Candida bloodstream infections in children are of special concern in neonatal and pediatric intensive care and patients with comorbidities. This study aimed to estimate the incidence and risk factors associated with mortality in candidemia cases occurring in a public children's hospital in Ribeirao Preto, Brazil. It is a retrospective transversal study. Every patient under the age of 18 admitted to the study facility from January 1, 2013, to December 31, 2019, was considered potentially eligible to be included if they had candidemia. We collected clinical data from medical records. We included 113 blood cultures yielding positive results for Candida. The incidence rate was 2.12 per 1000 admissions. The most common Candida species was Candida parapsilosis. Septic shock during the candidemia episode was the only clinical outcome associated with a relative risk-adjusted (RRa) of 2.77 with an interval >1 (1.12–6.85). Our findings show that the incidence rate and mortality rates of candidemia are in line with those in other children's services in Brazil. We found a global mortality rate of 28.31% (32/113) from candidemia episodes. We highlight the predominance of non-albicans Candida species including C. parapsilosis. Septic shock was the most important factor showing a significant risk of mortality.
La infección del torrente sanguíneo por Candida es un tema importante en las unidades de cuidados intensivos neonatales y pediátricas, y en niños con comorbilidades. El objetivo de este estudio fue estimar la incidencia de candidemia en un hospital público infantil de Ribeirão Preto, Brasil, e identificar los factores de riesgo de mortalidad por dicha afección. Se hizo un estudio retrospectivo y transversal en el que se incluyeron todos los pacientes menores de 18 años con candidemia admitidos en el hospital desde el 1 de enero de 2013 hasta el 31 de diciembre de 2019. Los datos se obtuvieron de las historias clínicas. Hubo 113 hemocultivos positivos para Candida. La tasa de densidad de incidencia global fue de 2,12 por 1.000 admisiones. La especie de Candida más común fue Candida parapsilosis. El shock séptico en el episodio de candidemia, que tuvo un riesgo relativo ajustado de 2,77 y un intervalo >1 (1,12-6,85), fue el único factor de riesgo asociado a la mortalidad en este análisis. Nuestros hallazgos muestran una incidencia y una mortalidad similares que las informadas por otros servicios para niños en Brasil. La tasa de mortalidad global por candidemia fue del 28,31% (32/113). Destacamos el predominio de especies de Candida no albicans, incluida C. parapsilosis. El shock séptico fue el factor más importante que mostró un riesgo significativo de mortalidad.
Candida species are fungi that can naturally colonize the skin, gastrointestinal mucosa, and genitourinary tract. However, they can also become pathogenic agents depending on imbalances in their microbiota and their association with comorbidities4,8. Nevertheless, in specific cases, they can enter the bloodstream such as in patients who are immunosuppressed, in intensive care or have local infections, mainly due to the presence of invasive devices7.
The incidence of Candida bloodstream infection (BSI) in children varies among hospitals worldwide. Candida species have accounted for 10% of all BSIs in children younger than 18 years of age in the USA3. In a multicenter study in the USA, the incidence of candidemia in neonates, infants, and non-infants decreased between 2012 and 20151. In a different multicenter study, European institutions reported rates of 0.47 cases per 1000 hospital discharges3. For 11 years, the median number of episodes admitted per year was 125 (range: 95–166; IQR: 31) and there was no significant difference in the median number of candidemia cases reported during both studies (2005–2010 and 2011–2015)19. In Latin America, the incidence rate was 1.18 cases per 1000 admissions, with 44.2% of the cases occurring in children (23.7% younger than 1 year)13. In Brazil, national surveillance was conducted, showing an incidence rate of 2.49 cases per 1000 admissions and 0.37 cases per 1000 patient days, of which admissions, 32% were children, and 21% were younger than 1 year old2. Moreover, in Brazil, a retrospective, cross-sectional, observational, and analytical study was conducted on a series of pediatric patients with clinical and laboratory diagnoses of candidemia, including a total of 94 cases between March 2014 and September 2017. The incidence rate was 1.13 cases per 1000patients/day, with a mortality rate of 14%. There was a predominance of non-albicans Candida (NAC) (71.3%) in candidemia cases16.
Neonates and patients with immune dysfunctions are the main groups affected by candidemia, which is especially important in critically ill patients due to the risks imposed on them4. Therefore, identifying the main agents, risk factors, and other associated factors is essential for the propaedeutic determination of the follow-up of these patients. A small number of studies have analyzed mortality risk factors among children in Brazil. One of them, conducted in the southwest of the country, identified risk factors such as mechanical ventilation and dialysis. Clinical outcomes such as sepsis, septic shock, and comorbidities such as acute renal insufficiency were associated with increased mortality12. Another study, conducted in the south of Brazil, identified clinical characteristics such as male sex, stay in the intensive care unit, and thrombocytopenia. Comorbidities such as cardiovascular disease and renal insufficiency; and risks such as mechanical ventilation and dialysis were associated with increased mortality16. The early identification of risk factors, Candida species, and susceptibility to antifungals will enable the early implementation of adequate propaedeutics to ensure better outcomes for patients. So this study aimed to estimate the incidence and risk mortality factors of Candida BSIs in a children's public hospital of Sao Paulo, Brazil.
Materials and methodsDesignA retrospective study, evaluating the incidence, general characteristics, and mortality risk factors for Candida BSIs.
SettingThis study was conducted in a public-affiliated tertiary-care facility with 121 active beds in Ribeirao Preto, Sao Paulo. Between 2013 and 2015, it was conducted in a general hospital with 5 beds for children's intensive care, 2 beds for neonatal intensive care, and 2 oncology beds. Between 2015 and 2019, the children's hospital was inaugurated with 11 beds for infant intensive care and 20 beds for neonatal intensive care, 8 oncology beds, and 2 beds for blood marrow transplant therapy. The facility offers acute care for a direct population of 600000 and a reference population of 1.4 million, and exclusively admits patients referred by primary and tertiary healthcare services.
Study populationEvery patient under the age of 18 admitted to the study facility from January 1, 2013, to December 31, 2019, was considered potentially eligible for inclusion in the study if they had positive blood cultures caused by Candida. Patients could be included more than once in the study if they had two episodes of infection with an interval of at least one month between them. Asymptomatic patients having positive blood cultures for Candida, but no symptoms or signs of sepsis were excluded.
Data collectionOur starting point was the microbiological laboratory data bank. We screened the blood sample culture results during the study period. Genus/species identification was automatically performed on the Vitek® 2 system device prior to version 8.01 before 2017. Demographic and clinical data were collected from the patients’ medical records.
Group classification of the isolatesAll isolates were divided into two groups; the first group included the number of patient deaths within 30 days of Candida BSI diagnosis, while the second group included the number of patients who survived within 30 days of the episodes.
Data analysisWe analyzed the temporal global incidence of infections related to the 2013–2016 and 2017–2019 periods. We compared all the patients included regarding mortality risk factors by dividing them into two groups. We conducted a previous analysis of variables using a tree-based method to avoid confounding among various risk factors. Moreover, we included general characteristics, comorbidities, patient's category with traditional Candida risk factors, and others identified in other studies and clinical features12,15. The variables abdominal surgery, CVC placement, previous vancomycin use, previous broad-spectrum antibiotic use, previous azole exposure, and previous bacteremia were considered in the 30 days before Candida BSIs. The variables fasting, use of parenteral nutrition, use of dialysis, neutropenia, and mechanical ventilation were considered risk factors if present in a candidemia episode. Sepsis was defined as having at least two criteria of the quick sequential failure assessment (qSOFA) score adjusted for pediatric age, while septic shock was checked with the use of vasoconstrictor agents17. Global mortality rate was determined by the number of patient deaths within 30 days of the candidemia diagnosis.
Statistical analysisSimple and multiple log-binomial regression models were adjusted to estimate the relative, gross, and adjusted risks. The covariables considered in the multiple models were dialysis, prematurity, malignancy, mechanical ventilation, and shock. The software used was SAS 9.4.
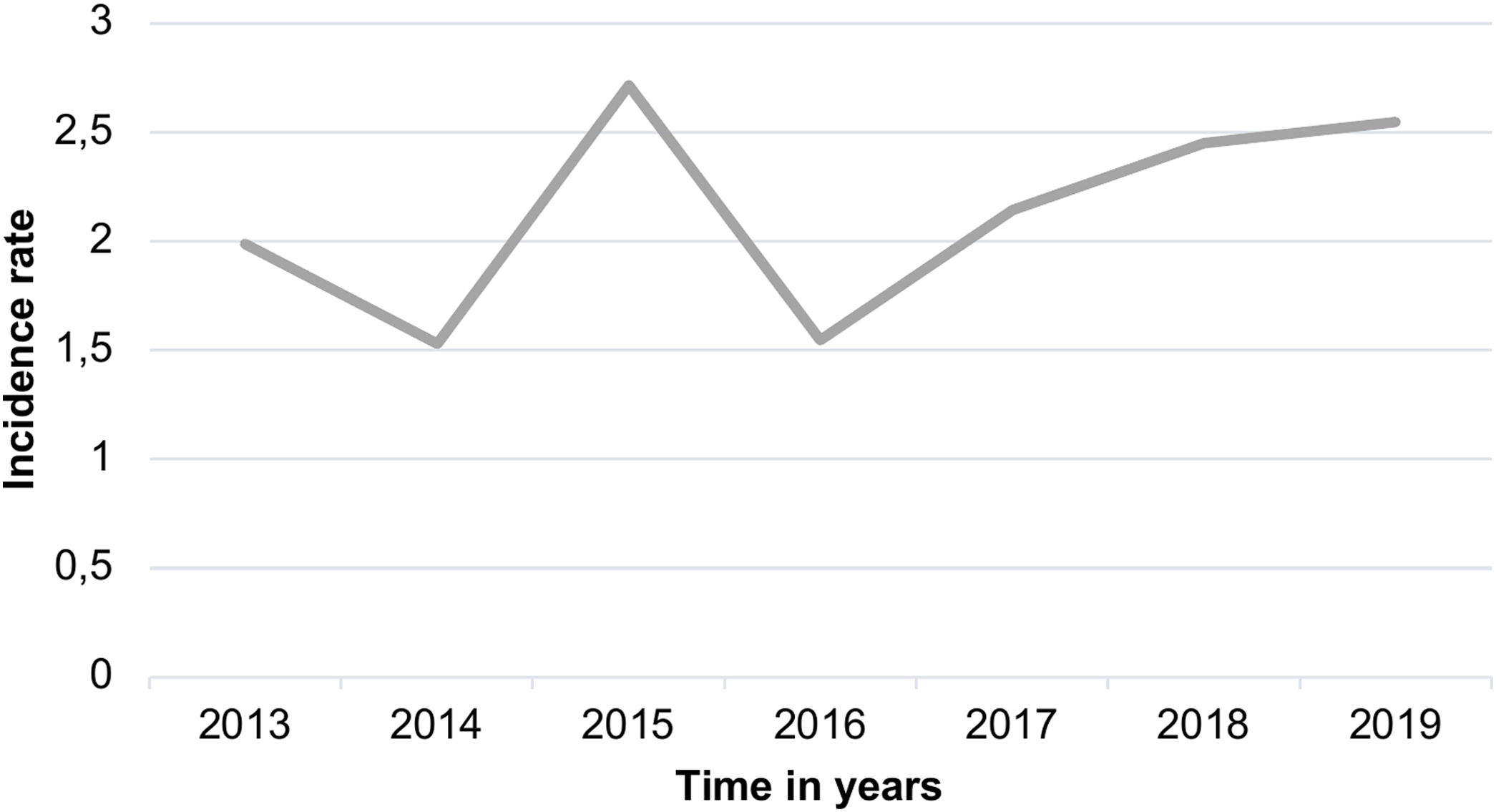
ResultsFrom the initially screened 115 blood cultures yielding a positive result for Candida in <18 years, 2 were excluded because the patients did not meet the clinical criteria for infection. The mean age of the patients was 36.2 months, and 56.6% of the patients were male. Therefore, 113 infection episodes were included in the study of 101 patients. The global mortality rate from candidemia episodes was 28.31% (32/113). Figure 1 shows the incidence rate per 1000 admissions during the study period. The incidence rate was 2.12 per 1000 admissions, which rose to 2.71 per 1000 admission in 2015. However, there were no significant differences in this incidence rate between the two periods (2013–2016) and (2017–2019) when using the incidence rate ratio (IRR) by person-year with a p-value of 0.285 (95% CI 0.565–1.183).
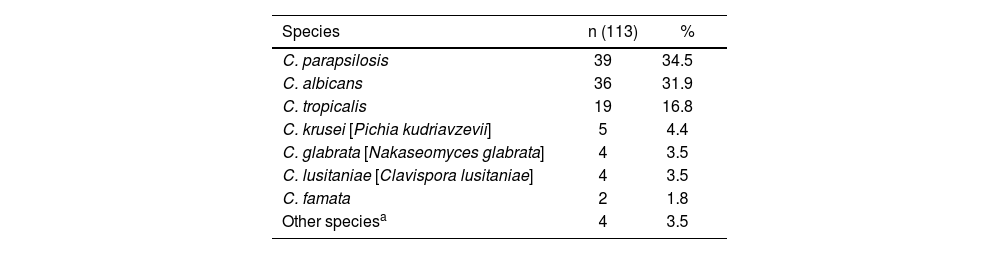
In the results (Table 1), of 113 positive blood culture samples, 11 species of Candida were isolated. Non-albicans Candida species (NAC) were the most frequent (64%). The distribution for other species was as follows: Candida parapsilosis was the most frequent (34.5%), followed by Candida albicans (31.9%), Candida tropicalis (16.8%), Candida krusei [Pichia kudriavzevii] (4.4%), Candida glabrata [Nakaseomyces glabrata] (3.5%), Candida lusitaniae [Clavispora lusitaniae] (3.5%), Candida famata (1.8%) and other rare species (3.5%). In one of the 113 positive samples, the Candida species was not identified due to technical reasons.
Species distribution of candidemia episodes identified by Vitek® 2.
| Species | n (113) | % |
|---|---|---|
| C. parapsilosis | 39 | 34.5 |
| C. albicans | 36 | 31.9 |
| C. tropicalis | 19 | 16.8 |
| C. krusei [Pichia kudriavzevii] | 5 | 4.4 |
| C. glabrata [Nakaseomyces glabrata] | 4 | 3.5 |
| C. lusitaniae [Clavispora lusitaniae] | 4 | 3.5 |
| C. famata | 2 | 1.8 |
| Other speciesa | 4 | 3.5 |
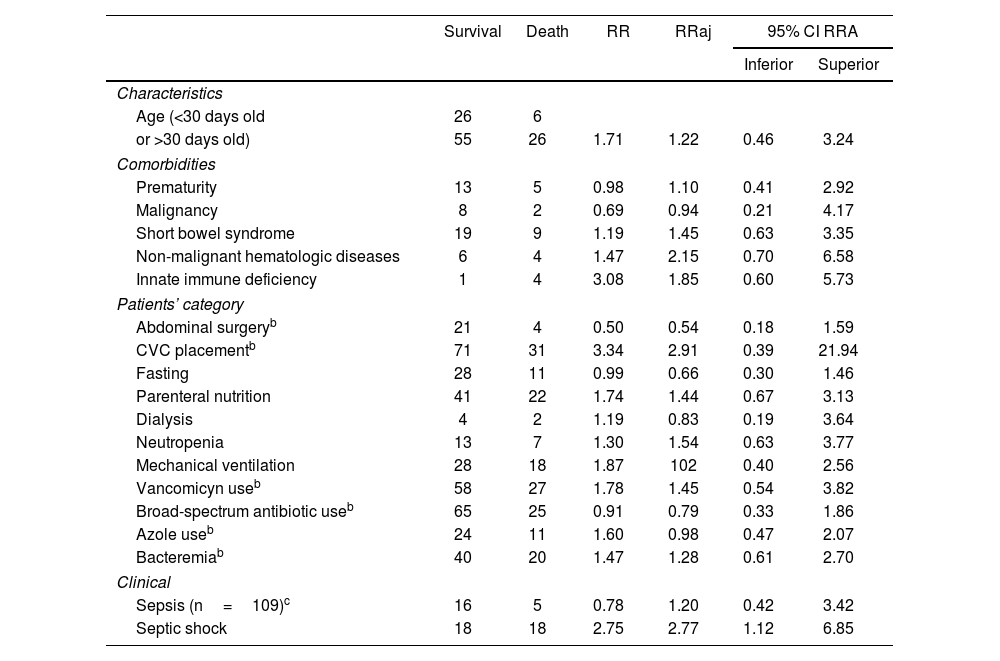
With regard to the mortality risk factors tested and highlighted in Table 2, we divided them into general characteristics, comorbidities, patient category, and clinical features. Septic shock during the candidemia episode was the only clinical outcome associated with a relative risk-adjusted (RRa) of 2.77 with an interval >1 (1.12–6.85).
Mortality risk factors of pediatric patients with candidemiaa
| Survival | Death | RR | RRaj | 95% CI RRA | ||
|---|---|---|---|---|---|---|
| Inferior | Superior | |||||
| Characteristics | ||||||
| Age (<30 days old | 26 | 6 | ||||
| or >30 days old) | 55 | 26 | 1.71 | 1.22 | 0.46 | 3.24 |
| Comorbidities | ||||||
| Prematurity | 13 | 5 | 0.98 | 1.10 | 0.41 | 2.92 |
| Malignancy | 8 | 2 | 0.69 | 0.94 | 0.21 | 4.17 |
| Short bowel syndrome | 19 | 9 | 1.19 | 1.45 | 0.63 | 3.35 |
| Non-malignant hematologic diseases | 6 | 4 | 1.47 | 2.15 | 0.70 | 6.58 |
| Innate immune deficiency | 1 | 4 | 3.08 | 1.85 | 0.60 | 5.73 |
| Patients’ category | ||||||
| Abdominal surgeryb | 21 | 4 | 0.50 | 0.54 | 0.18 | 1.59 |
| CVC placementb | 71 | 31 | 3.34 | 2.91 | 0.39 | 21.94 |
| Fasting | 28 | 11 | 0.99 | 0.66 | 0.30 | 1.46 |
| Parenteral nutrition | 41 | 22 | 1.74 | 1.44 | 0.67 | 3.13 |
| Dialysis | 4 | 2 | 1.19 | 0.83 | 0.19 | 3.64 |
| Neutropenia | 13 | 7 | 1.30 | 1.54 | 0.63 | 3.77 |
| Mechanical ventilation | 28 | 18 | 1.87 | 102 | 0.40 | 2.56 |
| Vancomicyn useb | 58 | 27 | 1.78 | 1.45 | 0.54 | 3.82 |
| Broad-spectrum antibiotic useb | 65 | 25 | 0.91 | 0.79 | 0.33 | 1.86 |
| Azole useb | 24 | 11 | 1.60 | 0.98 | 0.47 | 2.07 |
| Bacteremiab | 40 | 20 | 1.47 | 1.28 | 0.61 | 2.70 |
| Clinical | ||||||
| Sepsis (n=109)c | 16 | 5 | 0.78 | 1.20 | 0.42 | 3.42 |
| Septic shock | 18 | 18 | 2.75 | 2.77 | 1.12 | 6.85 |
The incidence of BSIs in children due to Candida varies among hospitals worldwide. The incidence rate in our study was higher than that reported in Latin America13 and slightly lower than the one reported in Brazil2. It should be highlighted that the Brazilian study had a limitation as the incidence rate included adults and children. The incidence rate in our study was 2.12 per 1000 admissions. National Brazilian surveillance data showed an incidence rate of 2.49 cases per 1000 admissions and 0.37 cases per 1000patients/day, of which 32% of the cases were children2. Furthermore, in South Brazil, two studies involving pediatric patients reported incidence rates of 1.13 cases per 1000patients/day16 and 0.23 cases per 1000patients/day12.
Candida species identification is very important to determine the best approach and treatment for candidemia. Historically C. albicans used to be the most frequent Candida species causing BSIs. However, with the increasing use of azoles, the NAC species has recently become an important concern in the hospital environment8,18. In our study, NAC species were the most frequent, C. parapsilosis being the most common species identified. In Brazil, similar studies in children have confirmed this trend11,14.
In addition to treatment, we should focus on infection control strategies for preventing candidemia in pediatric patients, including rigorous hand hygiene, strict environmental cleaning protocols, hospital-wide implementation practices for central-line insertion and maintenance bundles that ensure full sterile barrier precautions, meticulous skin preparation during line insertion, and daily consideration of catheter necessity10.
Resistance to widespread and tested antifungals proved to be low in other national and international studies15. Our study found 4.8% of resistance rates to fluconazole, 1% resistance to amphotericin B, and non-resistance to echinocandins.
Some risk factors have been studied for a long time, especially among patients hospitalized for prolonged periods and those in pediatric intensive care units. They are at the highest risk for candidemia, and unfavorable outcomes associated with it20,21. In neonatal intensive care, some studies have shown risk factors such as the use of antibiotics for >2 weeks, maternal vulvovaginitis candidiasis, repeated tracheal intubation, and the use of glycopeptides9, and worse outcomes when compared to children patients. In very-low-birth-weight neonates, mechanical ventilation, intubation, use of carbapenem antibiotics, total parenteral nutrition, and prolonged hospitalization have shown to be predictors of candidemia5,6. Nonetheless, our study included a small number of candidemia episodes in neonates and did not show evidence of an increased mortality risk factor due to candidemia in these patients.
In Brazil, classic mortality risks factors for candidemia in neonatal and pediatric patients are the use of a central venous catheter, mechanical ventilation, parenteral nutrition, diagnosis of neoplastic diseases, and use of antibiotics with coverage for anaerobes or vancomycin for more than three days12,16. Our findings only showed similarity with septic shock when using amines but did not confirm other conditions as risk factors. It is also not possible to lose sight of the fact that patients with the conditions described above are usually in critical condition and have multiple factors associated with worse prognosis.
Some limitations of our study include its lack of originality, the small sample size, the short-follow up time, and possible inconsistencies in the collected information due to the retrospective nature of the study. The inauguration of the children's hospital during our study might have complicated the comparison of incidence due to changes in the hospital structure. In addition, the identification of Candida was performed by Vitek® 2 system prior to version 8.01 before 2017, which was unable to identify important species such as Candida auris and some cryptic species. Other limitations had been discussed earlier.
ConclusionThe knowledge of the hospital's flora distribution is extremely important for the implementation of infection control strategies within the institution. Our study highlighted the predominance of NAC species, including C. parapsilosis. Moreover, other factors could also be analyzed and together they could help us to draw up guidelines for treating candidemia cases in critically ill patients. Considering that patients diagnosed with candidemia are typically severally ill, requiring intensive care and being equipped with multiple invasive devices, the related factors emphasize the necessity of implementing infection control strategies to effectively prevent candidemia and mitigate unfavorable outcomes.
Conflict of interestThe authors declare that they have no conflicts of interest.