Cardiac allograft vasculopathy is the leading cause of graft failure and death after the first year of heart transplantation. The optimal therapy for unprotected left main coronary artery disease in transplanted hearts has not been established. We report a case of emergency unprotected left main percutaneous coronary intervention in a transplanted heart after an aborted sudden death in a patient who was waiting for coronary artery bypass graft surgery.
Intervenção Coronária Percutânea Emergencial de Tronco de Coronária Esquerda Não-Protegido em Paciente com Transplante do Coração
A doença vascular do enxerto cardíaco é a principal causa de falência do enxerto e morte depois do primeiro ano após o transplante. O melhor tratamento para lesões de tronco de coronária esquerda não-protegido em corações transplantados ainda não está estabelecido. Descrevemos o caso de uma intervenção coronária percutânea emergencial de tronco de coronária esquerda não-protegido em coração transplantado após morte súbita revertida com sucesso em paciente que aguardava cirurgia de revascularização do miocárdio.
Cardiac allograft vasculopathy is the leading cause of graft failure and death after the first year of transplantation. There is gradual increase in the prevalence of vasculopathy in these patients: 8% in one year, 32% in five years, and 43% in eight years. After detection of diffuse or multivessel disease, the risk of death or retransplantation is extremely high.1
Drug treatment has shown significant improvement in the prevention of cardiac allograft vasculopathy, and consists in the aggressive control of classic risk factors for cardiovascular disease and treatment of acute rejection and infection by cytomegalovirus. Therapeutic options include percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) surgery, or a new heart transplant.
CABG may not be the best option, as diffuse intimal involvement frequently occurs, affecting distal vessels. Furthermore, perioperative mortality rates range between 40% and 80%, and graft patency rates in the long-term are unknown.2,3 A new heart transplant is hindered by the scarcity of donors and high perioperative mortality. The survival rate for patients who undergo repeat cardiac transplantation (75% in one year) is lower when compared to the first procedure, and half of these patients develop recurrent cardiac allograft vasculopathy.2,3 For these two types of surgical reintervention, there is a higher chance of complications associated with repeat sternotomy, due to mediastinal fibrosis and risk of infection related to immunosuppressive therapy.2,3
PCI also has limitations in cases of diffuse cardiac allograft vasculopathy; however, it can constitute the definite treatment in selected patients, and it is the best alternative in emergency cases. The present study reports a case of emergency unprotected left main percutaneous coronary intervention in a transplanted heart after a successfully reversed sudden death in a patient awaiting CABG surgery.
CASE REPORTFemale patient, 50 years-old, former smoker, dyslipidemic, with peripheral arterial failure, had a past history of CABG in 1993 and heart transplant in 2001, due to ischemic heart disease with heart failure classified as New York Heart Association (NYHA) functional class IV, and Canadian Cardiovascular Society (CCS) class IV angina. She used atenolol, losartan, amiodarone, acetylsalicylic acid (ASA), atorvastatin, deflazacort, cyclosporine, mycophenolate, cilostazol, and omeprazole.
From September 2012 onwards, she began to notice oppressive precordial discomfort chest pain and dyspnea on exertion, with progressive symptom worsening. An echocardiogram performed in October 2012 evidenced left ventricle with preserved dimensions and systolic function; ejection fraction of 59%; mild mitral regurgitation; dilated right ventricle, with moderate to severe dysfunction; moderate tricuspid regurgitation; and estimated pulmonary artery systolic pressure of 40mmHg. The changes found were the same observed in the echocardiography performed in 2006.
A subsequent cardiac catheterization showed 50% lesion in the distal portion of the left main coronary artery, 40% lesions in the proximal third, and 70% and 95% lesions in the distal third of the left anterior descending artery. The left circumflex artery showed only mild and diffuse parietal irregularities, and the right coronary artery showed a 30% lesion in its distal third. The left ventricle showed normal end-diastolic volume and contractile function. The mitral valve did not allow for regurgitation. The recorded pressures were 85/12mmHg in the left ventricle and 85/59mmHg in the aorta. During the same procedure, three endomyocardial fragments were removed, whose histopathological analysis showed subendocardial fibrosis and absence of signs of rejection. In the previous catheterization, performed in 2008, the coronary arteries and the left ventricle showed no changes, and the biopsy found no rejection.
Her case was discussed at a meeting with the cardiology, hemodynamics, and cardiovascular surgery teams in November 2012. There were discordant opinions: the interventionists indicated PCI for the left main coronary artery lesion, whereas surgeons and clinicians preferred surgical treatment. As there was a predominance of the second option, the procedure was scheduled for January 2013.
On December 25th, 2012, the patient went into cardiorespiratory arrest in asystole, successfully reversed. Upon arriving at the emergency room, she was ventilated through an endotracheal tube and received fentanyl, midazolam, and dopamine through infusion pumps. Vital signs showed blood pressure of 106/60mmHg, heart rate of 73bpm, and 93% oxygen saturation. Segmental assessment showed isochoric and photoreactive pupils, rhythmic and muffled heart sounds with no murmurs, discrete crackling rales at the bases, abdomen without visceromegaly, lower limbs without edema and with good perfusion. The electrocardiogram showed no acute ischemic changes. Initial laboratory assessment showed the following significant changes: creatine phosphokinase (CPK)=526 U/L (24 U/L to 170 U/L), creatine kinase – MB fraction (CK-MB)=30.7 U/L (< 25 U/L), troponin=0.18mg/L (< 0.01g/L), and D-dimer=5,681.3ng/mL (68ng/mL to 494ng/mL).
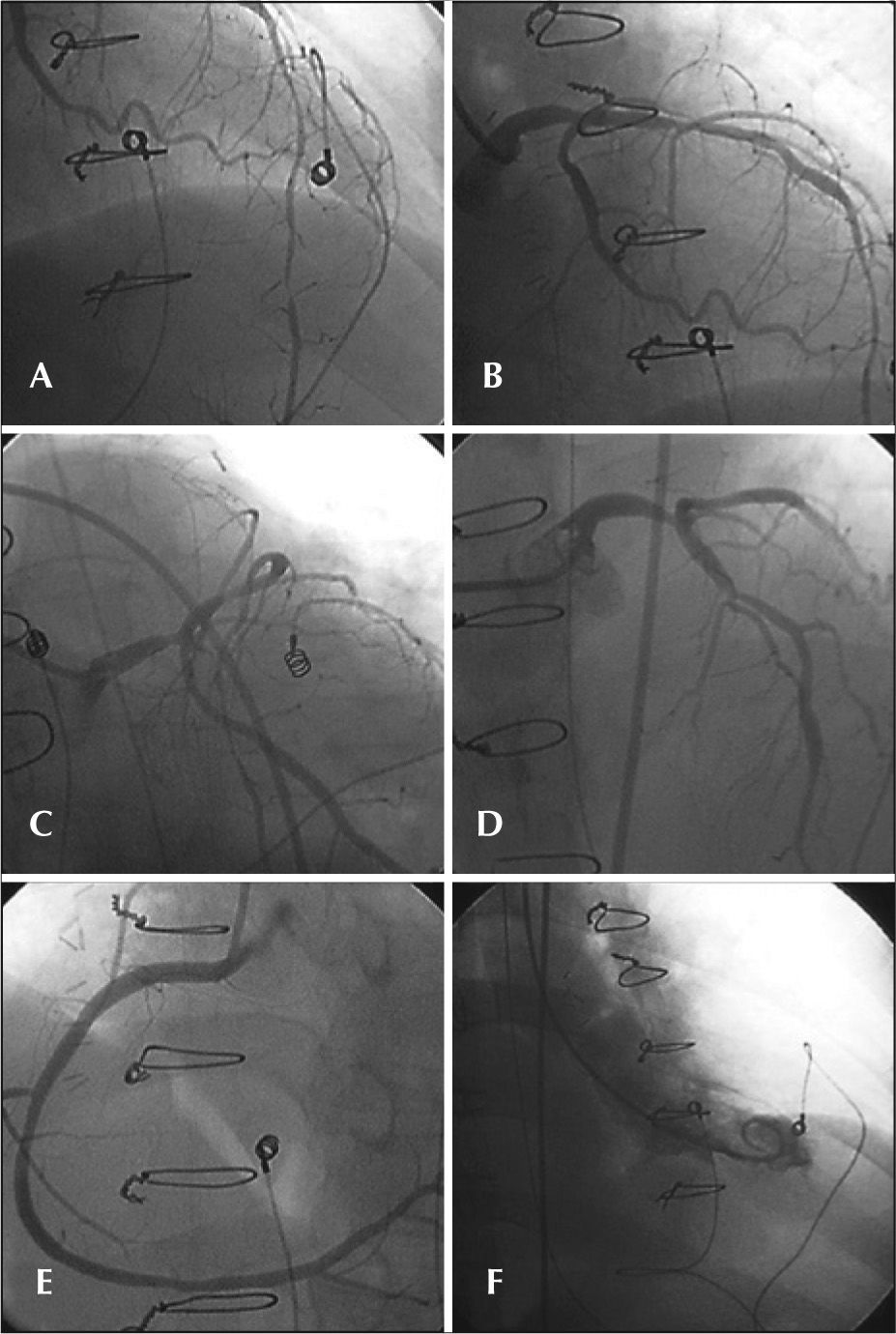
Due to the evolution with persistent hypotension, requiring start of noradrenaline infusion, the hemodynamic team was called to perform an emergency catheterization. The procedure, performed through the femoral artery, was uneventful and showed a 60% lesion in the distal third of the left main coronary artery, 60% lesions in the proximal third, 70% and 95% lesions in the distal third of the left anterior descending artery, 30% lesion in the proximal third of the left circumflex artery, and 40% lesions in the middle and distal thirds of the right coronary artery. There were no new segmental contractility alterations in the left ventricle (Figure 1). The recorded pressures were 172/14mmHg in the left ventricle and 172/83mmHg in the aorta, and the norepinephrine infusion velocity was reduced. An arteriography was performed to rule out the suspicion of pulmonary thromboembolism; no thrombus or areas of peripheral hypoperfusion were found. The pressure measured in the left main coronary artery was 48/21mmHg, with a mean of 31mmHg.
– In A, B, C, and D, left coronary artery in left-anterior oblique, caudal right-anterior oblique, caudal left-anterior oblique, and cranial left-anterior oblique projections, respectively. In E, right coronary artery in cranial left-anterior oblique projection. In F, left ventricular systole in the right-anterior oblique projection.
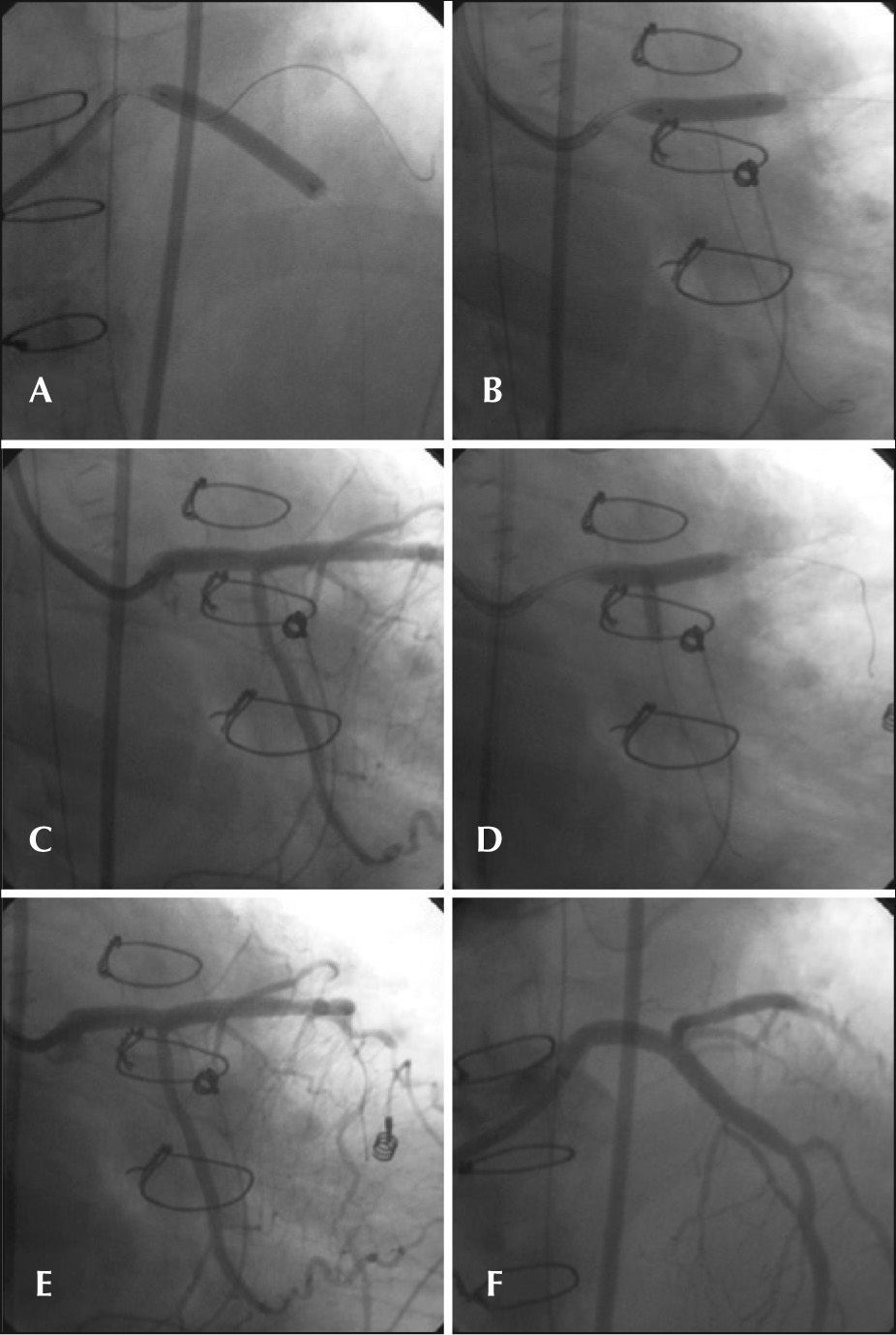
Regarding the adjuvant medication, the patient had already received ASA 200mg and clopidogrel 300mg. The dose of heparin was completed to 100 U/kg intravenously, and one ampoule of isosorbide mononitrate was administered by intracoronary route. The therapeutic catheter chosen was a JL 3.5 8F. Moderate support guidewires with hydrophilic and non-hydrophilic tips were positioned, respectively, in the distal portions of the left anterior descending artery and of the left circumflex artery. Subsequently, a 4.0/33mm everolimuseluting stent was implanted at 12atm, covering the left main coronary artery and the proximal left anterior descending artery lesions. The circumflex artery guidewire was then retreated and repositioned in its distal third, penetrating the stent mesh. It was decided to post-dilate the stent with a 4.0/20mm compliant balloon at 16atm. A 3.0/10mm compliant balloon was positioned at the left circumflex artery ostium and the procedure was finished with a kissing balloon, both inflated to 10atm, yielding satisfactory angiographic result (Figure 2). PCI was not performed in the other left anterior descending artery lesions, as they were located in the distal third, in a fine-calibre segment.
– In A, stent implantation in left main coronary artery lesions and proximal left anterior descending artery. In B, stent post-dilation. In C, control angiography in caudal right anterior oblique projection. In D, kissing balloon. In E, the final result in caudal right anterior oblique projection. In F, the end result in cranial projection.
At the end of the procedure, the patient was hypertensive, allowing for a gradual reduction in the norepinephrine dose, which was suspended on the next day. Markers of myocardial necrosis showed a slight increase, reaching maximum values on December 25th, 2012 at the collection performed 3.5hours after the PCI (CPK=671 U/L and CK-MB=48.1 U/L). The following samples showed a progressive decrease in these values.
Pneumonia was diagnosed on December 27th, and treatment with piperacillin and tazobactam was initiated. On the following day, the patient developed supraventricular tachycardia reversed with electrical cardioversion. On the same day, the patient once more had hypotension, requiring reintroduction of noradrenaline. There was progressive clinical and laboratory worsening, and the patient went into cardiac arrest with pulseless electrical activity on December 31st, 2012.
DISCUSSIONCardiac allograft vasculopathy is not a single disease entity, but rather a set of combined changes characterized by intimal fibromuscular hyperplasia, atherosclerosis (accelerated, affecting both adults and children), and vasculitis (inflammation of one or more layers of the vessel wall). Moreover, the veins are also affected, making the use of the term arteriopathy inaccurate.4
The most significant change in cardiac allograft vasculopathy is the intimal fibromuscular hyperplasia. By definition, it consists in intimal thickening promoted by fibromuscular proliferation, and not by the formation of atheromas or inflammation. Atherosclerotic plaques tend to be eccentric, to affect proximal epicardial vessels and spare the intramyocardial arteries. Intimal fibromuscular hyperplasia tends to be circumferential and may involve diffusely large and small epicardial coronary arteries, as well as intramyocardial vessels. This pattern of diffuse involvement of small epicardial arteries appears to cause most of the arterial occlusions. The intramyocardial arteries are affected in their medial or subepicardial portions, making it unlikely that the endomyocardial biopsy will obtain samples with the involved vessels. It is important to note that calcification, unlike atherosclerosis, is not a prominent finding in intimal fibromuscular hyperplasia, even in severely affected vessels.4
Many risk factors for the development of cardiac allograft vasculopathy have been identified: systemic arterial hypertension, diabetes mellitus, dyslipidemia, obesity, insulin resistance, infection by cytomegalovirus, specific serology for leukocyte antigens, donor’s age, donor’s brain death, and occurrence of cell or humoral rejection.5 Symptom onset may be insidious and, since the heart is denervated, patients often do not report angina. Its existence suggests myocardial reinnervation, which occurs in 10% to 30% of recipients in the long-term. Manifestations may include silent ischaemia, congestive heart failure, ventricular arrhythmias, myocardial infarction, and sudden death.6,7
The diagnosis of cardiac allograft vasculopathy is hindered by silent ischemia and low sensitivity of non-invasive tests. Intravascular ultrasound (IVUS) is more sensitive than coronary angiography to detect early disease and allows for the evaluation of both the arterial lumen and vessel wall. However, only the proximal portions can be evaluated, which limits its role in cases of diffuse vasculopathy.8
The best treatment for cardiac allograft vasculopathy has not been established, but there is evidence that myocardium revascularization procedures are effective in transplanted hearts with evidence of ischaemia and/or high-risk lesions. Patients free of these conditions have a good prognosis; however, those with diffuse disease with no possibility of revascularisation have unfavoura unfavorable ble evolution.3
In transplanted patients, PCI presents excellent angiographic success rates, ranging from 90% to 100%, rare complications, and restenosis rates with drugeluting stents between 9% and 19%, lower than those of bare-metal stents (which range between 18% and 31%).7−12 It is also a good therapeutic option for patients with left main coronary artery lesions. An international retrospective multicenter registry included 21 transplant patients undergoing unprotected left main coronary artery PCI from 1997 to 2009. Of the total procedures, 13 (62%) were elective and eight (38%) were emergency, of which three (14%) were in cardiogenic shock. Ten individuals (48%) had bifurcation lesions in the left main coronary artery. Angiographic success was attained in all patients, of whom 14 (67%) received drug-eluting stents. In a mean follow-up time of 4.9±3.2years, three patients (14 %) died, one patient (5%) had myocardial infarction, and four (19%) had restenosis requiring target-lesion revascularization. There were no cases of stent thrombosis. One individual (5%) was submitted to CABG and five (24%), to a new transplant.11
In a review totaling ten publications and 34 patients undergoing left main coronary artery PCI in the transplanted heart, including the publication mentioned in the previous paragraph, four subjects (11.8%) died within a mean follow-up of 43.1±32.4months. Considering that 12 participants (35.3 %) had distal lesions in the left main coronary artery and 18 (52.9%) received bare-metal stents, repeat revascularization with PCI or CABG was required in nine subjects (26.4%). At 60 months, death- and revascularisation-free survival was 66%.12 To the present date, most articles published on unprotected left main coronary artery PCI in patients undergoing cardiac transplantation consist in a limited number of case reports and small series of patients.11,12
In conclusion, PCI with drug-eluting stents is a therapeutic option for transplanted patients with unprotected left main coronary artery lesions, especially in emergency cases, and can delay the need for a new heart transplant. The procedure is safe and has high rates of immediate success; however, the long-term evolution requires further studies.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.