Percutaneous renal sympathetic denervation was developed as an adjunct method to treat clinical conditions associated to sympathetic hyperactivity. Percutaneous renal sympathetic denervation increases the renal blood flow and reduces vasoconstriction. The effects of percutaneous renal sympathetic denervation in renal artery diameter have not been reported. Our objective was to evaluate such effects by quantitative angiography.
MethodsProspective, observational, study including consecutive patients undergoing percutaneous renal sympathetic denervation.
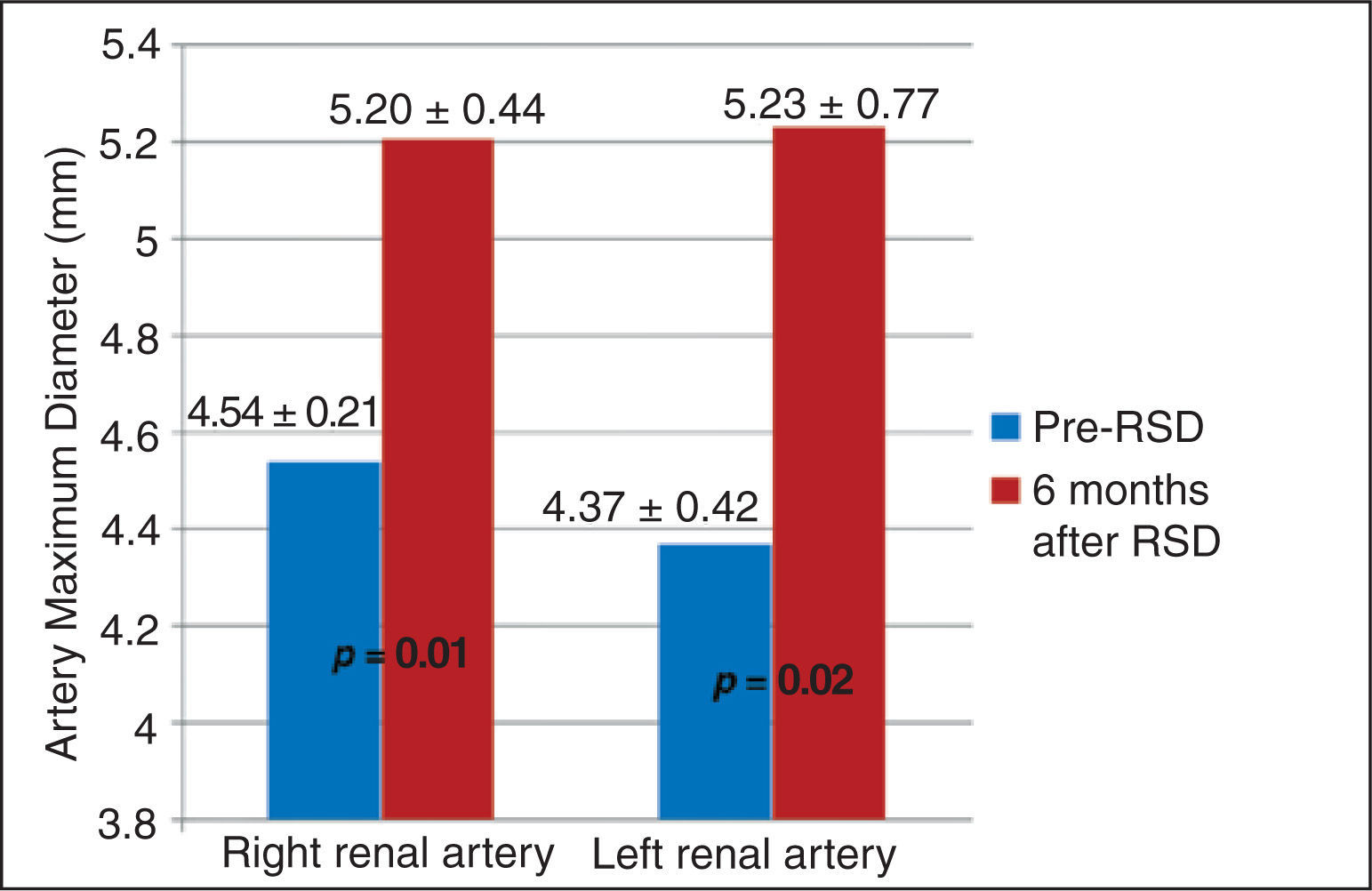
ResultsThirty-one patients were selected, 21 were submitted to percutaneous renal sympathetic denervation to control resistant arterial hypertension and 10 to control refractory ventricular arrhythmias. Seventeen patients did not perform renal arteriography in the follow-up due to clinical contraindications or because they did not complete the 6-month period established by the protocol. In addition, one patient performed a unilateral percutaneous renal sympathetic denervation and was also excluded from this analysis. Therefore, 52 renal angiographies (26 pairs) of 13 patients were analyzed. Mean maximal diameter of the right renal artery before the procedure was 4.54±0.21mm and increased to 5.2±0.44mm at 6 months (p=0.01). Likewise, there was a significant increase in the diameter of the left renal artery at 6 months of follow-up, increasing from 4.37±0.42 to 5.23±0.77mm (p=0.02).
ConclusionsThe results of this analysis illustrate the significant increment in renal artery diameter after percutaneous renal sympathetic denervation. Randomized controlled clinical trials are required to consolidate our observations.
RESUMOEfeitos da Denervação Simpática Renal no Diâmetro da Artéria Renal Avaliados Pela Angiografia Quantitativa
IntroduçãoA denervação simpática renal percutânea surgiu como método adjunto no controle de condições clínicas as- sociadas à hiperatividade simpática. Ela resulta em aumento do fluxo sanguíneo renal e em redução da vasoconstrição. Os efeitos da denervação simpática renal percutânea no diâmetro da artéria renal ainda não foram descritos. Nosso objetivo foi avaliar tais efeitos por meio da angiografia quantitativa. Métodos: Estudo prospectivo e observacional que incluiu pacientes consecutivos submetidos à denervação simpática renal percutânea.
ResultadosSelecionamos 31 pacientes, sendo 21 submetidos à denervação simpática renal percutânea para controle da hipertensão arterial resistente e 10 para controle de arritmias ventriculares refratárias. Dezessete pacientes não realizaram arteriografia renal no seguimento por não completarem o período protocolar de 6 meses, ou por contraindicação clínica. Adicionalmente, uma paciente realizou denervação simpática renal percutânea unilateral, sendo também excluída desta análise. Assim, 52 angiografias renais (26 pares) de 13 pacientes foram analisadas. A média do diâmetro máximo da artéria renal direita, antes do procedimento, foi de 4,54±0,21mm e aumentou para 5,2±0,44mm aos 6 meses (p=0,01). Da mesma forma, observou-se aumento significativo do diâmetro da artéria renal esquerda aos 6 meses de seguimento, ampliando de 4,37±0,42 para 5,23±0,77mm (p=0,02).
ConclusõesOs resultados desta análise ilustram o incremento significativo dos diámetros das artérias renais após denervação simpática renal percutânea. Ensaios clínicos randomizados e controlados são necessários para consolidar nossas observações.
Approximately one billion people suffer from systemic arterial hypertension (SAH) worldwide.1 It is well established that an increase in blood pressure is associated with an increased risk of cardiovascular disease. Conversely, small reductions in blood pressure (BP) substantially decrease the rates of stroke, coronary artery disease, and heart failure, also denoting a reduction in the number of hospitalizations, their related costs, and deaths, as well as an improvement in quality of life in patients with SAH.2,3
Based on the knowledge of the importance of hyperactivity of the sympathetic autonomic nervous system in the pathophysiology of SAH, percutaneous renal sympathetic denervation (RSD) has emerged as an adjunctive therapeutic strategy in selected groups of refractory hypertensive patients.4 Benefits have also been observed with this method in other clinical contexts associated with chronic hyperactivity of the sympathetic nervous system (SNS), such as cardiac arrhythmias,5 obstructive sleep apnea syndrome,6 heart failure,7 and metabolic syndrome.8
Despite the promising initial results of percutaneous RSD related to SAH, discrepancies have been observed among studies, in large part reflecting the variation in the technique used the learning curve, the type of device used, and, especially, the different populations studied, with a wide variation of the importance of the sympathetic system in the genesis of SAH.
Sympathetic activity in the context of percutaneous RSD is measured by microneurography and by norepinephrine spillover.9 However, although available, these procedures are not adopted routinely in clinical practice, due to limitations in their execution.
Chronic SNS stimulation progresses with an increase in plasma neurohormones, which in turn results in vascular (vasoconstriction, increased thickness, and reduced vascular compliance) and heart (development of myocardial hypertrophy, ischemia, arrhythmias, and heart failure) effects. In kidneys, an increased sympathetic activity leads to the activation of the renin-angiotensin- aldosterone system, increased reabsorption of sodium and water, reduction of renal blood flow and glomerular filtration rate, ischemia, and renal failure.10
Percutaneous RSD aims to reduce sympathetic activity. As a consequence, there is an increase in renal blood flow9 and arterial vasodilation.10 In this study; the aim was to evaluate the effects of percutaneous RSD on the diameter of the renal arteries, through the use of quantitative angiography.
METHODSSelection of patientsThirty-one consecutive patients undergoing percutaneous RSD at the Instituto Dante Pazzanese de Cardiologia were selected. The indication for the procedure was for control of refractory hypertension in 21 of these patients and for control of refractory ventricular arrhythmias in the other ten.
ProcedureIn 26 patients, an open-irrigated tip catheter (Ther- moCool® or Therapy Cool Path®) was used and, in five, the dedicated system EnligHTN® (St. Jude Medical Inc®, Westford, United States).
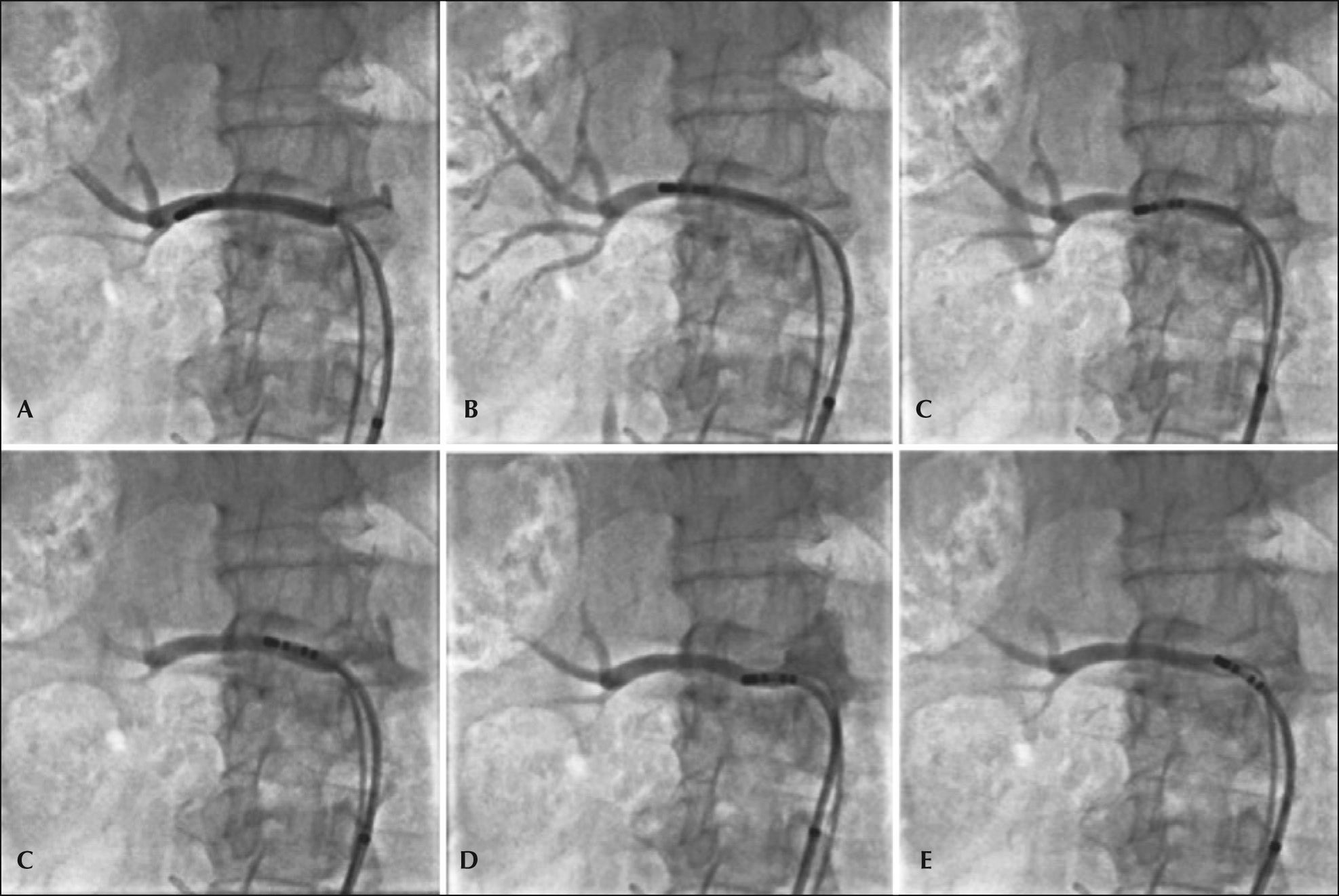
Four to six radiofrequency applications were performed in each renal artery with the irrigated catheter, starting at the most distal portion of the vessel, close to the bifurcation, toward the aorta, respecting the minimum distance of 5mm between each application and the helical arrangement of them. The detailed technique for a percutaneous RSD with an irrigated catheter has been described previously elsewhere by our group.11Figure 1 shows a case in which six radiofrequency applications were conducted in the right renal artery.
EnligHTN® is a dedicated system for percutaneous RSD, designed with the aim of generating better-distributed renal artery lesions, and, therefore, four radiofrequency applications are performed with a minimal manipulation of the catheter. The device consists of a catheter with a basket at its distal end, which has four electrodes that sequentially emit the radiofrequency. Initially, the device was placed distally in the renal artery, wherein a first section of application was performed. After this step, the device was collapsed and retracted by about 1cm, for a new application – a total of eight applications per treated renal artery were performed. The technique outlined for percutaneous RSD with the EnligHTN® dedicated system has been described previously elsewhere by the present group.12
Quantitative angiographyOf those 31 patients selected, 17 did not undergo renal arteriography under the protocol during follow- up, or because they did not complete the six-month period, or due to clinical contraindication. Fourteen patients underwent angiography 6 months after percutaneous RSD, which allowed for the comparison of this examination versus the initial examination performed before the ablation. One case was excluded from this analysis, because the patient was submitted to ablation of only one renal artery. Thus, 52 renal arteriographies (26 pairs) from 13 patients were analyzed by quantitative angiography.
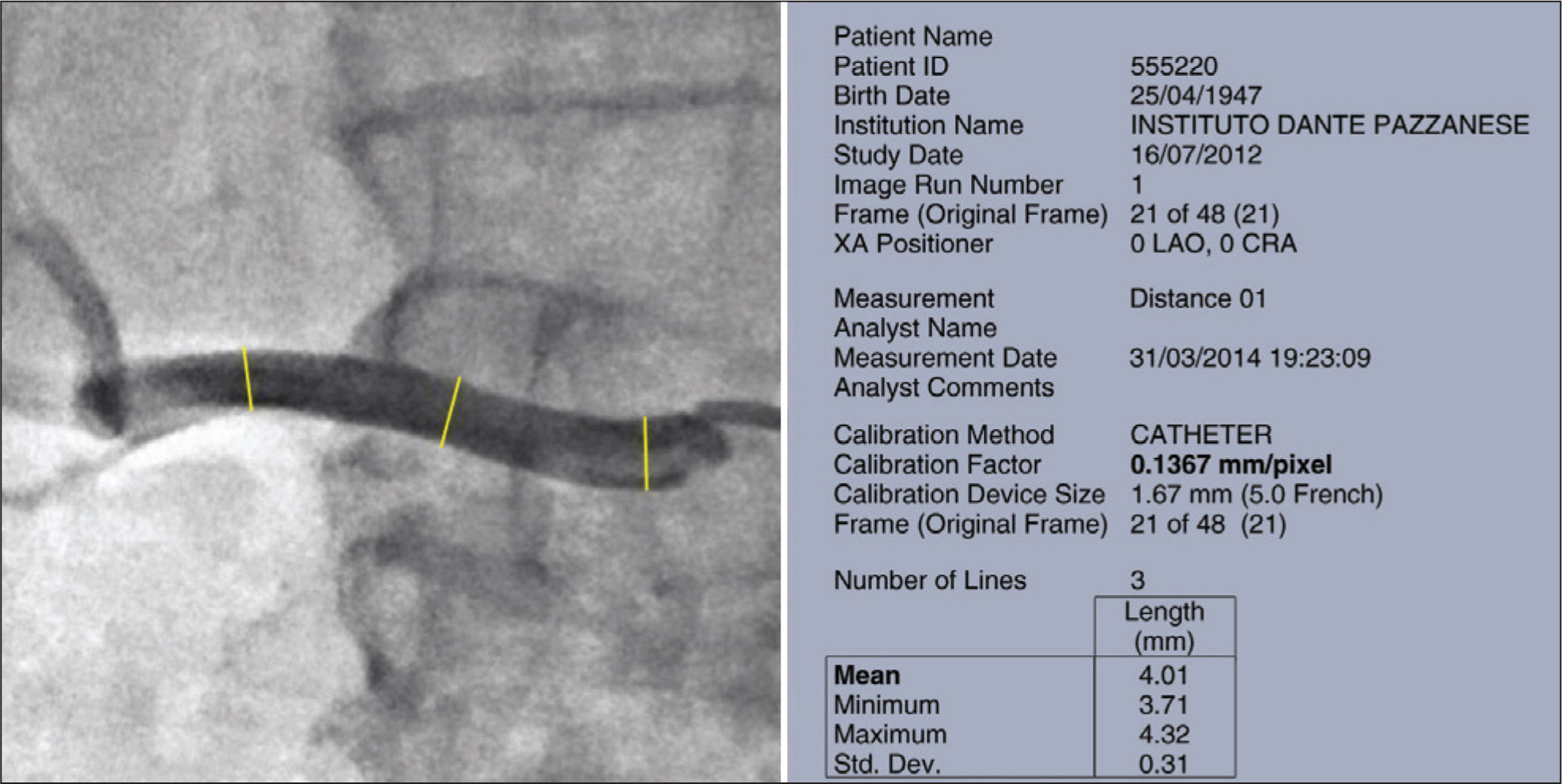
The quantitative renal angiography was interpreted with the help of QAngio XA software, version 7.3 (Medis Medical Imaging Systems BV, Leiden, The Netherlands). The maximum diameters of each renal artery were measured at three points: proximal, middle and distal thirds. The mean measure was calculated automatically by the program (Figure 2).
Statistical AnalysisContinuous variables were expressed as mean and standard deviation and compared using Student’s t-test. Categorical variables were presented as absolute and relative frequencies. For all parameters compared, p- values<0.05 were considered statistically significant. Data were analyzed using SPSS®, version 16.0 for Windows® (SPSS® Inc., Chicago, Illinois, United States).
RESULTSFifty-two renal angiograms were analyzed. The mean age was 49±10 years and 11 (84.6%) patients were female. The baseline characteristics of all patients included in the study are shown in Table 1.
Baseline characteristics of patients undergoing quantitative renal angiography before and 6 months after renal sympathetic denervation
| Characteristic | n=13 |
|---|---|
| Age, years | 49±10 |
| Female, n (%) | 11 (84.6) |
| Diabetes mellitus, n (%) | 4 (30.7) |
| Dyslipidemia, n (%) | 10 (76.9) |
| Smoking, n (%) | 0 |
| Prior myocardial infarct, n (%) | 1 (7.6) |
| Previous stroke, n (%) | 0 |
| Peripheral arterial disease, n (%) | 0 |
| Family history of cardiovascular disease, | 4 (30.7) |
| n (%) |
The volume of contrast used was 87.7±29.7mL and the fluoroscopy time was 21.4±6.6minutes. On average, five applications were performedon each renal artery.
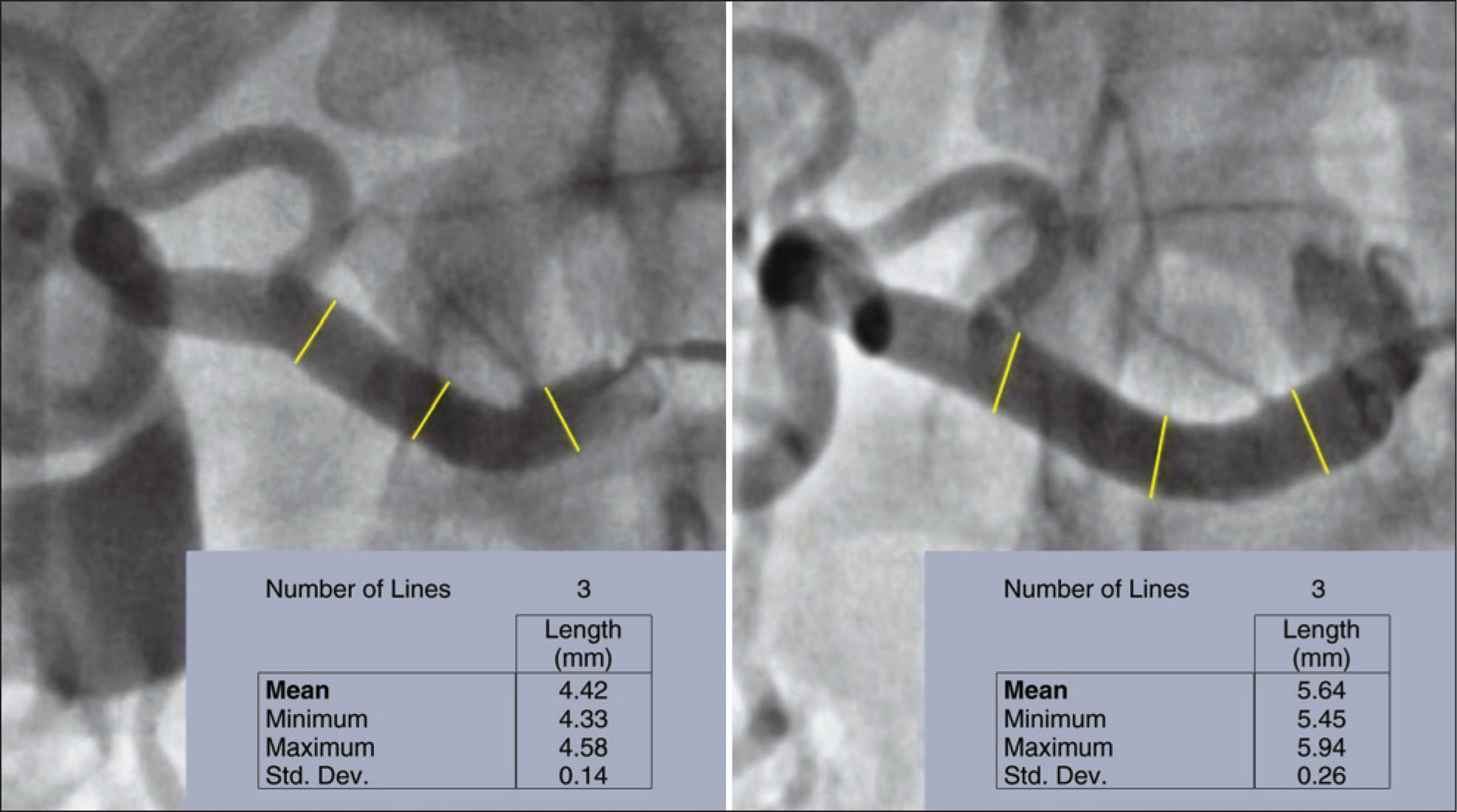
Quantitative angiographyFigure 3 exemplifies a quantitative angiography in right renal artery before and 6 months after percutaneous RSD.
The length of the left and right renal arteries was 26.9±8.9mm and 36.3±7.4mm, respectively.
The maximum diameter of the right renal artery before the procedure was 4.54±0.21mm, showing an increase to 5.20±0.44mm at 6 months (p=0.01). Similarly, an increase was observed in the diameter of the left renal artery at 6 months of follow-up, which changed from 4.37±0.42mm to 5.23±0.77mm (p=0.02) (Figure 4).
DISCUSSIONThis was the first study which prospectively assessed the effects of percutaneous RSD in the diameter of renal arteries. It was observed, using quantitative angiography, a significant increase in diameter, probably through two mechanisms: (1) increased renal blood flow and (2) decreased vasoconstriction, both as a result of blocking or reduction of sympathetic activity, caused by percutaneous RSD.
Coronary angiography is the gold standard of imaging for analysis of coronary anatomy; this technique provides definition of the extent and precise location of coronary artery disease. A well-performed coronary angiography requires a thorough knowledge of anatomy, including its variations, and a protocol for systematic acquisition of sequential images that allows the visualization of all coronary segments, especially in areas with overlapping vessels, bifurcations, and tortuous anatomy. Late lumen loss, a procedure applied for years for quantification of neointimal hyperplasia, has become one of the most sensitive and surgeon-independent angiographic evaluations of the effectiveness of coronary stents, both for drug-eluting devices or other types. Classically defined as the difference between the minimum lumen diameter (MLD) obtained immediately after the procedure (final MLD) and the MLD obtained during follow-up, late lumen loss is an angiographic measure of the absolute degree of vascular restenosis, different from the binary restenosis calculation.13,14 Renal artery angiography is used in a similar fashion as coronary angiography, and is useful for an accurate analysis of anatomy and also as an adjunctive tool in the evaluation of the results of interventions in this territory by means of quantitative angiography. In the present study, accurate renal angiographies were performed, with appropriate angles for better visualization of the renal arteries. The estimates applied in the initial tests were carefully repeated at the six-month follow-up in all patients involved in the study. The analyzes were interpreted with the aid of a contemporary program, QAngio XA, version 7.3. Measurements of maximum diameters in the proximal, middle, and distal segments of each renal artery before and 6 months after percutaneous RSD were performed. These procedures allowed us to assess the changes that took place, by comparing the averages of these measures.
The evaluation of sympathetic activity can be obtained directly by microneurography, usually of the fibular nerve, and indirectly by means of norepinephrine spillover.9 Although available, these methods are not routinely adopted in clinical practice, due to limitations in their implementation.
The development of microneurography, in which the neural activity can be recorded directly by means of microelectrodes inserted percutaneously into a peripheral nerve in humans, has provided valuable information on the control of sympathetic outflow to the muscle. This method allows for measuring the postganglionic efferent sympathetic nerve traffic to skeletal muscles, the so-called muscle sympathetic nerve activity (MSNA). The MSNA in different pathologies has expanded the knowledge about the functioning of the SNS. Although the quantification of MSNA is still largely limited to measures of frequency and incidence of bursts (bursts/min and bursts/100 heartbeats, respectively), the development of unit records of MSNA has provided more detailed information on the operation the SNS.15 In healthy subjects, MSNA is activated by decreases in cardiac filling pressure, exercise, hypoxia, hypercapnia, hyperpnoea, and sleep disorders, and is inhibited by lung inflation. Among the main clinical applications of sympathetic microneurography, the authors must emphasize the elucidation of the neural mechanisms of control of SAH and of thermoregulation. Despite obtaining direct measures of sympathetic activity (with the result that sympathetic microneurography is regarded as the gold standard), this technique has specific limitations, such as training and experience of the researcher, critical to the acquisition of high quality signals; reliance of the bursts’ amplitude relative to the position where the electrodes are applied; and the fact that the measure of MSNA in skeletal muscle may not reflect changes in renal and cardiac sites. Furthermore, this is a painful, time-consuming, and impractical method for use in the clinical environment, more suitable for research purposes.16
Historically, the methods used to measure changes of SNS in humans have consisted of determinations of norepinephrine, the primary neurotransmitter released from sympathetic postganglionic nerve endings. Today, the measurement of the excretion of this neurotransmitter in urine is a method into disuse as a test of sympathetic activity, while plasma concentration assays, although widely used yet, have two important limitations. The first of them is that these tests do not provide information on regional sympathetic function, and it is known that the sympathetic responses exhibit regional differences that can only be detected by techniques accessing organ- specific sympathetic function. The second limitation is that the plasma norepinephrine concentration depends not only of the sympathetic tone and the amount released, but also of its extraction from plasma.17
The overflow of norepinephrine (spillover) toward plasma, based on the dilution of radiotracers, is one of the safest ways of assessing regional sympathetic function. Renal norepinephrine spillover consists of the administration of the tritium-marked neurotransmitter at a known concentration, accompanied by the collection of blood samples from renal veins.18 This is a complex method, with little application in clinical practice.
Therefore, the authors believe that, if properly performed by experienced operators and with a careful attention to all the recommendations already well established, quantitative angiography of renal arteries can be a practical tool, easy to implement and of low cost, which will allow a better understanding of the results of percutaneous RSD.
LimitationsThis study has limitations, for instance, the small sample size, as well as the inherent limitations of observational studies; in particular, the non-use of established methods of measurement of sympathetic activity for comparison with quantitative angiography.
CONCLUSIONSThe results of this analysis illustrate the significant increase in diameter of the renal arteries after a renal sympathetic denervation procedure, probably due to the increase of renal blood flow and decreased vasoconstriction, both as a result of blockage or reduction of sympathetic activity. Randomized controlled trials are needed to consolidate these observations.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCENone.