Ceftobiprole is a fifth-generation cephalosporin with activity against methicillin-resistant Staphylococcus aureus (MRSA). This drug is currently approved in over 17 European countries and two Latin-American countries for the treatment of pneumonia, and skin and soft tissue infections4,5. The aim of this study was to determine ceftobiprole susceptibility in S. aureus isolates obtained in Mexico.
Isolates of S. aureus attributed to clinically significant specimens were collected from January 2019 to December 2021 from Hospital Universitario “Dr. José Eleuterio González” (HU) in Monterrey, and from Hospital Civil de Guadalajara “Fray Antonio Alcalde” (HC) in Guadalajara. Both are third-level teaching hospitals that offer healthcare to patients from northeastern and western regions of Mexico.
The strains were identified by MALDI-TOF MS (Bruker Daltonics, Bremen). In our study, susceptibility to ceftobiprole (Liofilchem) was determined by disk diffusion using cutoff values established by EUCAST guidelines3. Susceptibility to penicillin and cefoxitin (Oxoid Ltd.) was also determined by disk diffusion according to the CLSI breakpoints. Quality control strains were S. aureus ATCC 25923 and Escherichia coli ATCC 259222.
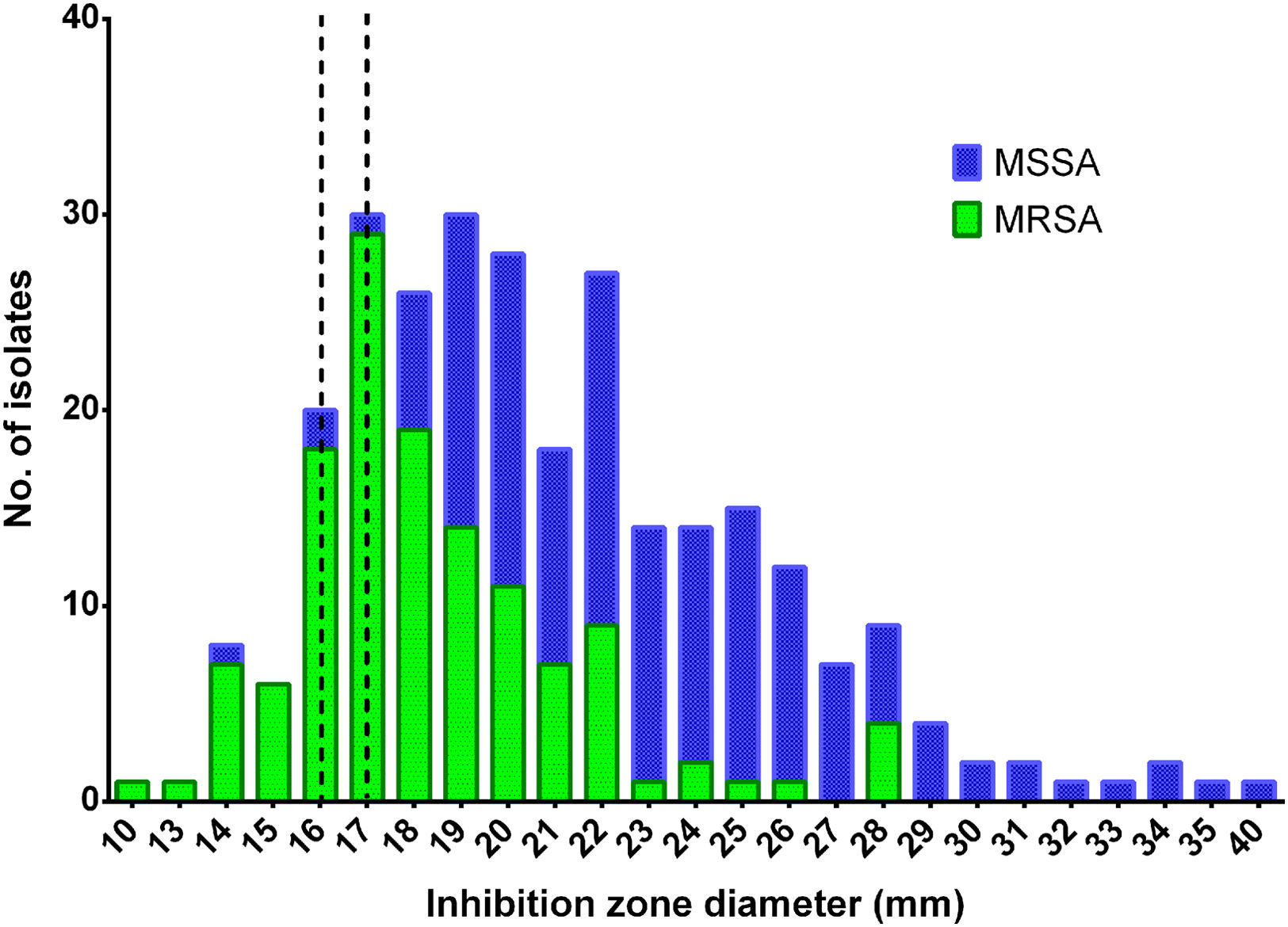
Our results show that of 280 strains identified as S. aureus, 69.6% (n=195) were isolated from HU and 30.4% (n=85) from HC. Of the total strains, 87.1% (n=244) were susceptible to ceftobiprole, 85.1% of which (n=166/195) were from HU and 91.7% (n=78/85) from HC. In addition, 17.9% of the strains exhibited an inhibition zone diameter between 16 and 17mm, which is considered an Area of Technical Uncertainty (ATU) by the EUCAST, indicating an area of interpretive difficulties and, therefore, higher complexity in ensuring reliable test results.
Among the strains tested, penicillin resistance was observed in 86.8% of the strains, with 46.8% identified as MRSA. Ceftobiprole susceptibility was observed in 74.8% of MRSA strains. On the contrary, 98.0% of methicillin-susceptible S. aureus (MSSA) strains were susceptible to ceftobiprole (Fig. 1) and 2.0% of MSSA isolates showed resistance to both ceftobiprole and penicillin. Of the 50 isolates exhibiting ATU (16–17mm) values to ceftobiprole, 94.0% were identified as MRSA.
Distribution of inhibition zone diameters to ceftobiprole for methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Methicillin-susceptible (blue) and resistant S. aureus (green) isolates are shown. The dotted line indicates the Area of Technical Uncertainty based on the criteria established by the EUCAST. Susceptibility cutoff values were ≥17mm for ceftobiprole.
In our current study, we observed that 87.1% of the analyzed strains showed susceptibility to ceftobiprole. In comparison, studies conducted in Europe1 and the United States5 reported higher susceptibility percentages of 99.7%. Moreover, susceptibility to ceftobiprole for MSSA in a study conducted in Europe1 was reported to be 100% among the 3498 isolates tested, whereas in our study, it was 98.0%. In vitro susceptibility results of ceftobiprole against MRSA were notably different in our study (74.8%) compared to previous studies conducted by Farrell et al.4, Pfaller et al.5 and Canton et al.1, in which ceftobiprole susceptibilities were 98.3%, 99.3%, and 99.5%, respectively. However, in these studies, different antimicrobial susceptibility testing methodologies were used. Furthermore, ceftobiprole resistance was not expected to be found in Mexican isolates because this drug has not been used in Mexico yet; therefore, patients have not been previously exposed to this drug.
The ceftobiprole resistance rates detected in this study were higher than 85% and, highlight the importance of antimicrobial susceptibility testing prior to ceftobiprole treatment.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interestsNone declared.
We are grateful to Xochitl Yañez-Melendez for technical support.