Pseudomycetomas are rare fungal subcutaneous infections caused by dermatophytes, which are mainly observed in immunocompromised patients. Mycobacterium genavense is considered an opportunistic pathogen in people living with HIV/AIDS (PLWHA), clinically resembling the presentation of Mycobacterium avium complex (MAC). Here, we describe the case of a 26-year-old PLWHA with a 3-month history of a 4cm tumoral, duroelastic and painful lesion located on the back. Histopathology of the tumoral lesion revealed chronic granulomatous inflammation with grains composed of PAS-positive and Grocott-positive septate hyphae, as well as acid-fast bacilli (AFB). Culture on Sabouraud and lactrimel agar developed colonies that were later identified as Microsporum canis. In successive samples, the AFB were identified as M. genavense by restriction analysis of PCR products. Immunocompromised PLWHA not only suffer increased susceptibility to diseases due to unusual pathogens but also atypical clinical presentation of frequently encountered pathogens.
Los seudomicetomas son infecciones fúngicas subcutáneas causadas por dermatofitos. Son infrecuentes; se observan, principalmente, en inmunocomprometidos. Mycobacterium genavense es considerado un patógeno oportunista en las personas afectadas por el virus de la inmunodeficiencia humana (VIH), su presentación es similar a las Mycobacterium avium complex (MAC). Presentamos el caso de una persona VIH de 26 años con una lesión tumoral de 4cm, de 3 meses de evolución, de consistencia duroelástica y dolorosa a la palpación, localizada en la espalda. En la histopatología se observó inflamación crónica granulomatosa, con granos constituidos por elementos compatibles con hifas tabicadas, tinciones PAS y Grocott positivas, así como bacilos ácido alcohol resistentes (BAAR). El cultivo en agar Sabouraud y Lactrimel evidenció colonias que se identificaron como Microsporum canis y, en sucesivas muestras, se identificaron BAAR como M. genavense por análisis de restricción de productos de PCR. El inmunocompromiso en las personas VIH ha provocado un aumento de las enfermedades por patógenos poco frecuentes, así como presentaciones inusuales de patógenos habituales.
Severely immunocompromised people living with HIV/AIDS (PLWHA) are at risk of suffering co-infections, which confound clinical manifestations and diagnostic procedures13.
Pseudomycetomas are rare fungal subcutaneous infections caused by dermatophytes, characterized by the presence of a chronic granulomatous or pyogranulomatous reaction surrounding fungal hyphae. They usually occur in immunosuppressed patients15. Several species of dermatophytes, including Microsporum canis, Trichophyton tonsurans and Trichophyton mentagrophytes have the ability to produce grains in tissues9. Although their clinical presentation is similar to that of eumycetomas, pseudomycetomas usually affect the scalp and lack a history of trauma for their inoculation9.
Mycobacterium genavense was first discovered in 1992 in a PLWHA from Geneva, hence its name. Since its discovery, it has been reported to cause disseminated infections in severely immunocompromised patients. Its clinical presentation in PLWHA resembles that of Mycobacterium avium complex, which is characterized by fever, weight loss and abdominal involvement7.
We report the case of a PLWHA who concurrently presented with disseminated pseudomycetoma caused by M. canis and disseminated M. genavense infection.
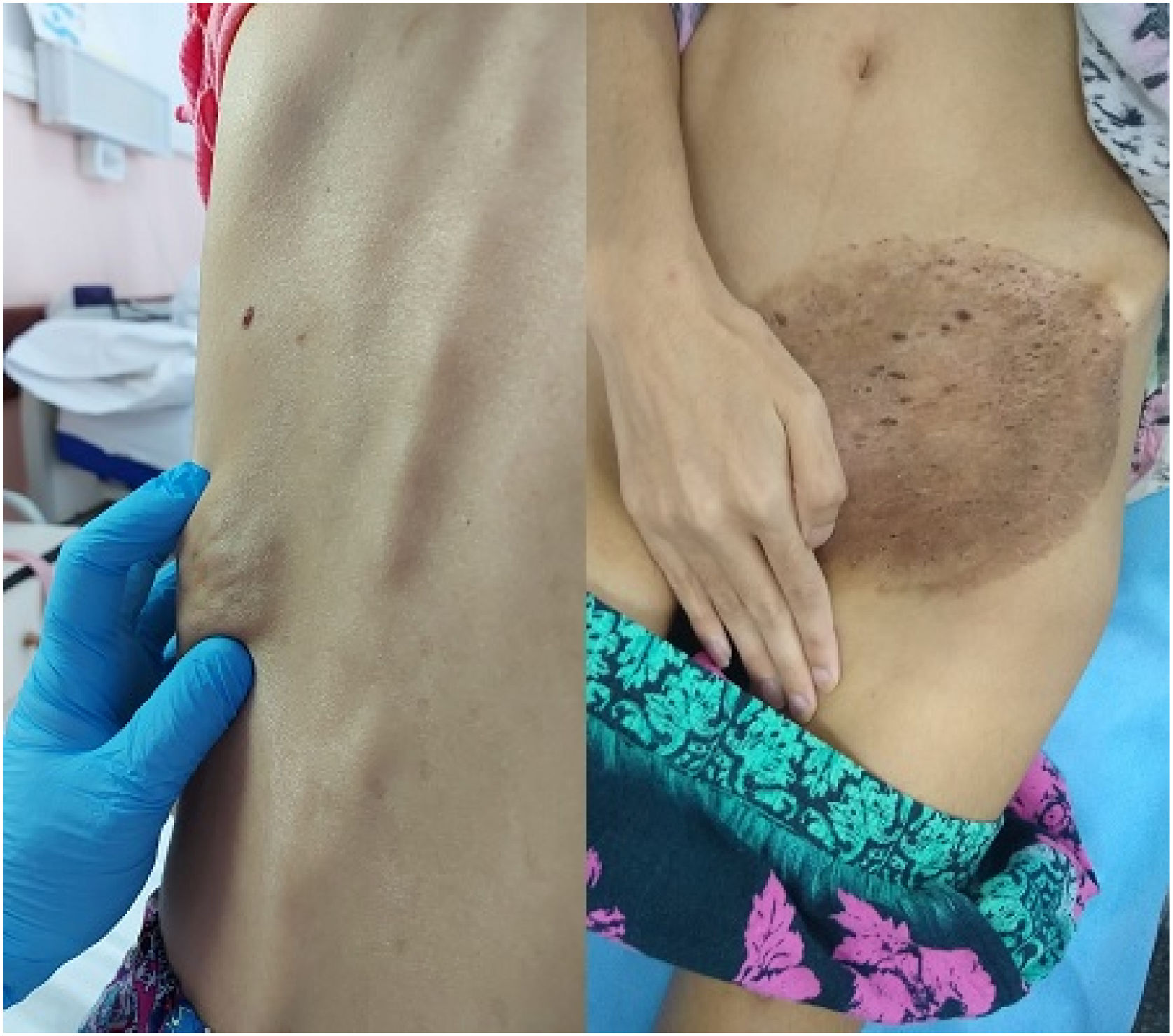
A 26-year-old female with congenital HIV infection and a history of poor adherence to antiretroviral treatment presented with a 3-month history of a tumoral lesion on her back. The lesion was approximately 4cm in diameter, and was soft and painful when touched. She also exhibited an extensive desquamative and pruritic plaque on her left groin (Fig. 1) and showed signs of a wasting syndrome. Her CD4 cell count was 19cells/ml, and her logarithmic HIV viral load was 4.7 log. She also had a history of chronic kidney disease, calcification of the abdominal aorta and femoral arteries, meningeal cryptococosis and tinea cruris caused by M. canis.
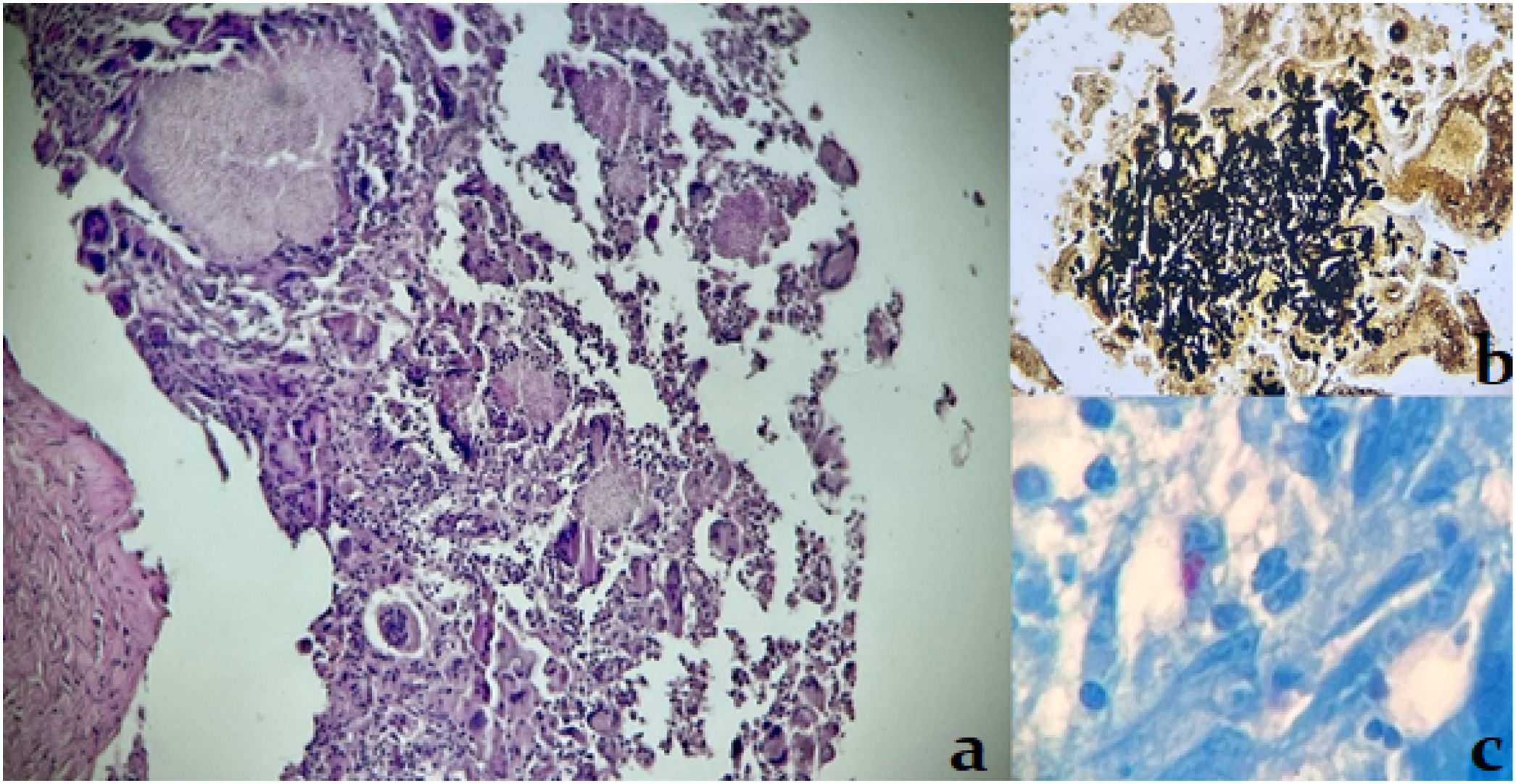
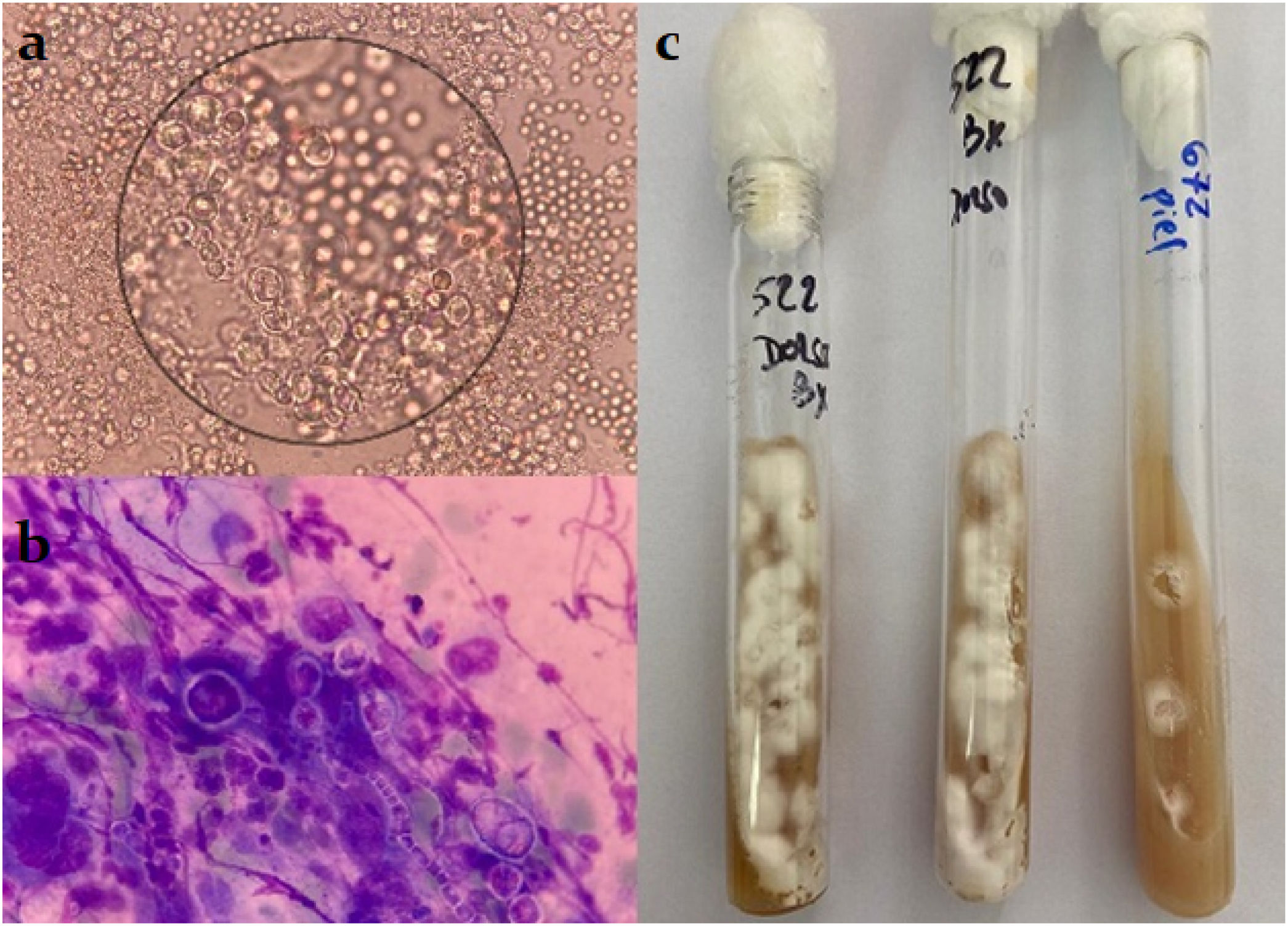
On ultrasonography, the tumoral lesion appeared hypoechoic and vascularized, with lobulated contours, with a thickness of 4.5mm, localized on the subcutaneous tissue. Hepatosplenomegaly and multiple hypoechoic mesenteric lymph nodes were also detected. A lesion biopsy revealed chronic granulomatous inflammation with grain-like structures composed of septate hyphae, as well as positive staining for acid-fast bacilli (Fig. 2). Hyaline, branched and septate hyphae were identified both in the microscopic examinations of wet mount preparations from the skin lesion samples and in the Giemsa stain from the same samples. The samples were cultured on Sabouraud and lactrimel agar, and incubated at 28°C and 37°C. The development of cottony, white colonies was observed on the culture at 28°C (Fig. 3). After the dissociation of these colonies, no fructification was observed. Molecular techniques, Malditof MS® and sequencing of ITS1 and ITS2 genes, identified the organism as M. canis. The samples were also cultured on Löwenstein Jensen and Stonebrink solid media and modified Middlebrook 7H9 liquid media for MGIT system, yielding no growth.
The tumoral lesion was surgically removed, and treatment with itraconazole and antitubercular drugs (isoniazid, rifabutin, ethambutol, pirazynamide and clarithromycin) was initiated, with poor adherence. New similar lesions developed on her forehead and left jaw area, with M. canis growing on culture after fine needle aspiration. The patient's clinical condition worsened, developing ascites, pancytopenia and bilateral pneumonia. She deteriorated further, experiencing septic shock that led her to her death. On direct examination of ascitic fluid, acid-fast bacilli were observed using the Ziehl-Neelsen stain. This sample was immediately cultured on Löwenstein Jensen and Stonebrink solid media, and modified Middlebrook 7H9 liquid media for MGIT system and no growth was observed in any of these media after 60 days of incubation. Later, a new sample of ascitic fluid was submitted to the laboratory to perform a direct PCR targeting a 439bp segment of the hsp65 gene. The obtained amplicon was subsequently digested by restriction enzymes BstEII and HaeIII. Restriction profiles analysis allowed for the identification of M. genavense. In addition, this identification was confirmed by the amplification and sequencing of the 16S rRNA gene in the same sample, exhibiting 100% identity with the standard strain M. genavense ATCC 51234.
Dermatophyte infection is almost exclusively a superficial cutaneous mycosis, usually confined to the stratum corneum of skin, nails and hair of normal hosts. Chronic inflammatory and invasive forms of dermatophytosis are the result of an intense hypersensitivity reaction to the fungal infection, more frequently observed in immunocompromised individuals2,5. Pseudomycetoma is an extremely rare mycosis, caused by the penetration of dermatophytes into the tissue, as a result of the rupture of infected follicular epithelium. Mycelium aggregates formed by the dermatophytes are denominated pseudograins, in contrast to grains in mycetomas4,8. M. canis appears to be the dermatophyte most frequently associated with pseudomycetoma9,14. As has been previously described, our patient did not have a history of trauma, and although in most case reports, pseudomycetoma involves the scalp, other areas of the body and even disseminated involvement were described in our patient's case11. Even though antifungal treatment is usually insufficient and surgical excision is necessary to resolve pseudomycetoma, in our patient poor adherence seemed to be the cause of the new lesions. Previous or concomitant dermatophytosis could raise suspicion of pseudomycetoma in immunocompromised individuals with a compatible clinical presentation.
Although M. genavense infection has been described in a number of PLWHA, skin and soft tissue involvement appears to be unusual. The first report of this type of clinical presentation was submitted by Fournier et al. in 1998, and few cases have been published since then1,6,7. In our case, the patient exhibited confirmed skin and soft tissue involvement by histopathology accompanied by more frequent abdominal, bone marrow and lymph node involvement. M. genavense identification is challenging as it does not grow on conventional solid and liquid media; therefore, it usually requires either molecular techniques for diagnosis3,10 or the use of supplemented culture media for fastidious mycobacteria species, which are not commonly available in routine diagnostic laboratories. Multiple samples from different tissues were directly observed and unsuccessfully cultured until M. genavense could finally be identified from an ascitic fluid sample. In severely immunocompromised PLWHA with compatible clinical features and acid-fast bacilli on direct examination with negative cultures, M. genavense should be suspected and molecular studies should be promptly performed. The broader use of this type of studies in recent years may increase the number of diagnosed M. genavense infections. In our institution, two additional cases were diagnosed in the last two years, with one of them being currently unpublished12.
Co-infection not only complicates the diagnosis of opportunistic infections in PLWHA, but also presents a greater challenge in treatment. Drug interactions may occur, in this case, rifampicin could not be used as treatment, requiring treatment with itraconazole, and the need of more pills poses a risk for adequate adherence.
In PLWHA with a low CD4 cell count, a high level of suspicion for multiple opportunistic diseases must be kept. Atypical clinical features and unusual microorganisms may be present, which might lead to the need of repeated samplings or more complex diagnostic procedures.
FundingNone declared.
Conflict of interestThe authors declare that they have no conflict of interest.