobacillus casei | |||||
| Co-adhesion | Preformed | Co-adhesion | Preformed | |||
| Escherichia coli O157:H7 | Apple | 1.85±0.21 | ND | ND | ND | ND |
| Grape | 3.89±0.04 | 2.94±0.34** | ND | 2.75±0.21** | ND | |
| Salmonella enterica | Apple | 2.81±0.47 | ND | ND | ND | ND |
| Grape | 4.53±0.45 | 3.93±0.28ns | 3.96±0.01ns | 4.69±0.06ns | ND | |
| Listeria monocytogenes | Apple | 2.48±0.74 | ND | ND | ND | ND |
| Grape | 4.33±0.19 | ND | ND | ND | ND | |
Results are expressed as log CFU/cm2±SD. Significant differences are expressed through the following nomenclature *p<0.05, **p<0.01, and nsnot significant. ND: not detected, below limit of detection.
In the case of G juice, complete elimination or at least significant reductions of E. coli and L. monocytogenes counts were achieved for both LAB in competition and exclusion assays. In addition, S. enterica evidenced a significant decrease when faced with L. casei pre-established biofilm. In contrast, both LAB failed to reduce the number of S. enterica sessile cells in competition assays (co-adhesion). Similarly, Kamal et al.24, observed that the addition of L. rhamnosus to yoghurt failed to inhibit S. typhimurium in co-culture until 72h of cold storage. Alvarez-Ordóñez et al.2, concluded that this behavior can be attributed to the phenotypic acid tolerance response of Salmonella spp. Several investigations have demonstrated that Salmonella spp. can survive in acidic foods at low pH values for long-term periods. Similar to the considerations taken into account by Cisneros et al.11, when analyzing the exclusion potential of certain LAB strains against enterohemorrhagic E. coli (EHEC) in a meat-based medium, the number of pathogenic cells attached, for example, to a stainless steel pipeline in a real scenario in juice processing industries would be significantly lower than the inoculum used, and therefore would be appropriate to hypothesize that LAB will be able to completely exclude E. coli, S. enterica and L. monocytogenes. In fact, the results obtained suggest that L. rhamnosus and L. casei are able to restrain the adhesion and proliferation of the three pathogens tested in Ap. Nevertheless, as evidenced in the case of G juice, the food matrix is a factor that must be taken into consideration before selecting the biocontrol strategy to be used as it is a factor that proved to influence the final outcome. As observed in G juice, L. casei prevented the proliferation of pathogens whereas the effect of L. rhamnosus depended on the pathogenic species to which it was confronted. Furthermore, and in line with the results obtained in the present study, Hascoët et al.22, and Toushik et al.44, advocated two possible hypotheses regarding the effect that a LAB preformed biofilm could have on pathogens: (i) non-specific interaction (competition, displacement and exclusion) hypothesis where the pre-established biofilms of other microorganisms affect the implantation of the pathogen on the surface by occupying the available space; and (ii) specific competition hypothesis in which pre-existing microorganisms affect the development of the pathogen biofilm by competing for nutrients or producing anti-microbial substances such as bacteriocins, anti-quorum sensing compounds, and enzymes, among others. On the other hand, the inhibitory performance of LAB according to Arena et al.4, could be due to the organic acids released by several lactobacilli that could also ascribed to or potentiate the effect over their anti-adhesive properties. Tejero-Sariñena et al.43, observed that the growth-inhibiting activity of different LAB, i.e., strains belonging to Lactobacillus, against pathogens such as S. typhimurium and E. coli was attributed to a pH reduction and/or to the production of organic acids, including lactate and acetic acid.
Effect of LAB over juice physicochemical propertiesData from Table 2 compiles the results obtained after evaluating the potential effect that L. casei and L. rhamnosus had on the food matrix. The parameters tested were the ones mostly observed as indicators of spoilage by this type of microorganisms when exposed to juice: pH, °Brix, TA and turbidity. No significant differences (p>0.05) were observed between controls and samples in contact with pre-adhered Lactobacillus cells. LAB species, such as lactobacilli, leuconostocs, and weissellas, have usually been associated with spoilage of fruit juices through the production of turbidity and excessive acidity35. On the contrary, no substantial changes to the food matrix were noted when using LAB. Indeed, LAB had aroused much interest as biocontrol agents on surfaces, packaging and as bioconservatives in the food itself26,27. The present study allowed to delve into the study of LAB as biocontrol agents against foodborne pathogens and their application as a “green” alternative in juice processing facilities, demonstrating an alternative growth and adhesion control of pathogens using an ecofriendly strategy through microbial implementation.
Physicochemical parameters of apple juice after exposure to L. casei ATCC 393 and L. rhamnosus ATCC 53103 biofilms.
| Condition | Contact time (h) | pH | Brix | Turbidity (NTU) | TA (% lactic acid) |
|---|---|---|---|---|---|
| Control | 3.59±0.05 | 10.43±0.60 | 0.23±0.12 | 3.22±0.09 | |
| L. casei | 24 | 3.65±0.09 | 10.20±0.42 | 2.95±1.52 | 2.37±0.77 |
| 120 | 3.45±0.21 | 10.50±0.71 | 0.43±0.17 | 4.27±1.30 | |
| 168 | 3.30±0.23 | 10.01±0.27 | 0.50±0.18 | 3.59±0.85 | |
| L. rhamnosus | 24 | 3.62±0.07 | 11.00±0.71 | 0.92±1.22 | 2.63±1.39 |
| 120 | 3.63±0.12 | 10.50±0.71 | 0.24±0.27 | 3.98±1.35 | |
| 168 | 3.53±0.04 | 10.00±0.41 | 0.49±0.06 | 3.02±0.38 | |
TA: titratable acidity.
Antimicrobial intervention technologies through the application of decontamination treatments or antimicrobial procedures for inhibition or reduction of microbial growth are gaining interest in order to reduce bacterial contamination8. Based on the data obtained in this study, L. casei and L. rhamnosus could be considered good candidates for applications as natural barriers or competitive-exclusion microorganisms to control E. coli, S. enterica and L. monocytogenes biofilm formation. The integral combination of factors such as target microorganism and nature of the food matrix clearly influences the performance that Lactobacillus strains have and thus might influence their effectiveness. In this sense, several studies conducted previously10,17,29,32 have highlighted the role of food matrices in both biofilm formation and efficacy of biocontrol treatments reinforcing the need to a deeper study, especially in juice production lines where small amount of information is available about biocontrol.
ConclusionsThis study highlights the potential that the implementation of a protective biofilm of LAB has to inhibit growth and adhesion of E. coli O157:H7, S. enterica and L. monocytogenes on surfaces commonly used in apple and grape juice production settings. Regardless of the type of juice used, L. rhamnosus and L. casei showed that can be considered good candidates for controlling the adhesion of these foodborne pathogen in juice-related industries, mainly when used in a protective biofilm form.
Even though both LAB showed that, under the conditions tested, they can be considered an ecofriendly alternative to mitigate the poor response that biofilms currently have to conventional control methods, further analyses should be performed to confirm that the application of these strains as potential biocontrol agents does not alter the sensory properties of the final products on an industrial scale.
Authors’ contributionsTarifa María Clara: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. Agustín María del Rosario: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. Brugnoni Lorena Inés: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Funding acquisition.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank the Agencia Nacional de Promoción Científica y Tecnológica, Ministerio de Educación, Ciencia y Tecnología de la República Argentina (PICT 2015 N̊ 0156), for funding this research.