The Xience VTM everolimus-eluting stent is a new generation drug-eluting stent (DES) that incorporates a low profile cobalt-chromium platform (81μm) and a highly biocompatible polymer (fluoropolymer), which carries and controls the release of everolimus. Recent studies have demonstrated sustained safety and efficacy of the Xience VTM in the treatment of real-world populations. Our aim was to report the clinical results of 12 months of the BRAVO Brazilian protocol.
MethodsThe BRAVO Registry was a prospective, non-randomized, single-arm, multicenter (25 centers) study that evaluated the late clinical results of 535 minimally selected patients treated with the drug- eluting stent Xience VTM in Brazilian daily practice.
ResultsOverall, 40% of patients had diabetes, 25% prior myocardial infarction, and 42% presented with acute coronary artery syndrome. The majority of lesions (69%) was highly complex (ACC/AHA type B2 or C). The mean length and the nominal stent diameter were 19.9 ± 5.3mm and 3.0 ± 0.4mm, respectively. The angiographic and procedural successes were 99.7 and 98%, respectively. At 12 months, the cumulative rate of major adverse cardiac events, available in 100% of patients, was 5.6% (cardiac death: 1.3%; acute myocardial infarction: 3.0%; revascularization of the target lesion: 2.2%). Stent thrombosis occurred in 5 patients (0,9%), and only 1 case was reported between 6 and 12 months.
ConclusionsThe drug-eluting stent Xience VTM demonstrated sustained safety and efficacy up to 12 months in the treatment of complex coronary lesions in patients from daily practice.
O stent liberador de everolimus XIENCE V® é um stent farmacológico de nova geração que incorpora uma plataforma de cromo-cobalto de baixo perfil (81μm) e um polímero de elevada biocompatibilidade (fluoropolímero), o qual carreia e controla a liberação do fármaco everolimus. Estudos recentes demonstram segurança e eficácia sustentadas do dispositivo XIENCE V® no tratamento de populações da prática clínica. Nosso objetivo foi reportar resultados clínicos de 12 meses do protocolo brasileiro BRAVO.
MétodosO registro BRAVO foi um estudo prospectivo, não randomizado, de braço único, multicêntrico (25 centros), que avaliou os resultados clínicos tardios de 535 pacientes minimamente selecionados, tratados com o stent farmacológico XIENCE V®.
ResultadosCerca de 40% dos pacientes tinham diabetes, 25% infarto agudo do miocárdio prévio e 42% apresentaram-se com síndrome coronária aguda. A maioria das lesões (69%) era de elevada complexidade (ACC/AHA tipo B2/C). As médias da extensão e do diâmetro nominais dos stents foram, respectivamente, 19,9 ± 5,3mm e 3,0 ± 0,4mm. Os sucessos angiográfico e de procedimento foram de 99,7% e 98%, respectivamente. Aos 12 meses, a taxa cumulativa de eventos cardíacos adversos maiores, disponível em 100% dos pacientes, foi de 5,6% (morte cardíaca: 1,3%; infarto agudo do miocárdio: 3,0%; revascularização da lesão-alvo: 2,2%). Já a trombose de stent ocorreu em cinco pacientes (0,9%), sendo reportada apenas uma ocorrência entre 6 e 12 meses.
ConclusõesO stent farmacológico XIENCE V® demonstrou segurança e eficácia sustentadas ao final de 12 meses no tratamento de lesões coronárias complexas em pacientes da prática diária.
In general, the new generation of drug-eluting stents (DES) has shown high efficacy and safety in the treatment of patients with coronary lesions in daily practice.1–3 Compared with older DES,4,5 these devices are characterized by the presence of smaller profile metallic components, drug carrier systems with optimized biocompatibility and potent antiproliferative agents.6–13The XIENCE VTM stent (Abbott Vascular, Santa Clara, USA) incorporates an 81μm thick cobalt-chromium platform with a non-thrombogenic polymer (fluoropolymer), which carries and controls the everolimus release.6 Recent studies with the XIENCE VTM stent, including real-world populations, have shown low occurrence of major adverse cardiac events (MACE) and late and very late stent thrombosis.14–19Specifically in the 6 month follow-up of BRAVO registry (BRazil XIENCE VTM Everolimus-Eluting Coronary Stent System “Real-World” Outcomes Registry), which evaluated 535 patients, the cumulative rates of MACE (4.3%), cardiac death (1.1%) and stent thrombosis (0.8%) were relatively low, especially considering a complex Brazilian cohort treated at multiple centers in daily practice.20 However, long-term follow-up data are still needed to better assess the impact of this device in our population. Thus, our objective was to report the clinical results of the 12 month follow-up of patients included in the BRAVO protocol.
MethodsProtocol and procedureThe protocol details and the initial results of the BRAVO registry had been previously reported.20 In short, this was a prospective, non-randomized, single-arm, multicenter (25 centers), national, post-marketing clinical trial, which used a validated electronic data capture system, independently managed by a Representative Organization of Clinical Research in São Paulo (SP). The objective of the study was to assess the late clinical outcomes in minimally selected patients (exclusion: saphenous graft and life expectancy < 24 months) undergoing percutaneous coronary intervention (PCI) with XIENCE VTM DES in Brazilian daily practice. Patients with at least one lesion with stenosis ≥ 50%, located in a native coronary artery with favorable anatomy for PCI with stenting were included. In general, PCI procedures were performed according to current guidelines,21 and the final strategy was left to the operator's discretion. The available stents had the following measurements: 2.5, 3.0 and 3.5mm in diameter, and 8, 12, 15, 18, 23 and 28mm in length. In relation to dual antiplatelet therapy (DAPT) recommendations after the procedure, aspirin was prescribed indefinitely and thienopyridine (clopidogrel) was prescribed for at least 12 months.
Clinical outcomes and definitionsThe primary study outcome was the occurrence of MACE in the 12 months of follow-up. The considered late clinical outcomes included target-lesion revascularization (TLR) at 6 and 12 months, stent thrombosis and major bleeding up to 12 months. The composite MACE outcome was defined as cardiac death, acute myocardial infarction (AMI) and TLR. Target-vessel revascularization (TVR) included cases of TLR.
Stent thrombosis was defined according to the criteria of the Academic Research Consortium (ARC).22 Major bleeding followed the Thrombolysis in Myocardial Infarction (TIMI) criteria, including intracranial hemorrhage or absolute decrease ≥ 5g/dL in hemoglobin concentrations or absolute decrease ≥ 15% in hematocrit levels.23
Clinical follow-up was scheduled at 30 days, 6, 12 and 24 months post-procedure and consisted of medical consultations or telephone contact carried out according to a predefined protocol. A total of 535 patients were enrolled between September 2008 and September 2010, and all (100%) completed the follow-up at 12 months.
All adverse events had their information directly verified in the source documentation and were later adjudicated by an independent Clinical Events Committee, which included three experienced professionals from the Clinical and Invasive Cardiology areas. For the current analysis, clinical results were reported up to 12 months.
Statistical analysisCategorical variables are shown as frequencies and percentages. Quantitative variables are shown as mean and standard deviation.
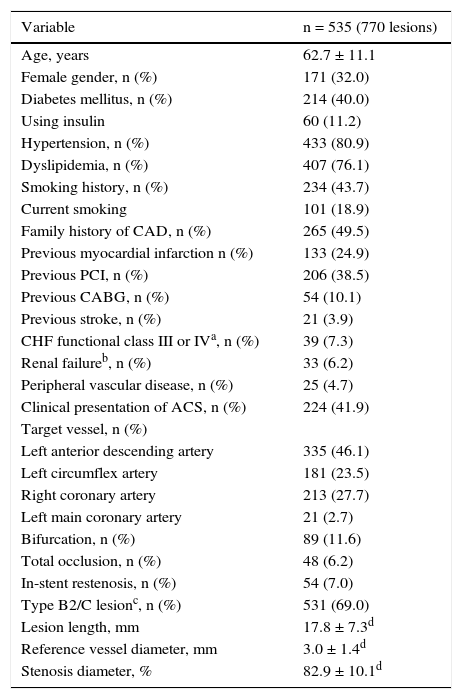
ResultsBaseline and procedure characteristicsThe baseline clinical and angiographic characteristics are shown in Table 1. Overall, 40% of patients had diabetes, 25% had a previous AMI, and 42% had acute coronary syndrome (ACS), including 29% with unstable angina, 9% with AMI without ST-segment elevation, and 4% with AMI with ST-segment elevation. Regarding the PCI procedure, 14% received multiple stents, 42% had post-dilation with balloon catheter and the mean nominal length and nominal diameter of the stents were, respectively, 19.9 ± 5.3mm and 3.0 ± 0.4mm. At the end of the procedure, the angiographic and procedure success were 99.7% and 98%, respectively.
Baseline clinical and angiographic characteristics.
| Variable | n = 535 (770 lesions) |
|---|---|
| Age, years | 62.7 ± 11.1 |
| Female gender, n (%) | 171 (32.0) |
| Diabetes mellitus, n (%) | 214 (40.0) |
| Using insulin | 60 (11.2) |
| Hypertension, n (%) | 433 (80.9) |
| Dyslipidemia, n (%) | 407 (76.1) |
| Smoking history, n (%) | 234 (43.7) |
| Current smoking | 101 (18.9) |
| Family history of CAD, n (%) | 265 (49.5) |
| Previous myocardial infarction n (%) | 133 (24.9) |
| Previous PCI, n (%) | 206 (38.5) |
| Previous CABG, n (%) | 54 (10.1) |
| Previous stroke, n (%) | 21 (3.9) |
| CHF functional class III or IVa, n (%) | 39 (7.3) |
| Renal failureb, n (%) | 33 (6.2) |
| Peripheral vascular disease, n (%) | 25 (4.7) |
| Clinical presentation of ACS, n (%) | 224 (41.9) |
| Target vessel, n (%) | |
| Left anterior descending artery | 335 (46.1) |
| Left circumflex artery | 181 (23.5) |
| Right coronary artery | 213 (27.7) |
| Left main coronary artery | 21 (2.7) |
| Bifurcation, n (%) | 89 (11.6) |
| Total occlusion, n (%) | 48 (6.2) |
| In-stent restenosis, n (%) | 54 (7.0) |
| Type B2/C lesionc, n (%) | 531 (69.0) |
| Lesion length, mm | 17.8 ± 7.3d |
| Reference vessel diameter, mm | 3.0 ± 1.4d |
| Stenosis diameter, % | 82.9 ± 10.1d |
CAD: coronary artery disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; CHF: congestive heart failure; ACS: acute coronary syndrome.
a According to the New York Heart Association classification.
b Defined by the measurement of baseline serum creatinine ≥ 1.5mg/dL.
c According to the modified classification of the American College of Cardiology/American Heart Association.
d Estimated by visual analysis, as reported by the operator.
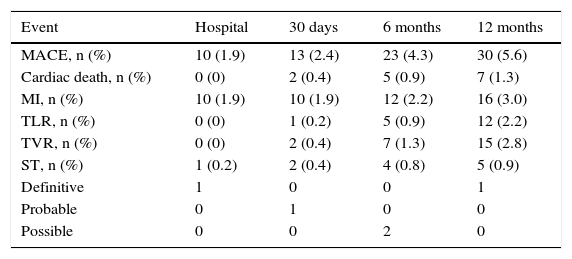
Table 2 shows the cumulative rates of adverse events up to 12 months of clinical follow-up. Between 6 and 12 months, there were seven new cases of MACE, including two cardiac deaths. In the first case, a 53 year-old patient with multiple risk factors (systemic arterial hypertension – SAH, diabetes, dyslipidemia, smoking, acute myocardial infarction and previous coronary artery bypass graft – CABG), had initially two lesions treated in the right coronary artery in the presence of ACS, but developed inferior AMI with ST-segment elevation followed by death 245 days after the procedure. In the second case, a 70 year-old female, with a history of SAH, dyslipidemia and diabetes, had the left anterior descending artery treated in the index procedure due to the clinical picture of stable angina functional class III; however, she showed recurrence of the lesion in the treated vessel and progression of atherosclerotic disease, being submitted to CABG; she developed postoperative complications and died on the day 289. Additionally, three patients had non-cardiac death due to the development of pancreatic cancer (day 246), multiple trauma (day 280) and respiratory failure (day 295).
Cumulative rates of adverse events up to 12 months of follow-up (n = 535).
| Event | Hospital | 30 days | 6 months | 12 months |
|---|---|---|---|---|
| MACE, n (%) | 10 (1.9) | 13 (2.4) | 23 (4.3) | 30 (5.6) |
| Cardiac death, n (%) | 0 (0) | 2 (0.4) | 5 (0.9) | 7 (1.3) |
| MI, n (%) | 10 (1.9) | 10 (1.9) | 12 (2.2) | 16 (3.0) |
| TLR, n (%) | 0 (0) | 1 (0.2) | 5 (0.9) | 12 (2.2) |
| TVR, n (%) | 0 (0) | 2 (0.4) | 7 (1.3) | 15 (2.8) |
| ST, n (%) | 1 (0.2) | 2 (0.4) | 4 (0.8) | 5 (0.9) |
| Definitive | 1 | 0 | 0 | 1 |
| Probable | 0 | 1 | 0 | 0 |
| Possible | 0 | 0 | 2 | 0 |
MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularization; TVR: target vessel revascularization; ST: stent thrombosis.
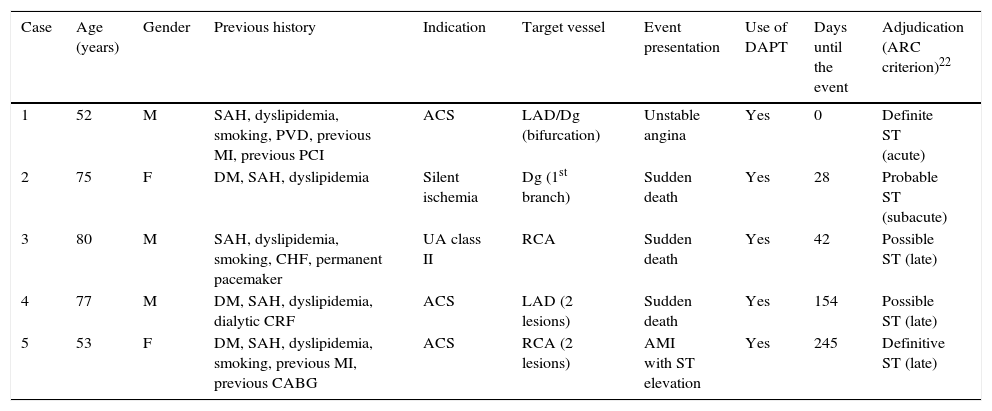
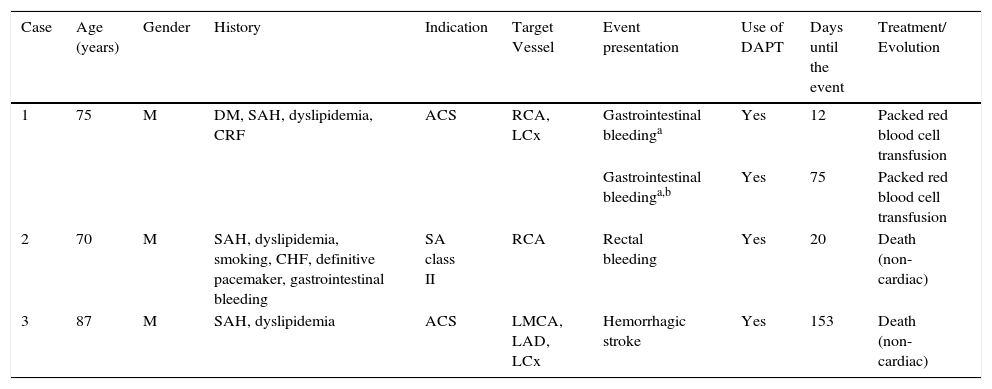
The cumulative rate of any stent thrombosis reported up to 12 months was 0.9%, totaling five cases; the rate of definitive/probable stent thrombosis was 0.6% (three cases). The detailed description of the cases is shown in Table 3. Finally, cases of major bleeding according to the TIMI criteria were reported in three patients up to 12 months of follow-up and, in one case, the patient had two distinct episodes of gastrointestinal bleeding (Table 4).
Description of stent thrombosis cases reported up to 12 months of follow-up (organized by the time up to the event from the index procedure).
| Case | Age (years) | Gender | Previous history | Indication | Target vessel | Event presentation | Use of DAPT | Days until the event | Adjudication (ARC criterion)22 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | SAH, dyslipidemia, smoking, PVD, previous MI, previous PCI | ACS | LAD/Dg (bifurcation) | Unstable angina | Yes | 0 | Definite ST (acute) |
| 2 | 75 | F | DM, SAH, dyslipidemia | Silent ischemia | Dg (1st branch) | Sudden death | Yes | 28 | Probable ST (subacute) |
| 3 | 80 | M | SAH, dyslipidemia, smoking, CHF, permanent pacemaker | UA class II | RCA | Sudden death | Yes | 42 | Possible ST (late) |
| 4 | 77 | M | DM, SAH, dyslipidemia, dialytic CRF | ACS | LAD (2 lesions) | Sudden death | Yes | 154 | Possible ST (late) |
| 5 | 53 | F | DM, SAH, dyslipidemia, smoking, previous MI, previous CABG | ACS | RCA (2 lesions) | AMI with ST elevation | Yes | 245 | Definitive ST (late) |
DAPT: dual antiplatelet therapy; ARC: Academic Research Consortium; M: male; SAH: systemic arterial hypertension; PVD: peripheral vascular disease; AMI: acute myocardial infarction; PCI: percutaneous coronary intervention; ACS: acute coronary syndrome; LAD: left anterior descending artery; Dg: diagonal branch: ST: stent thrombosis; F: female; DM: diabetes mellitus; CHF: congestive heart failure; SA: stable angina; RCA: right coronary artery; CRF: chronic renal failure; CABG: coronary artery bypass graft surgery.
Description of major bleeding cases according tothe TIMI17 criterion up to 12 months of follow-up (organized by time up to the event from the index procedure).
| Case | Age (years) | Gender | History | Indication | Target Vessel | Event presentation | Use of DAPT | Days until the event | Treatment/ Evolution |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | DM, SAH, dyslipidemia, CRF | ACS | RCA, LCx | Gastrointestinal bleedinga | Yes | 12 | Packed red blood cell transfusion |
| Gastrointestinal bleedinga,b | Yes | 75 | Packed red blood cell transfusion | ||||||
| 2 | 70 | M | SAH, dyslipidemia, smoking, CHF, definitive pacemaker, gastrointestinal bleeding | SA class II | RCA | Rectal bleeding | Yes | 20 | Death (non-cardiac) |
| 3 | 87 | M | SAH, dyslipidemia | ACS | LMCA, LAD, LCx | Hemorrhagic stroke | Yes | 153 | Death (non-cardiac) |
DAPT: dual antiplatelet therapy; M: male; DM: diabetes mellitus; SAH: Systemic arterial hypertension; CRF: chronic renal failure; ACS: acute coronary syndrome; RCA: right coronary artery; LCx: left circumflex artery; CHF: congestive heart failure; SA: stable angina; LMCA: left main coronary artery; LAD: left anterior descending artery.
a Event associated with the decrease in serum hemoglobin levels ≥ 5g/dL.
b Second bleeding event in the same patient (case 1).
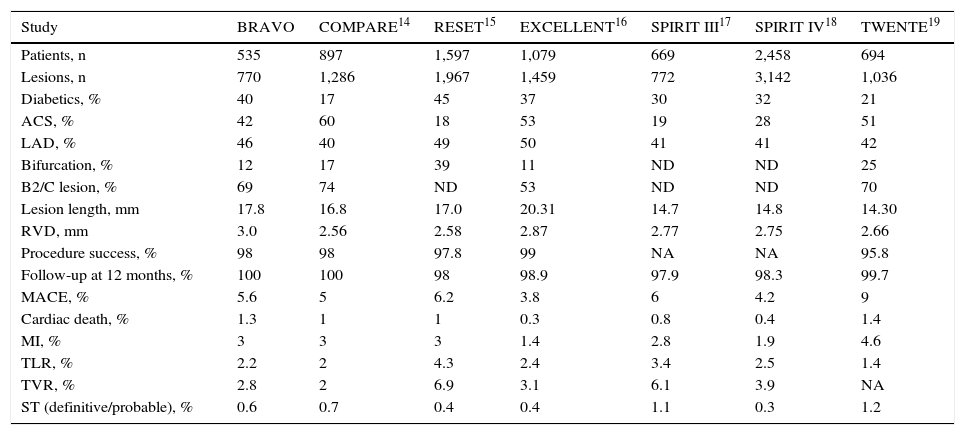
In general, the clinical results at 12 months in the current analysis, which involved a relatively large number of minimally selected patients (n = 535) obtained from the national daily practice, confirmed the safety and efficacy profile previously observed in our registry at 6 months.20 They were also comparable to a series of reported studies with the XIENCE VTM device in real-world populations, which showed high rates of procedure success (96% to 99%), as well as clinical safety and effectiveness that persisted during the late and very late follow-up (Table 5).14–19 These findings unequivocally suggest a very favorable performance of this second-generation everolimus-eluting DES in different clinical settings, including high-risk subgroups.
Comparison of clinical studies with XIENCE VTM Everolimus-eluting stent.
| Study | BRAVO | COMPARE14 | RESET15 | EXCELLENT16 | SPIRIT III17 | SPIRIT IV18 | TWENTE19 |
|---|---|---|---|---|---|---|---|
| Patients, n | 535 | 897 | 1,597 | 1,079 | 669 | 2,458 | 694 |
| Lesions, n | 770 | 1,286 | 1,967 | 1,459 | 772 | 3,142 | 1,036 |
| Diabetics, % | 40 | 17 | 45 | 37 | 30 | 32 | 21 |
| ACS, % | 42 | 60 | 18 | 53 | 19 | 28 | 51 |
| LAD, % | 46 | 40 | 49 | 50 | 41 | 41 | 42 |
| Bifurcation, % | 12 | 17 | 39 | 11 | ND | ND | 25 |
| B2/C lesion, % | 69 | 74 | ND | 53 | ND | ND | 70 |
| Lesion length, mm | 17.8 | 16.8 | 17.0 | 20.31 | 14.7 | 14.8 | 14.30 |
| RVD, mm | 3.0 | 2.56 | 2.58 | 2.87 | 2.77 | 2.75 | 2.66 |
| Procedure success, % | 98 | 98 | 97.8 | 99 | NA | NA | 95.8 |
| Follow-up at 12 months, % | 100 | 100 | 98 | 98.9 | 97.9 | 98.3 | 99.7 |
| MACE, % | 5.6 | 5 | 6.2 | 3.8 | 6 | 4.2 | 9 |
| Cardiac death, % | 1.3 | 1 | 1 | 0.3 | 0.8 | 0.4 | 1.4 |
| MI, % | 3 | 3 | 3 | 1.4 | 2.8 | 1.9 | 4.6 |
| TLR, % | 2.2 | 2 | 4.3 | 2.4 | 3.4 | 2.5 | 1.4 |
| TVR, % | 2.8 | 2 | 6.9 | 3.1 | 6.1 | 3.9 | NA |
| ST (definitive/probable), % | 0.6 | 0.7 | 0.4 | 0.4 | 1.1 | 0.3 | 1.2 |
ACS: acute coronary syndrome; LAD: left anterior descending artery; NA: not available; RVD: reference vessel diameter; MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularization; TVR: target vessel revascularization; ST: stent thrombosis.
In the BRAVO registry, the MACE rate at 6 months was 4.3%20; however, there were seven new occurrences of this outcome between 6 and 12 months, representing a small increase (approximately 1.3%) during the period. It is noteworthy the fact that the new revascularization, due to the late stenosis recurrence or disease progression, was the main determining factor in six of seven new reported cases. In the era of balloon angioplasty, the main late recurrence mechanism seemed to be vascular remodeling or recoil, with the binary angiographic restenosis occurring in up to 50% of the cases. Regarding the cases of post-bare metal stent recurrence, they have been associated mainly with neointimal hyperplasia, with rates ranging between 20% and 30%.4,5,24,25
It was noteworthy that, in both situations, the incidence peak seemed to occur at the similar time, between 3 and 6 months.26 However, with the DES, the formation of neointimal tissue is significantly inhibited – binary angiographic restenosis usually < 10% and such process is also delayed due to the local action of the antiproliferative drug.1,4–12,27 Therefore, albeit unlikely, cases of recurrence may occur at a later stage (> 6 months) in comparison with bare-metal stents (≤ 6 months). However, the predictors classically associated with post-PCI restenosis (diabetes, long lesions, small-caliber vessels, complex lesions, etc.) remain the same.28,29
Interestingly, in addition to neointimal hyperplasia, recent studies have demonstrated a new post-stent recurrence mechanism, including the neoatherosclerosis process, which can occur even in the presence of second-generation DES.30,31 Serial analyses of quantitative coronary angiography in the SPIRIT II and III studies showed relatively low rates of binary angiographic restenosis in the segment treated with XIENCE VTM stent (3.4 to 4.7% at 6 and 8 months, respectively), which were associated with TLR rates of 1.8% at 6 months (SPIRIT II) and 3.4% at 12 months (SPIRIT III).17,18 Similarly, clinical restenosis rates in the BRAVO registry were 0.9% at 6 months and 2.2% at 12 months, despite the high prevalence of diabetes, ACS and complex lesions. These findings demonstrate the clinical effectiveness of the second-generation DES, especially of the everolimus-releasing system, even in more complex populations (Table 5).
Despite the remarkable efficacy in reducing neointimal hyperplasia, restenosis, and thus the need for new revascularization demonstrated by the first-generation DES vs. bare-metal stents,4,5 safety-related concerns, particularly in late and very late stages,3 eventually prompted the research and development of new DES systems, aiming at a better safety profile. A series of studies showed, quite clearly, the multifactorial profile associated with the genesis of thrombosis post-DES implantation.3,32,33 Of note, among others, are the characteristics associated with: comorbidities and clinical presentation (diabetes, renal failure, ACS, etc.); lesion morphology and procedure (complex lesions, bifurcation, thrombus, multivessel PCI, stent underexpansion, dissection, etc.); DAPT interruption (mainly in the first 6 months); and stent type.
Nevertheless, even considering the current evidence, it is not completely determined to which extent (or to what degree) one or another of these characteristics, or a combination of them, may influence and/or predict the occurrence of stent thrombosis. Regarding DES, strut thickness, biocompatibility of the carrier systems and the action mechanism of the antiproliferative drug have been considered decisive components for performance and safety profile, both early and late/very late. Among the most used second-generation DES, the thin chrome-cobalt and platinum-cobalt metallic platforms (74-91 microns) are commonly found.6,9,10,13 In relation to the carrier systems, they incorporate “non-thrombogenic” or biodegradable polymers with a better biocompatibility profile, when compared to those durable polymers used in older DES.6–13,34
Finally, drugs from the “limus” family currently prevail.6–13 Three randomized trials comparing the XIENCE VTM stent vs. Taxus™ paclitaxel-eluting stent (Boston Scientific, Natick, USA) showed lower rates of MACE and stent thrombosis with the first one in the 12 months of follow-up.14,17,18 Three other randomized studies showed non-inferiority of everolimus-releasing DES vs. CYPHERTM sirolimus-releasing stent (Cordis Corp., Warren, USA) regarding the clinical outcomes at 12 or 18 months of follow-up;15,16,35 however, very late stent thrombosis rate (> 1 year) seems to be lower with the everolimus-releasing system.3
Stent thrombosis rates reported up to 1 year with the XIENCE VTM stent ranged from 0.4% to 1.2%. In our population, this rate (definite/probable) was 0.6%, despite the high prevalence of diabetic patients (40%), ACS (42%) and complex lesions (69% B2/C type). Considering only the late (between 1 and 12 months) stent thrombosis (definite/probable), the rate was extremely low (0.2%), representing only one occurrence.
It is noteworthy the fact that most patients that had any stent thrombosis (n = 5) had multiple clinical comorbidities and ACS in the index procedure, although they were being treated with DAPT at the time of the thrombotic event. Nonetheless, the evaluation of event incidence in the very late clinical follow-up (not yet reported) is required, so the sustainability of these results can be verified.
ConclusionsResults of the late clinical follow-up of the BRAVO Brazilian multicenter registry showed sustained safety and efficacy of the XIENCE VTM everolimus-eluting stent in the treatment of complex coronary lesions in minimally selected patients from the national daily practice, including relatively low stent thrombosis (< 1%) and target-lesion revascularization rates (2.2%) after 12 months.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
The following investigators are part of the BRAVO study: Alexandre Abizaid Instituto Dante Pazzanese de Cardiologia, São Paulo (SP), Brazil; Amanda G. M. R. Sousa Instituto Dante Pazzanese de Cardiologia, São Paulo (SP), Brazil; Andrea S. Abizaid Instituto Dante Pazzanese de Cardiologia, São Paulo (SP), Brazil; Antônio C. N. Ferreira Hospital Mater Dei S.A., Belo Horizonte, MG, Brazil; Ari Mandil Hospital Lifecenter, Belo Horizonte, MG, Brazil; César R. Medeiros Hospital Rede D’Or, Unidades Copa, Barra e Quinta D’Or, Rio de Janeiro, RJ, Brazil; Costantino R. Costantini Hospital Cardiológico Costantini, Curitiba, PR, Brazil; Décio Salvadori Hospital Beneficência Portuguesa, São Paulo (SP), Brazil; Erlon O. de Abreu-Silva Cardiovascular Research Center, São Paulo (SP), Brazil; Flávio R. A. Oliveira Maximagem Diagnóstico por Imagem Ltda., Recife, PE, Brazil; Gilberto Nunes Santa Casa de Misericórdia, Porto Alegre, RS, Brazil; Hélio Castello Jr. Hospital Bandeirantes, São Paulo (SP), Brazil; Heloísa M. S. Guimarães Centro Integrado de Medicina Intervencionista, Belém, PA, Brazil; José Airton Arruda CIAS Unimed, Vitória, ES, Brazil; José Armando Mangione Hospital Beneficência Portuguesa, São Paulo (SP), Brazil; José Eduardo Sousa Instituto Dante Pazzanese de Cardiologia, São Paulo (SP), Brazil; Juliana P. de Castro Cardiovascular Research Center, São Paulo (SP), Brazil; Luiz A. Mattos Instituto Dante Pazzanese de Cardiologia, São Paulo (SP), Brazil; Marco Perin Hospital Israelita Albert Einstein, São Paulo (SP), Brazil; Marco V. Wainstein Hospital Moinhos de Vento, Porto Alegre, RS, Brazil; Marcos Marino Hospital Madre Teresa, Nova Lima, MG, Brazil; Maurício L. Prudente Encore Cardiologia e Radiologia Intervencionista, Goiânia, GO, Brazil; Newton Stadler S. Filho Instituto de Neurologia de Curitiba, Curitiba, PR, Brazil; Norberto T. Duda Hospital São Vicente de Paulo, Passo Fundo, RS, Brazil; Paulo Caramori Hospital São Lucas da Pontifícia Universidade Católica de Porto Alegre, Porto Alegre, RS, Brazil; Ricardo A. Costa Instituto Dante Pazzanese de Cardiologia, São Paulo (SP), Brazil; Roberto Botelho Instituto do Coração do Triângulo Mineiro, Uberlândia, MG, Brazil; Rodrigo F. Cardoso Hemocor, Rio de Janeiro, RJ, Brazil; Rogério Sarmento-Leite Instituto de Cardiologia/Fundação Universitária de Cardiologia, Porto Alegre, RS, Brazil.