Endovascular interventions in the superficial femoral artery for the treatment of peripheral arterial occlusive disease have increased over the last decades. The first- and second-generation stents in the superficial femoral artery have failed to demonstrate improved patency of the treated vessel due to high fracture rates. The aim of this study was to evaluate the clinical, short-term outcomes of using third-generation superflexible nitinol stents in the treatment of atherosclerotic lesions in the superficial femoral artery.
MethodsThis was a retrospective study carried out in a single center, from June 2013 to May 2014. A total of 27 patients underwent angioplasty with third-generation, superflexible nitinol stents in atherosclerotic lesions of the superficial femoral artery.
ResultsThe mean age was 68 ± 12 years, 55.6% were females, and 74.1% were diabetics. Patients were classified as TASC B and C in 77.7% of cases. Technical success was 100%. There was an increase in the ankle-brachial index from 0.35 ± 0.1 before the intervention to 0.75 ± 0.2 at hospital discharge. The mean follow-up of patients was 6.7 ± 2.3 months. The primary patency rate was 96.3%. The limb salvage rate was 100%. There were no stent fractures documented by X-rays.
ConclusionsAngioplasty with third-generation superflexible nitinol stent placement was shown to be effective in the treatment of atherosclerotic lesions of the superficial femoral artery.
As intervenções endovasculares na artéria femoral superficial para o tratamento da doença arterial oclusiva periférica têm crescido nas últimas décadas. A primeira e a segunda geração de stents na artéria femoral superficial falharam em demonstrar a melhora da perviedade do vaso tratado, devido às altas taxas de fratura. O objetivo deste estudo foi avaliar os desfechos clínicos no curto prazo com o uso de stents de nitinol superflexíveis de terceira geração no tratamento de lesões ateroscleróticas na artéria femoral superficial.
MétodosTrata-se de um estudo retrospectivo, realizado em único centro, no período de junho de 2013 a maio de 2014. Um total de 27 pacientes foi submetido à angioplastia com stents de nitinol superflexíveis de terceira geração em lesões ateroscleróticas da arterial femoral superficial.
ResultadosA média de idades foi de 68 ± 12 anos, 55,6% eram do sexo feminino e 74,1%, diabéticos. Os pacientes foram classificados em TASC B e C em 77,7% dos casos. O sucesso técnico foi de 100%. Houve aumento do índice tornozelo-braquial de 0,35 ± 0,1 pré-intervenção para 0,75 ± 0,2 na alta hospitalar. O seguimento médio dos pacientes foi de 6,7 ± 2,3 meses. A taxa de patência primária foi de 96,3%. A taxa de salvamento de membro foi de 100%. Não ocorreram fraturas de stent documentadas por raios X.
ConclusõesA angioplastia com uso de stent de nitinol superflexível de terceira geração demonstrou ser efetiva no tratamento das lesões ateroscleróricas da artéria femoral superficial.
Endovascular interventions for the treatment of peripheral arterial occlusive disease (PAOD) have grown exponentially over the past few decades.1 Around 40% of these procedures are performed in the femoral segment.2 However, despite the large number, these interventions still remain a challenge for the interventionists due to the biomechanical forces exerted by the muscle compartments on the vessel wall, promoting metal fatigue and stent fracture, in addition to restenosis.3
The first- and second-generation stents in the superficial femoral artery failed to demonstrate improved patency of the treated vessel when compared to conventional surgery, as even with the second-generation nitinol stents, fracture rates reach up to 20%.3,4
This study aimed to evaluate the short-term clinical outcomes of using superflexible third-generation nitinol stents in the treatment of atherosclerotic lesions in the superficial femoral artery.
MethodsType of study and populationThis was a retrospective, longitudinal, observational study carried out at a referral center for cardiovascular diseases from June 2013 to May 2014. The study included patients of both genders, with limiting intermittent claudication, pain at rest, or ulceration in the affected limb, with lesions limited to the superficial femoral arteries and with at least one leg artery left for distal run-off. Patients who had a history of severe allergy to iodinated contrast, significant atherosclerotic disease in aortoiliac territories, and with creatinine clearance < 30mL/kg/minute were excluded from the procedure.
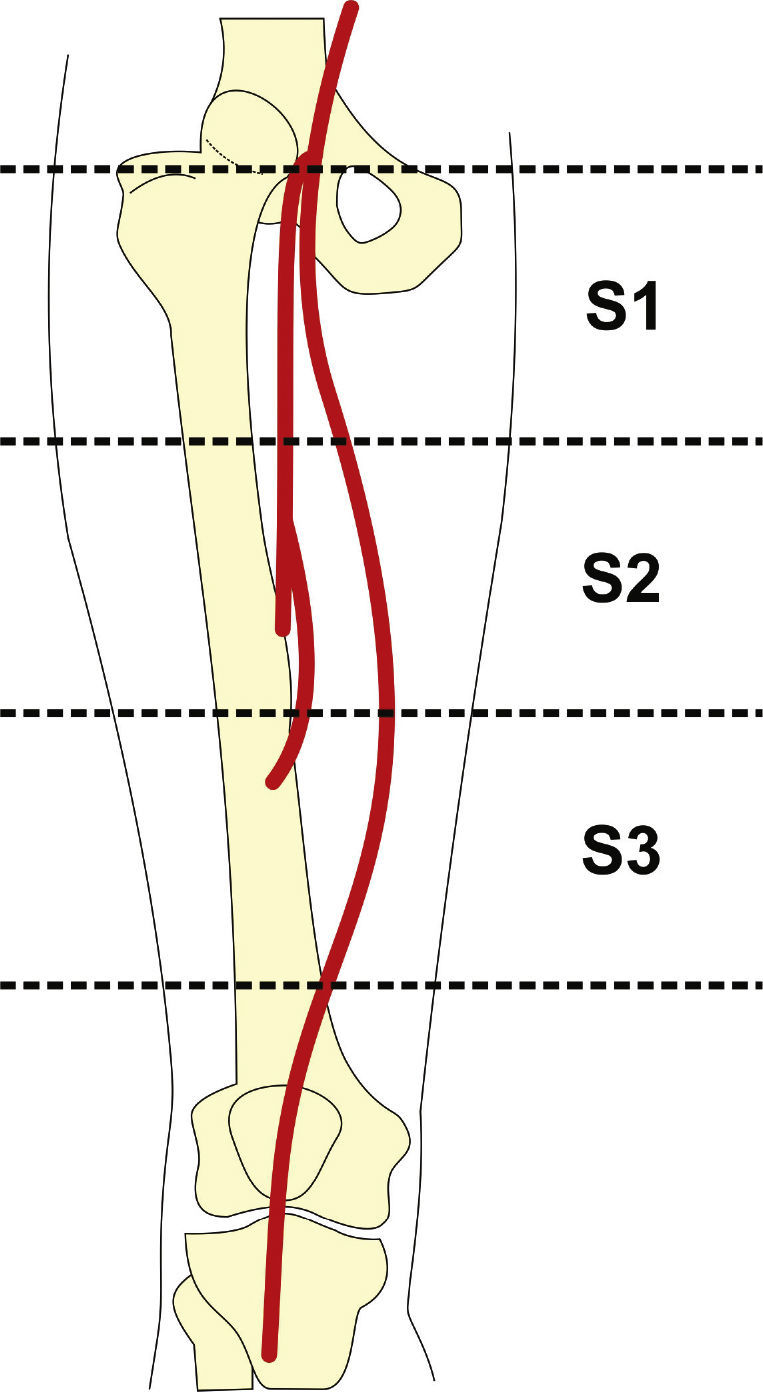
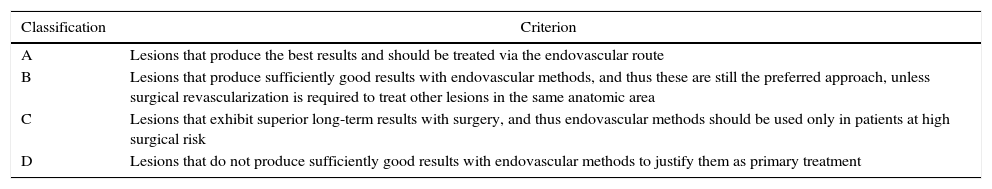
Preoperative arteriography was used to classify lesions according to: (1) the Trans-Atlantic Inter-Society Consensus II (TASC- II) A, B, C, and D criteria5 (Table 1); (2) the type of lesion (stenosis, occlusion, dissection, or restenosis); and (3) the location of the lesion in relation to the superficial femoral artery segments (Fig. 1).
Classification of lesions according to the Trans-Atlantic Inter-Society Consensus II (TASCII).
| Classification | Criterion |
|---|---|
| A | Lesions that produce the best results and should be treated via the endovascular route |
| B | Lesions that produce sufficiently good results with endovascular methods, and thus these are still the preferred approach, unless surgical revascularization is required to treat other lesions in the same anatomic area |
| C | Lesions that exhibit superior long-term results with surgery, and thus endovascular methods should be used only in patients at high surgical risk |
| D | Lesions that do not produce sufficiently good results with endovascular methods to justify them as primary treatment |
All procedures were performed in the Endovascular Intervention Center of Instituto Dante Pazzanese de Cardiologia. The patients received acetylsalicylic acid 100mg and clopidogrel 75mg daily, 3 days before the procedure. Clopidogrel was maintained for at least 30 days and acetylsalicylic acid was maintained indefinitely.
Patients were treated under local anesthesia. Antibiotic prophylaxis was performed with 1.5g of cefuroxime at the time of anesthesia induction. The preferred approach was through the ipsilateral common femoral artery for antegrade puncture, using a 6 F Prelude® valved sheath (Merit Medical Systems, South Jordan, USA). In failing to use this access route, or when it was not possible to cross the target lesion, the retrograde access through popliteal artery puncture was chosen, using a valved 5 F Prelude® sheath. Target lesions were crossed through luminal or subintimal route using a 0.035’ 150cm Radiofocus® hydrophilic guidewire (Terumo Interventional Systems, Somerset, USA) together with an MPA-1 5 F and/or STR 4 F diagnostic catheter (Cordis Corporation, Warren, USA). Pre-dilation was performed in cases of occlusion or when adequate stent positioning was not possible. The third-generation, superflexible sinus-SuperFlex (Optimed, Ettlingen, Germany) nitinol stent or Innova® self-expanding system (Boston Scientific®, Maple Grove, USA) were used in all cases.
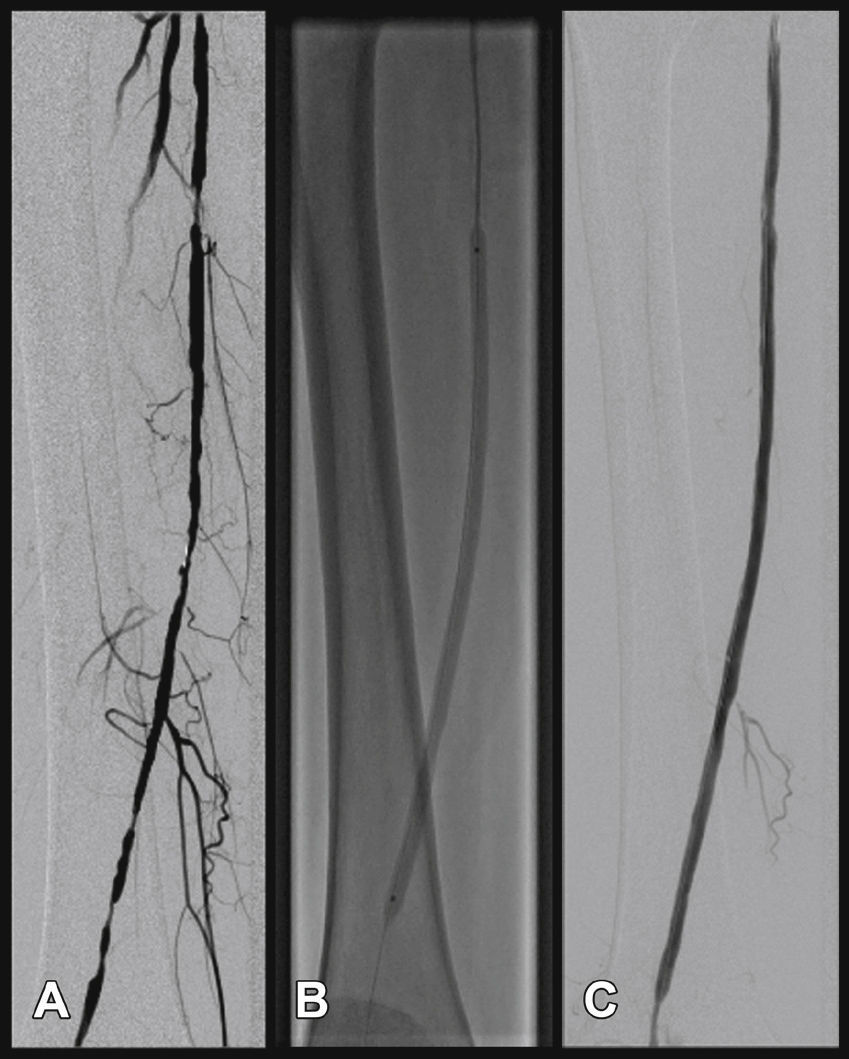
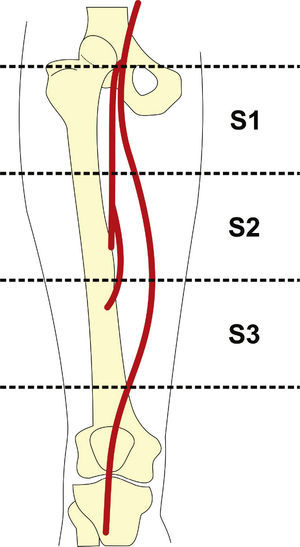
Radiographic control (Fig. 2) were conducted with a Siemens® Artis Flat Panel device or in the hybrid room, with a Siemens® Artis zeego Hybrid device.
The immediate postoperative period was carried out in the infirmary in all cases, and local hemostasis was performed by manual compression for 40minutes.
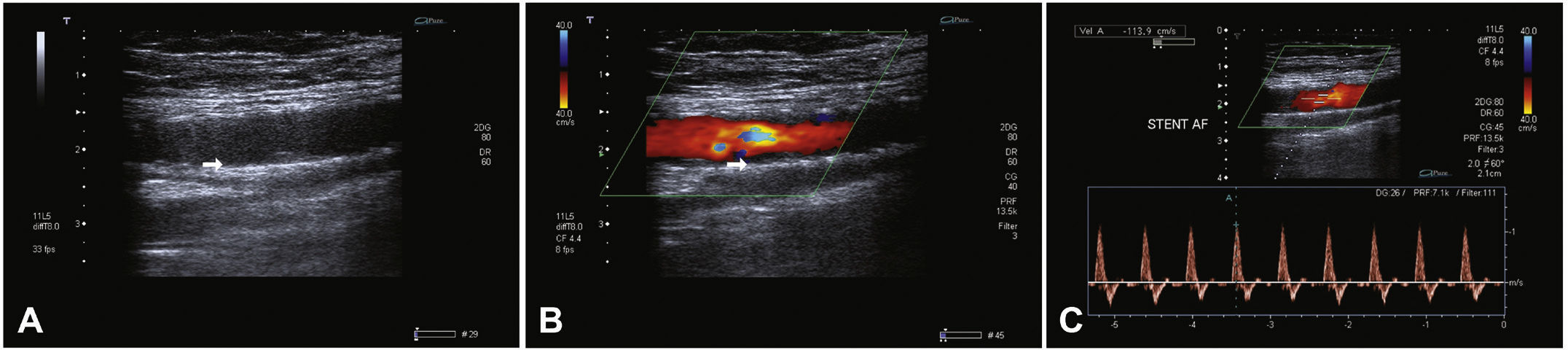
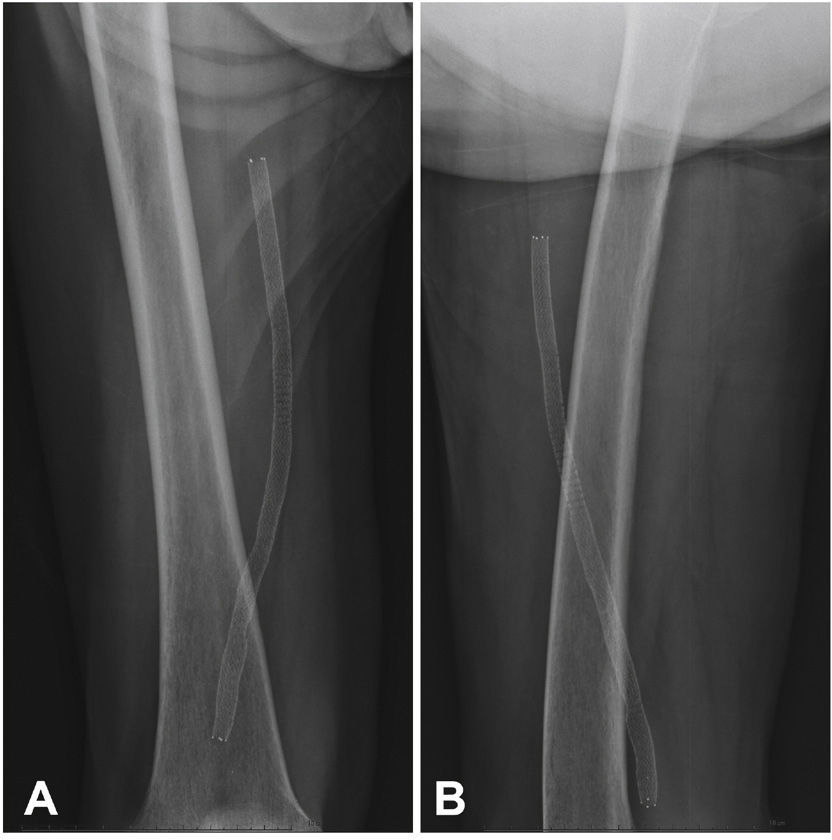
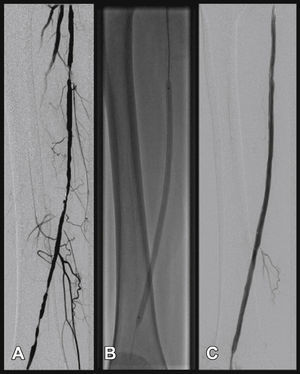
Postoperative follow-upPatients were followed through outpatient assessment with physical examination and ankle-brachial index (ABI) measurement at 15, 30, 90, and 180 days after the procedure. Control with Doppler ultrasound (USG-D) was performed at 30, 90, and 180 days after surgery, aiming to identify restenosis (Fig. 3). Radiographs of the knee joint in the posteroanterior and lateral views were performed at 30 and 180 days, aiming to identify stent fractures (Fig. 4).
The analyzed outcomes were: (1) immediate technical success, when the target lesion was treated as previously planned, with residual lesion < 30% in the angiographic control; (2) primary patency, which indicates uninterrupted permeability after the revascularization procedure; (3) secondary patency, which expresses the cases in which a new intervention is performed to open the occluded vessel after the primary procedure; (4) perioperative morbimortality for deaths and complications recorded up to 30 days postoperatively; (5) major amputations: transfemoral and transtibial amputations; (6) restenosis, for in-stent lesions > 50% at USG-D, with peak systolic velocity > 200cm/s or pre and post-stenosis velocity ratio ≥ 2; (7) fractures, for disconnection or twisting of stent meshes; and (8) limb salvage rate.
Continuous variables were expressed as means ± standard deviations and qualitative variables, as absolute values and percentages.
ResultsA total of 81 patients underwent angioplasty and stenting in the femoral artery segment, of whom 27 received third-generation superflexible nitinol stents and constituted the study population. In 16 cases (59.3%), the Innova® self-expanding stent system was used.
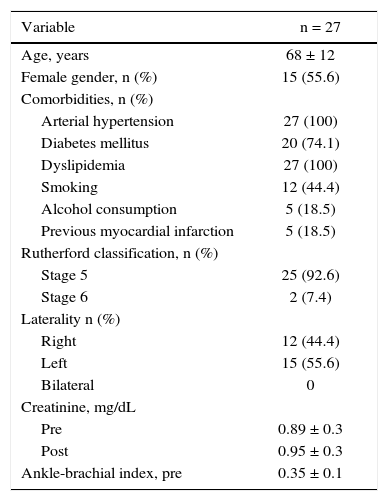
The demographic characteristics, comorbidities, and treatment indications are described in Table 2. The mean age was 68 ± 12 years; 55.6% were females, and 74.1% were diabetics. The left leg was the most frequently treated (15 cases, 55.6%) and most had ulceration, with little tissue loss (92.6%) (Rutherford 5). There were no cases of treatment for intermittent claudication.
Basal clinical characteristics.
| Variable | n = 27 |
|---|---|
| Age, years | 68 ± 12 |
| Female gender, n (%) | 15 (55.6) |
| Comorbidities, n (%) | |
| Arterial hypertension | 27 (100) |
| Diabetes mellitus | 20 (74.1) |
| Dyslipidemia | 27 (100) |
| Smoking | 12 (44.4) |
| Alcohol consumption | 5 (18.5) |
| Previous myocardial infarction | 5 (18.5) |
| Rutherford classification, n (%) | |
| Stage 5 | 25 (92.6) |
| Stage 6 | 2 (7.4) |
| Laterality n (%) | |
| Right | 12 (44.4) |
| Left | 15 (55.6) |
| Bilateral | 0 |
| Creatinine, mg/dL | |
| Pre | 0.89 ± 0.3 |
| Post | 0.95 ± 0.3 |
| Ankle-brachial index, pre | 0.35 ± 0.1 |
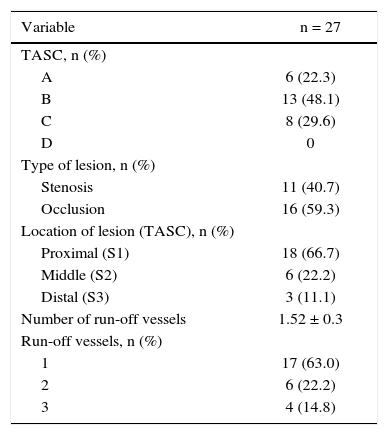
Patients were classified as TASC B and C in 77.7% of cases. No patient was classified as TASC D. Regarding the location, two-thirds of the lesions were in the distal segment of the superficial femoral artery (S3). In over half of the cases (59.3%), stents were implanted in previously occluded vessels and pre-dilation was carried out in all these lesions (Table 3).
Angiographic characteristics of lesions.
| Variable | n = 27 |
|---|---|
| TASC, n (%) | |
| A | 6 (22.3) |
| B | 13 (48.1) |
| C | 8 (29.6) |
| D | 0 |
| Type of lesion, n (%) | |
| Stenosis | 11 (40.7) |
| Occlusion | 16 (59.3) |
| Location of lesion (TASC), n (%) | |
| Proximal (S1) | 18 (66.7) |
| Middle (S2) | 6 (22.2) |
| Distal (S3) | 3 (11.1) |
| Number of run-off vessels | 1.52 ± 0.3 |
| Run-off vessels, n (%) | |
| 1 | 17 (63.0) |
| 2 | 6 (22.2) |
| 3 | 4 (14.8) |
TASC: Trans-Atlantic Inter-Society Consensus.
When evaluating the run-off bed, most patients had only one patent artery (59.3%), and the fibular artery was the most frequently found. A mean of 1.52 ± 0.3 pervious artery per treated limb was obtained.
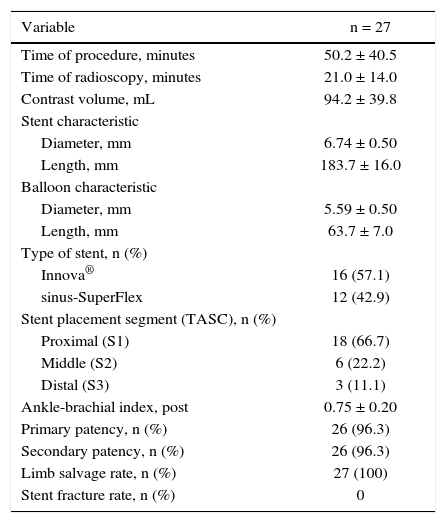
Target lesion revascularization was achieved using only one stent in 26 cases, with a technical success rate of 100%. The use of two stents was necessary in one case. The mean length of lesion coverage was 183.7 ± 16mm (120 to 200mm). The mean balloon diameter was 5.59 ± 0.5mm, with a mean length of 63.7 ± 7mm (40 to 100mm). It was necessary to carry out pre-dilation of the stenotic target lesion in only one patient, due to difficulties in guidewire progression. Twenty-one (75%) 7-mm diameter stents and seven (25%) 6-mm diameter stents were used. There were no cases of intraoperative embolization. The most frequent stent landing zones were the distal segment of the superficial femoral artery (S1) in 18 cases (66.7%), the middle segment (S2) in 6 cases (22.2%), and in 3 cases (11.1%), stent implantation occurred in the proximal segment (S3) (Table 4).
Characteristics of the procedure.
| Variable | n = 27 |
|---|---|
| Time of procedure, minutes | 50.2 ± 40.5 |
| Time of radioscopy, minutes | 21.0 ± 14.0 |
| Contrast volume, mL | 94.2 ± 39.8 |
| Stent characteristic | |
| Diameter, mm | 6.74 ± 0.50 |
| Length, mm | 183.7 ± 16.0 |
| Balloon characteristic | |
| Diameter, mm | 5.59 ± 0.50 |
| Length, mm | 63.7 ± 7.0 |
| Type of stent, n (%) | |
| Innova® | 16 (57.1) |
| sinus-SuperFlex | 12 (42.9) |
| Stent placement segment (TASC), n (%) | |
| Proximal (S1) | 18 (66.7) |
| Middle (S2) | 6 (22.2) |
| Distal (S3) | 3 (11.1) |
| Ankle-brachial index, post | 0.75 ± 0.20 |
| Primary patency, n (%) | 26 (96.3) |
| Secondary patency, n (%) | 26 (96.3) |
| Limb salvage rate, n (%) | 27 (100) |
| Stent fracture rate, n (%) | 0 |
TASC: Trans-Atlantic Inter-Society Consensus.
The procedure and fluoroscopy times were, respectively, 50.2 ± 40.5minutes (20 to 240minutes) and 21.0 ± 14minutes (5 to 53minutes). The mean volume of iodinated contrast was 94.2 ± 39.8mL. In one case, distal retrograde access was performed in the popliteal artery due to the impossibility of crossing the target vessel using the antegrade technique. No complications were observed at the puncture site.
Patient follow-upThe mean follow-up of patients was 6.7 ± 2.3 months (4 to 10.4 months). There were no perioperative deaths or deaths related to the procedure.
The limb salvage rate was 100%. There were no major amputations during the follow-up period. There was an increase in ABI from 0.35 ± 0.1 preoperatively to 0.75 ± 0.2 at the time of hospital discharge.
The primary patency rate was 96.3%. Stent occlusion was observed in only one case. In this patient, there was superficial femoral artery dissection during the initial procedure and thus a new stent was implanted in the dissected segment on the seventh day post-procedure; anticoagulation was chosen. However, on the 14th day after the initial procedure, the USG-D showed a new stent occlusion. The clinical treatment was chosen for this patient, who showed progressive healing of the lesion, not requiring further intervention. This event was responsible for a secondary patency rate of 96.3% in the present population. There were no stent fractures documented by radiography of the knee joint.
DiscussionThe endovascular treatment of the superficial femoral artery has shown a significant development in recent years.6 The continuity with the popliteal artery and the common femoral artery exposes the superficial femoral artery to stretching forces, and its trajectory and interaction with the surrounding muscles expose it to twisting and compressive forces.7 These forces, acting on the vessel wall, can result in material fatigue and stent fracture.8 The interaction of the vessel wall with the metal also triggers a potent inflammatory response, resulting in myointimal hyperplasia.9 The complexity of the inflammatory response and the diversity of forces to which the artery is subjected result in the main limitation of endovascular treatment of PAOD: restenosis.10 This has motivated the technological improvement of materials to achieve longer-lasting results.
The first generation of stents was balloon-expandable. Their high radial strength and little flexibility failed to properly comply with the biomechanical stress to which this artery is exposed.11,12 The failure of this material in the treatment of PAOD led to the development of self-expanding, metal-alloy stents with thermal memory – the nitinol stents. These stents are more flexible, and have shown greater resistance and stability to repeated biomechanical stress. Therefore, several randomized studies have demonstrated that the nitinol stent is the state-of-the-art in the treatment of PAOD in the femoropopliteal segment.1,13–19 However, even with their greater flexibility, fracture rates can reach up to 20%.3 The fracture rates and the consequent restenoses led to the development of the third-generation stents, which had the stent cell interconnection structures modified, resulting in greater flexibility and lower fracture rates (Table 5).6,20–22
Fracture rates in recent studies of percutaneous intervention with second- and third-generation nitinol stent in the superficial femoral artery.
| Studies | Year | Stent | n | Lesion length (cm) | Primary patency in 12 months | Fracture rate (%) |
|---|---|---|---|---|---|---|
| SIROCCO3 | 2006 | SMART | 46 | 8.1 | 68.1 (2 years) | 20 |
| SUPERA 5006* | 2013 | Supera | 490 | 12.6 | 83.3 | 0 |
| RESILIENT14 | 2010 | Life stent | 134 | 7.1 | 81.3 | 3.1 |
| FAST15 | 2007 | Luminexx 3 | 101 | 4.5 | 68.3 | 12 |
| SUMMIT20* | 2013 | Epic | 100 | 7 | 85.1 | 0 |
| COMPLETE SE21* | 2014 | Complete SE | 196 | 6.1 | 72.6 | 0 |
| MISAGO 222 | 2012 | Misago | 744 | 6.4 | 87.6 | 3.1 |
| DURABILITY 20024 | 2011 | Everflex | 100 | 24.2 | 64.8 | 6 |
* Studies with third-generation nitinol stents.
Three of the most recent studies have shown better results with the newest generation of stents. The SUMMIT20 study was a prospective, multicenter trial of the self-expanding, laser-cut nitinol stent EPIC® (Boston Scientific, Maple Grove, USA), which showed a 15.7% restenosis rate, with primary patency of 92% and no fractures in the radiographic follow-up in the one-year period. The COMPLETE SE21 multicentric study, using the Complete SE stent (Medtronic, Minneapolis, USA), showed primary patency rate of 72.6%, with a restenosis rate of 8.4% and no evidence of fractures in the 1-year follow up. The SUPERA 5006 study assessed 490 patients using the SUPERA® stent (IDEV Technologies Inc, Webster, USA), resulting in a primary patency of 83.3% and no fractures in the 1-year follow-up period of 304 stents with radiographic follow-up. In the present study, the use of two types of third-generation, superflexible nitinol stents led to a primary patency rate of 96.3%, with an occlusion rate of 3.7% due to the dissection of the femoral artery in the initial procedure, and no signs of significant restenosis or fracture in the 6 month follow-up observed with the USG-D and X-rays, respectively.
Currently, the only available data is from the TASC II5 consensus recommending the femoropopliteal angioplasty as the first choice in the categories TASC A and B; as a second option for the TASC C category; and not recommended for category D. However, this consensus already shows a significant gap due to the remarkable evolution of techniques and materials in recent years. It is a growing opinion that the TASC II needs to be revised, especially regarding angioplasty indications of AFS, a view shared by the present authors. In this study, 18 cases (66.7%) were classified as TASC A and B, but in 9 cases (33.3%), angioplasty with third-generation stent was performed in TASC C arteriographic lesions as the first option. Goltz et al.23 observed that TASC A and B patients obtained 79% of primary patency and those with TASC C and D obtained 68% of patency at 1 year. Of this group, 87% of patients had previous occlusion, and the rate of procedure-related complications was 7.5%. In the DURABILITY-200 study,24 100 patients were assessed, of whom 71% were treated for intermittent claudication and 29% for critical ischemia, with lesions classified as TASC C and D, in the femoropopliteal segment, obtaining a primary patency rate of 85.4% at 6 months and of 64.8% at 12 months. In the present study, most stents were implanted in the distal segment (S3) of the superficial femoral artery. We observed primary and secondary patency of 96.3% at six months of follow-up in a population consisting of patients with critical ischemia Rutherford stages 4 and 5. In the subgroup of patients TASC C, there were no occlusion events or restenosis during the 6 month follow-up, and no TASC D patient was treated.
During the clinical follow-up of patients, there was an improvement in the ABI from 0.35 ± 0.1 preoperatively to 0.75 ± 0.2 at the time of hospital discharge, in addition to a limb salvage rate of 100%. ABI measurement may have been overestimated due to the high prevalence of diabetes in the present population, because the prevalence of arterial calcification in the distal segments of the leg arteries of diabetic patients overestimates the measurement of this index.25
Study limitationsThe small number of cases, the heterogeneous group of treated segments in the superficial femoral artery and the short clinical follow-up, may have compromised the results of the present study. Additionally, the accuracy of the results may have been affected by the retrospective analysis of data.
ConclusionsIn the present study, angioplasty using a third-generation superflexible nitinol stent was shown to be effective in the treatment of atherosclerotic lesions of the superficial femoral artery. The patency rates in the treated artery demonstrate the need for stringent clinical follow-up of these patients in the medium and long terms.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.