We report a case of everolimus-eluting stent restenosis caused by neoatherogenesis. Optical coherence tomography indicated the presence of a superficial arch with high optical intensity in the in-stent mid-segment, followed by significant signal attenuation, with poorly defined borders, indicating the presence of lipid infiltration and/or necrotic core, similar to that observed in de novo coronary lesions. Signs suggesting macrophage/ foam cell infiltration were observed inside the fibrous cap, indicating the presence of local inflammatory activity. The development of new in-stent atherosclerosis at the site of a pre-existing neointimal tissue (neoatherosclerosis) was recently identified as an additional cause of coronary stent failure. The present report is one of the first to demonstrate the finding of neoatherosclerosis as a second generation drugeluting stent failure.
Neoaterosclerose Precoce como Causa de Reestenose de Stent Farmacológico de Segunda Geração
Relatamos um caso de reestenose de stent eluidor de everolimus causada por neoaterogênese. A tomografia de coerência óptica revelou, no segmento médio intrastent, presença de arco superficial com alta intensidade óptica, seguido por significativa atenuação do sinal luminoso, com limites mal definidos, indicando presença de infiltração lipídica e/ou núcleo necrótico, semelhante ao observado em lesões coronárias de novo. Sinais sugerindo infiltração de macrófagos/foam cells puderam ser observados no interior da capa fibrosa, denotando presença de atividade inflamatória local. O surgimento de nova aterosclerose intrastent, no local de um tecido neointimal já formado (neoaterosclerose), tem sido recentemente identificado como causa adicional de falência de stents coronários. O presente relato é um dos primeiros a demonstrar o achado de neoaterosclerose como falha de um stent farmacológico de segunda geração.
The case of a 58-year-old male patient is reported, an ex-smoker, with hypertension and prior coronary artery bypass grafting surgery, who presented with acute myocardial infarction without ST-segment elevation on January 1, 2012, and was submitted to percutaneous coronary intervention on January 18, 2012, with implantation of an everolimus-eluting stent in the middle third of the left circumflex artery.
Six months after the procedure, the patient returned complaining of stable angina class II according to the classification of the Canadian Cardiovascular Society (CCS).
A new angiography showed focal in-stent restenosis (Figure 1). An optical coherence tomography was performed (Figures 2 and 3) and demonstrated a heterogeneous vascular response pattern throughout the previously treated segment. The distal segment of the stent had a satisfactory vascular healing pattern, with a thin layer of neointimal hyperplasia with circumferential distribution and regular borders, in which the tissue had a homogeneous pattern of high optical intensity 1 (Figure 2, panel 1). In contrast, the proximal intrastent segment showed more pronounced neointimal suppression, with the heterogeneous distribution of a fine layer of neointimal tissue and presence of several struts with no tissue covering them (Figure 2, panel 3).
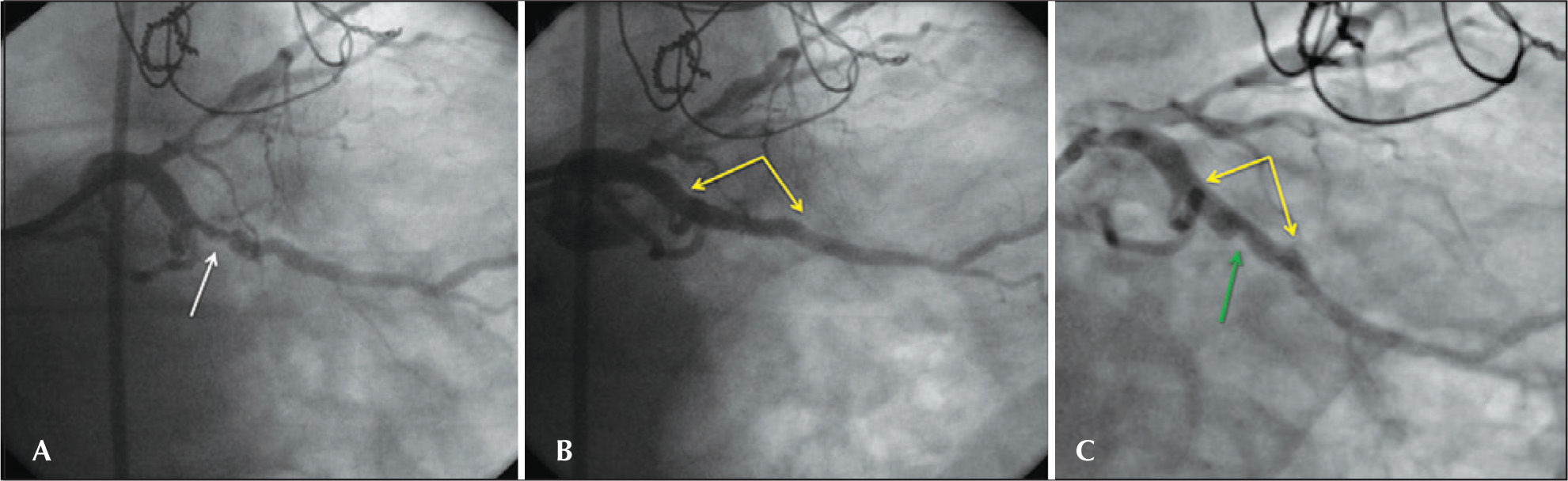
– Coronary angiography of the procedure and at 6 months. In A, angiography shows an eccentric lesion in the middle third of the left circumflex artery. The arrow points to the stenosis. In B, the final angiographic result after implantation of an everolimus-eluting stent of 3.5×23mm, post-dilated with a non-compliant balloon of 4×12mm up to 16atm. A satisfactory angiographic result without residual stenosis in the in-stent segment, no signs of injury at the borders and preserved distal epicardial flow (Thrombolysis In Myocardial Infarction – TIMI 3) were observed. Yellow arrows delimit the stent borders. In C, coronary angiography six months after the procedure with binary angiographic restenosis (stenosis diameter of 62%), focal, restricted to the in-stent segment is shown. Yellow arrows delimit the stent borders, and the green arrow points to the site of restenosis.
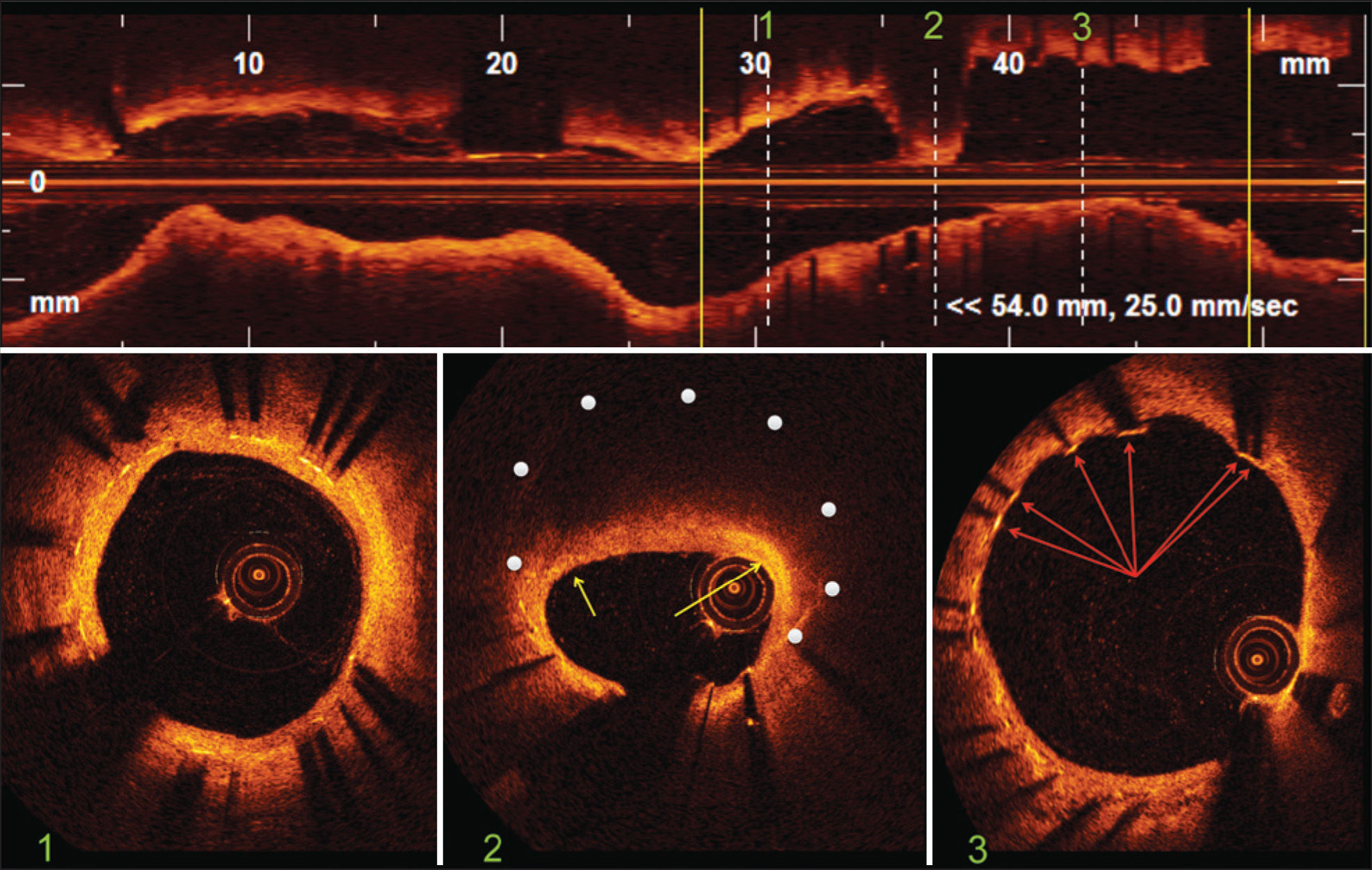
– Optical coherence tomography. In the top panel, longitudinal reconstruction of the left circumflex artery. The stent limits are identified by the yellow vertical bars. The white dotted vertical bars identify three representative images of the intrastent distal segment, site of restenosis and intrastent proximal segment corresponding to images of cross-sections of the vessel shown in the lower panel. Bottom Panel 1: distal segment of the stent showing a region with normal neointimal hyperplasia characterized by homogeneous circumferential distribution, a regular outline and high optical intensity. Bottom Panel 2: site of restenosis with eccentric distribution of neointimal tissue that has characteristics similar to those of a lipid plaque in de novo lesions. The white dots indicate the position of the stent struts that were not visualized due to important attenuation of the optical signal. The yellow arrows indicate areas of increased superficial brightness within the fibrous cap, suggesting the infiltration of macrophages/foam cells. Bottom Panel 3: proximal stent segment showing heterogeneous vascular healing with the presence of struts not covered by neointimal tissue (arrows).
– Three-dimensional reconstruction of optical coherence tomography images. In A, three-dimensional reconstruction of the stent revealing discontinuity of the stent structure at the site of restenosis through important attenuation of the optical signal promoted by fatty infiltration of the neointima. In B, longitudinal images of the open vessel in two orthogonal planes showing the eccentricity and the three-dimensional distribution of the restenosis point. The black arrows indicate the site of restenosis. The asterisk indicates the shadow caused by the presence of the 0.014-inch guidewire. In C, fly-through visualisation of the coronary vessel showing the spatial distribution of neointimal tissue at the site of restenosis. The darker neointimal tissue at the point of restenosis compared with other regions of the vessel is the result of the attenuation of the light signal by lipid infiltration. The asterisk indicates the shadow caused by the presence of the guidewire.
In the medium intrastent segment (Figure 2, panel 2), significant neointimal proliferation with expressive involvement of the luminal area was observed. A high optical intensity shallow arch, followed by significant attenuation of the light signal in which boundaries were poorly defined was observed, suggesting the existence of fatty infiltration and/or a necrotic core similar to de novo coronary lesions, 2 showing new intrastent atherosclerosis. 3 The optical signal attenuation was so expressive that it prevented the identification of the structure of the metallic stent struts. Signs of local inflammatory activity could be inferred by visualising a ‘flecked’ glow within the fibrous cap, with an optical intensity greater than the surrounding fibrous tissue, suggesting the infiltration of clusters of macrophages/ foam cells.
In the three-dimensional reconstruction of the optical coherence tomography images, there was a discontinuity image in the central region of the stent structure (restenosis site) resulting from the hyperattenuation of the optical signal by the neoatherogenic tissue (Figure 3A).
The development of new intrastent atherosclerosis at the site of already formed neointimal tissue (neoatheros clerosis) has recently been identified as an additional cause of failure (restenosis or thrombosis) of coronary stents. 4–6 A series of 299 autopsy cases demonstrated that the incidence of neoatherosclerosis is greater in lesions treated with first-generation drug-eluting stents (DES; 31%) compared with bare-metal stents (BMS; 16%), and the time to its onset is shorter after implantation of a DES (420days – 361 days to 683 days) when compared to BMS (2,160days – 1,800days to 2,880days).7 To date, descriptions of encountering neoatherosclerosis in second-generation DES are scarce. The present report is one of the first to report the finding of neoatherosclerosis as a second-generation stent failure. The prematurity of this phenomenon is noteworthy and deserves further investigation.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.