Medical applications are the main source of ionizing radiation exposure, and in this context the issue of occupational risk is particularly important. Although the different organs of the human body present different radiation sensitivities, specific assessments of the impact on the different regions of the interventionist's body with diverse radioprotection devices are rare in Brazil.
MethodsA scattered radiation test was performed using an ionization chamber in a fluoroscopy station, with standard radioprotection accessory kit of the equipment (lower skirt and upper movable shield, in two different positions), at sequential distances from the source, using acrylic phantoms as human chest simulation.
ResultsDifferences in radiation were identified in relation to distance and use of radioprotection devices. The median radiation reduction was 50.6% (interquartile range – IQ from 39.42% to 51.05%) using the lower skirt shield, 71.3% (IQ from 67.66% to 77.05%) with the addition of an upper shield in angulated position, and 84.7% (IQ from 83.75% to 85.87%) with the addition of an upper shield aligned with the lower shield. Significant differences were also found regarding height and distance from the source.
ConclusionsThe use of the assessed local radioprotection devices was effective in reducing the overall radiological impact to the interventionist. However, there were radiation escape routes, especially with non-ideal positioning, demonstrating the importance of the additional use of individual protection devices.
As aplicações médicas representam a maior fonte de exposição radiológica ionizante e, neste contexto, é de especial importância a questão do risco profissional. Embora existam diferentes sensibilidades à radiação dos distintos órgãos do corpo humano, avaliações específicas do impacto nas diversas regiões do corpo do operador, com diferentes dispositivos de radioproteção, são raras em nosso meio.
MétodosTeste de radiação espalhada foi realizado com câmara de ionização em estação de fluoroscopia, com jogo de acessórios de radioproteção padrão do equipamento (saia inferior e escudo móvel superior, em duas diferentes posições), a distâncias sequenciais em relação à fonte, utilizando fantoma de acrílico em simulação de tórax humano.
ResultadosForam identificadas diferenças na radiação em relação à distância e ao uso dos dispositivos de radioproteção. A redução mediana da radiação foi de 50,6% (intervalo interquartil − IQ de 39,42% a 51,05%) com uso do escudo saia inferior, 71,3% (IQ de 67,66% a 77,05%) com adição de escudo superior em posicionamento angulado e 84,7% (IQ de 83,75% a 85,87%) com adição de escudo superior em linha ao escudo inferior. Diferenças significativas foram encontradas ainda em relação à altura e à distância da fonte.
ConclusõesO uso dos dispositivos locais de radioproteção avaliados se mostrou efetivo na redução global do impacto radiológico ao operador, havendo, no entanto, vias de escape de radiação, especialmente com posicionamento não ideal, demonstrando a importância do uso adicional dos dispositivos de proteção individuais.
Currently, medical applications are the main source of artificial ionizing radiation exposure in the population. Among the medical application fields, the issue of occupational risk is especially important, considering that the higher the exposure to the radiation source and the longer the exposure time, the higher the risk. In this context, interventional cardiologists are routinely exposed to ionizing radiation; among the professionals exposed to radiation, interventional cardiologists are those who accumulate the highest load received, mainly due to exposure to the scattered radiation from the patient receiving the primary beam of X-rays.1 Consequently, interventionists that perform radiological interventional techniques adopt the as low as reasonably achievable (ALARA) principle,2 limiting the duration of the exposure, increasing the distance from the radiation source and maintaining shields of radiological protection.
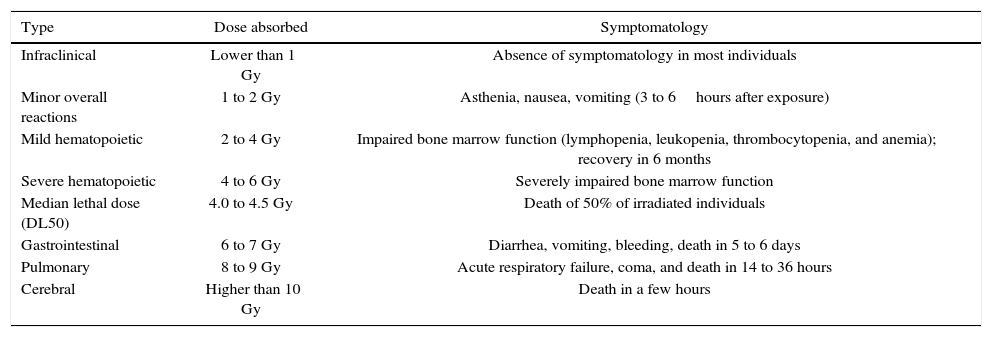
The national3–5 and international6–8 literatures describe the deleterious effects of ionizing radiation (Table 1), as well as maximum recommendation standards for cumulative occupational exposure, according to the affected area (Table 2).
Effect of acute radiation exposure in adults.3
| Type | Dose absorbed | Symptomatology |
|---|---|---|
| Infraclinical | Lower than 1 Gy | Absence of symptomatology in most individuals |
| Minor overall reactions | 1 to 2 Gy | Asthenia, nausea, vomiting (3 to 6hours after exposure) |
| Mild hematopoietic | 2 to 4 Gy | Impaired bone marrow function (lymphopenia, leukopenia, thrombocytopenia, and anemia); recovery in 6 months |
| Severe hematopoietic | 4 to 6 Gy | Severely impaired bone marrow function |
| Median lethal dose (DL50) | 4.0 to 4.5 Gy | Death of 50% of irradiated individuals |
| Gastrointestinal | 6 to 7 Gy | Diarrhea, vomiting, bleeding, death in 5 to 6 days |
| Pulmonary | 8 to 9 Gy | Acute respiratory failure, coma, and death in 14 to 36 hours |
| Cerebral | Higher than 10 Gy | Death in a few hours |
Limits of equivalent radiation doses for interventionists.
| Area of exposure | mSv/year |
|---|---|
| Crystalline lens | 20 |
| Thyroid | 150 |
| Skin | 500 |
| Annual Effective Dose | 20 for 5 consecutive years of work OR 50 in 1 year |
Source: Comissão Nacional de Energia Nuclear (CNEN portuguese for National Nuclear Energy Committee). Diretrizes Básicas de Proteção Radiológica. Rio de Janeiro: Ministério da Ciência, Tecnologia e Inovação; 2014 [cited 2016 Jan 30]. Available: http://appasp.cnen.gov.br/seguranca/normas/pdf/Nrm301.pdf
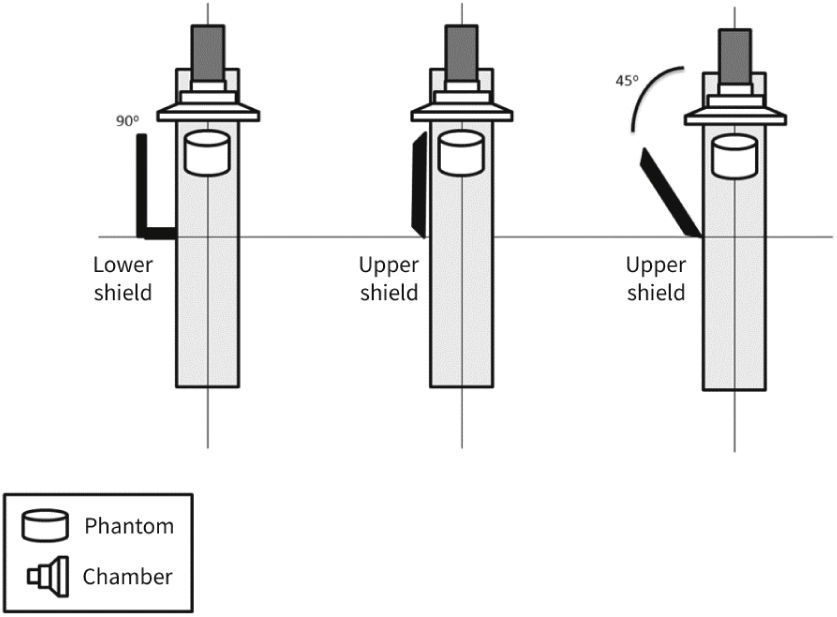
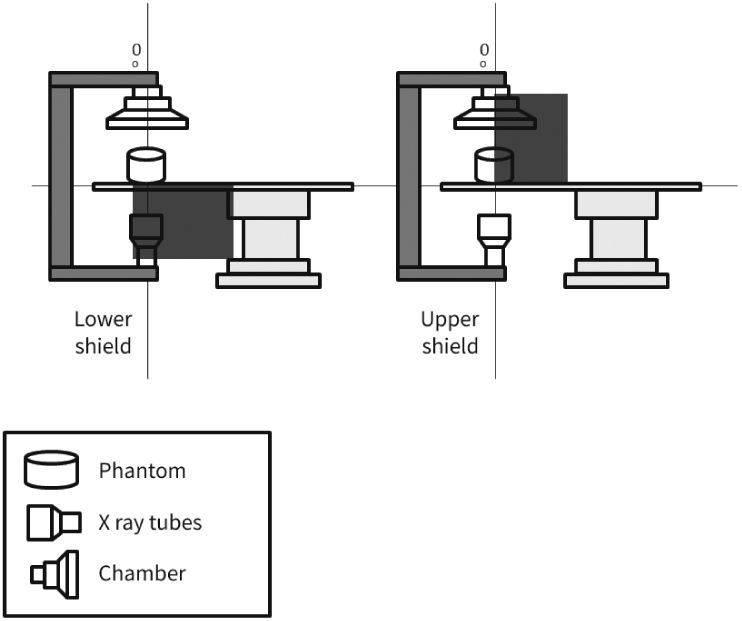
Usually, the standard radiation protection set includes individual protection equipment, such as radiation protection apron and thyroid collar, both with 0.5mm of lead equivalence, and goggles, constructed with 0.75mm lead glass. Moreover, local protection devices at the fluoroscopy station, such as a skirt-type lead vinyl shield in the lower region of the table (with or without an additional folding bulkhead), and movable suspended glass shield with lead vinyl curtain, both with 0.5mm of lead equivalence, are universally used, providing interventionists with protection against 95% of the total radiation to which they are exposed.9
The equivalent dose limits differ between the several regions of the interventionist's body, according to radiation sensitivity; the crystalline lens is considered the limiting organ. Although an international guideline10 recommends the use of three dosimeters to highly exposed individuals (including a personal dosimeter under the lead apron), the Brazilian guideline establishes a single measurement at chest level, outside the apron,4 and the individual dose or effective dose equivalent is estimated from the exposure measured by this single dosimeter. The calculation of the total impact is performed by multiplying the dose recorded in the chest by the correction factor for photons (factor f = 1.14Sv/Gy) and expressed in Sv.11
This is the most direct of the available measures of interventionists’ cancer risk in daily practice; this value is usually presented in the monthly reports of occupational exposure. A review of the risks and adverse effects of ionizing radiation in interventional cardiology has been recently published, presenting detailed aspects of Brazilian and international standards, with important recommendations for the protection of patients and staff.5
Although there are different sensitivities to radiation and more sensitive organs, such as the gonads, and thyroid, specific evaluations for the interventionist, focused on the different regions of the body, are rare.12 To the best of the authors’ knowledge, the literature does not present an evaluation of the differential impact in the most sensitive organs using the different local radioprotection devices available at the fluoroscopy station.
The objective of the present study was to evaluate the impact of scattered radiation with the use of different radiation protection equipment available at the fluoroscopy station (lead vinyl shield with lower skirt and movable glass shield with lead vinyl curtain), in a controlled simulated catheterization laboratory environment, testing variations regarding the height and the distance from the source.
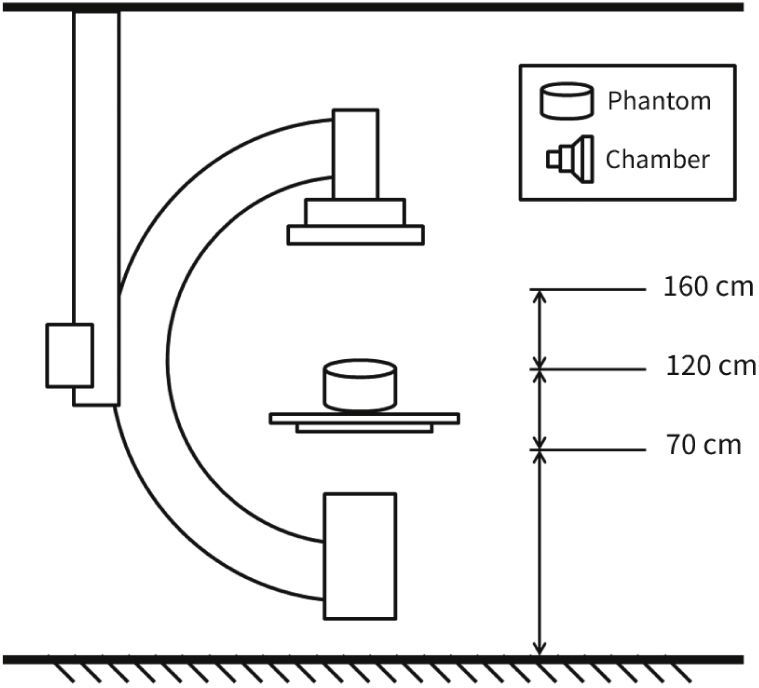
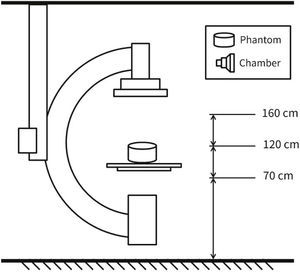
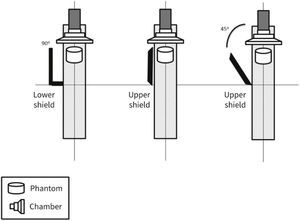
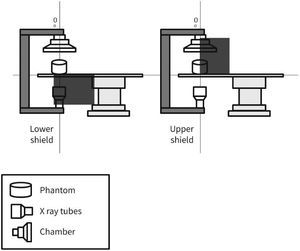
MethodsThe radiometric field test for scattered radiation was carried out using a RadCal 1,800cm3 ionization chamber and correction factor of 1, with 48% ambient air humidity, 93 Kpa atmospheric pressure, and 25°C temperature, in a previously calibrated fluoroscopy station (Philips, Allura Xper FD20), in the 48-cm field in standard georeset position, without angulation. The standard positioning of the X-ray tube in the georeset position was suspended 50cm from the ground, with a 40-cm distance between the table and the tube, and a flat detector positioned 30cm above the table, with a set of standard radiation protection accessory equipment, comprising the lower skirt and upper movable shield, both with a 0.5-mm lead equivalence, and positioned as shown in Figures 1 to 3. A 1.7-cm Al filter (the acronym refers to the aluminum thickness - Al) was used in the primary beam, kept stable at a rate of 15 frames per second, at 80kV and 14.3mA. A commercial acrylic phantom was positioned in the central axis of the beam, simulating the human thorax.
The acquisition sequence was carried out according to the distance to the source (axial axis between the primary beam and acrylic phantom) and measured at three defined height points, simulating the gonads, thorax, and the crystalline lens, assuming a 1.70-m tall interventionist. Three standard measures were then obtained at 70cm, 120cm, and 160cm in height. Measurements regarding distance from the source were detailed every 50cm (50 to 300cm) for scattered radiation relative to the primary beam, which was measured after beam stabilization in relation to time, as microSievert/minute (μSv/min) and in the diagonal direction at 45° from the table's axis, just posterior to the radiation protection devices.
Continuous variables were expressed as units and percentages, or medians and interquartile ranges (IQ), as required. The software used to create the spreadsheets and charts was Microsoft Excel, version 2010 (Microsoft Corporation, Redmond, USA).
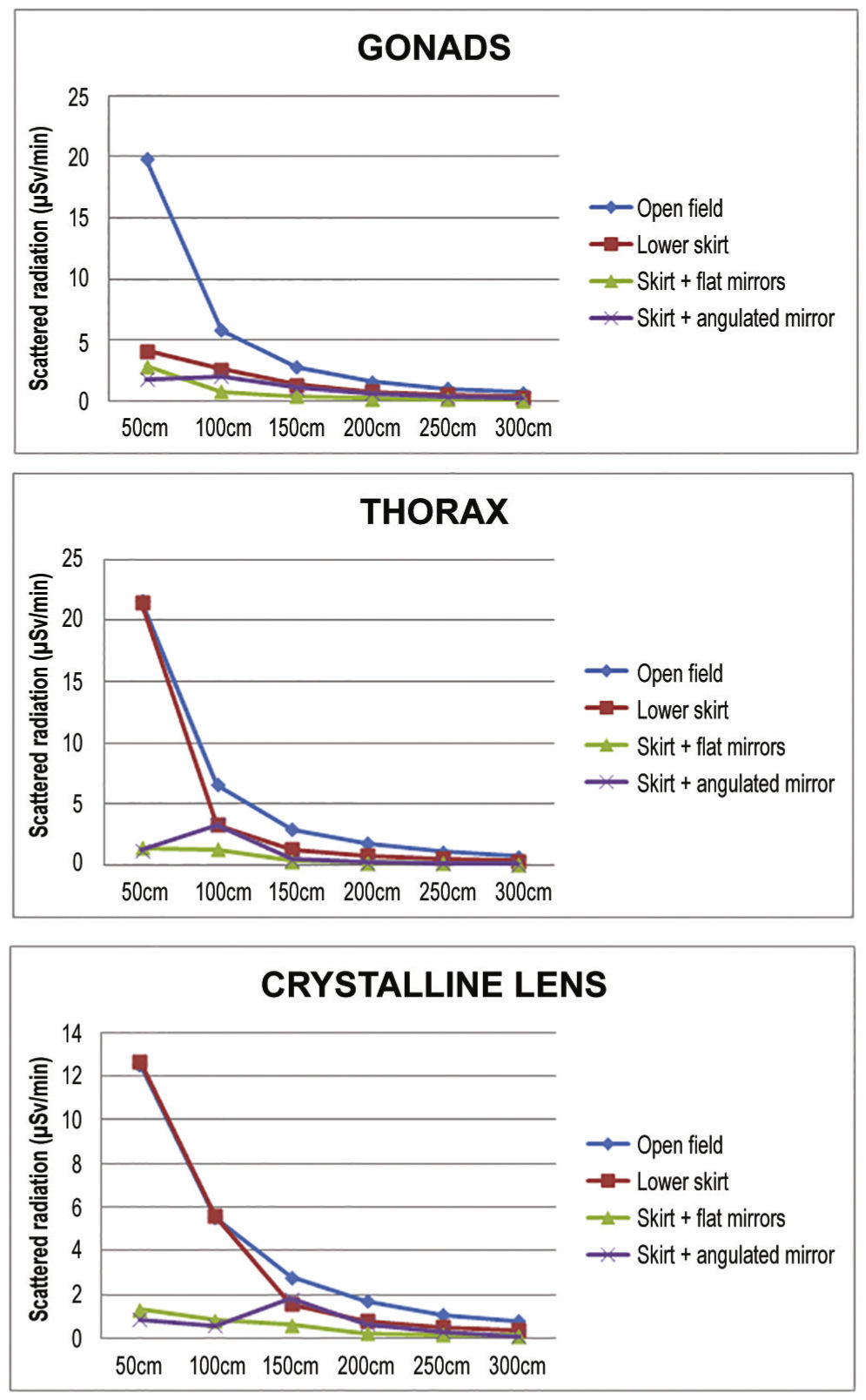
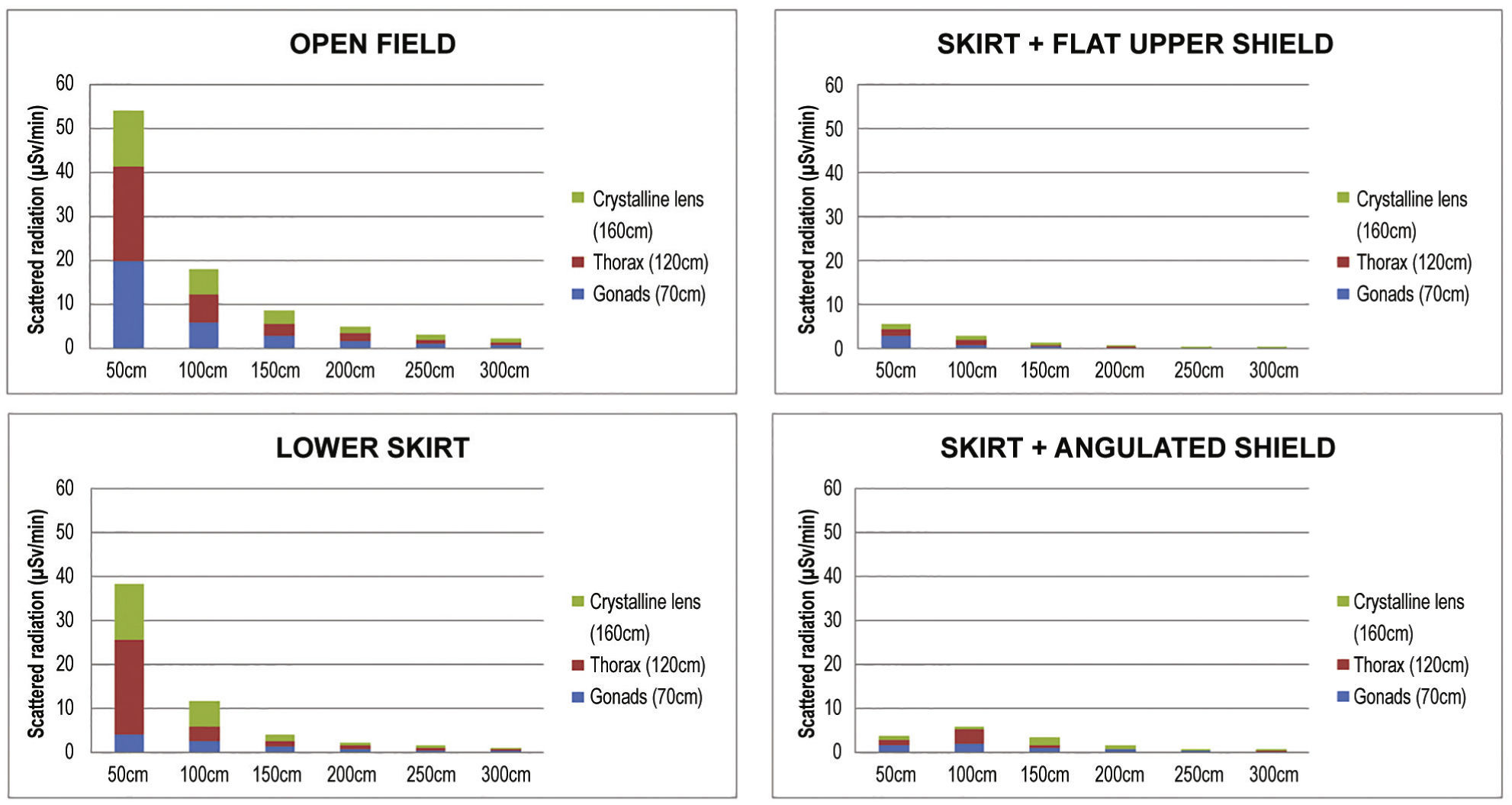
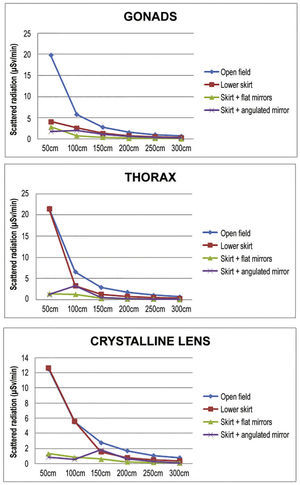
ResultsDifferences were observed between the groups regarding the level of radiation directed to the interventionist. The levels reached at the different measurement sites are presented in Table 3, and Table 4 presents their percentage reduction in relation to the open field [reduction% = (radiationopen field- with device) / radiationopen field × 100].
Scattered radiation in relation to distance to the source, in microSievert/minute, and to the use of the different protection devices.
| Assessed focus | Open field | Lower skirt | Skirt/flat shield | Skirt/angulated shield |
|---|---|---|---|---|
| Gonads (70 cm) | ||||
| 50 cm | 19.80 | 4.12 | 2.86 | 1.79 |
| 100 cm | 5.83 | 2.61 | 0.83 | 2.03 |
| 150 cm | 2.79 | 1.38 | 0.41 | 1.13 |
| 200 cm | 1.63 | 0.83 | 0.23 | 0.66 |
| 250 cm | 1.02 | 0.54 | 0.16 | 0.39 |
| 300 cm | 0.72 | 0.37 | 0.11 | 0.27 |
| Thorax (120 cm) | ||||
| 50 cm | 21.6 | 21.45 | 1.42 | 1.22 |
| 100 cm | 6.58 | 3.32 | 1.29 | 3.29 |
| 150 cm | 2.95 | 1.31 | 0.37 | 0.51 |
| 200 cm | 1.80 | 0.79 | 0.24 | 0.24 |
| 250 cm | 1.12 | 0.53 | 0.17 | 0.17 |
| 300 cm | 0.76 | 0.36 | 0.11 | 0.12 |
| Crystalline lens (160 cm) | ||||
| 50 cm | 12.53 | 12.70 | 1.34 | 0.87 |
| 100 cm | 5.54 | 5.59 | 0.84 | 0.60 |
| 150 cm | 2.80 | 1.56 | 0.63 | 1.84 |
| 200 cm | 1.70 | 0.79 | 0.24 | 0.66 |
| 250 cm | 1.08 | 0.51 | 0.17 | 0.31 |
| 300 cm | 0.79 | 0.38 | 0.12 | 0.10 |
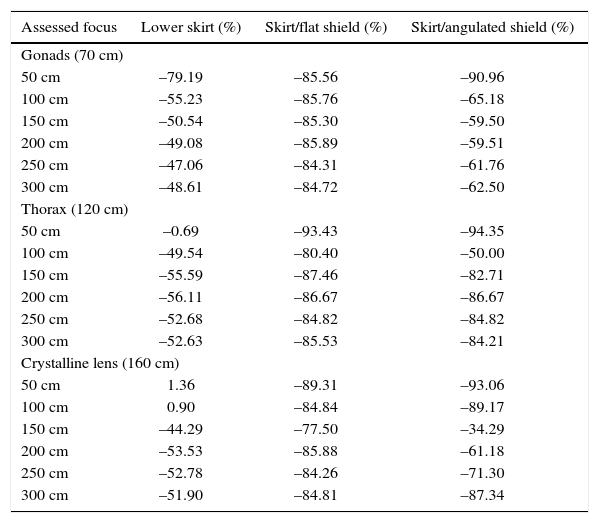
Reduction of scattered radiation in relation to distance to the source and use of different protection devices.
| Assessed focus | Lower skirt (%) | Skirt/flat shield (%) | Skirt/angulated shield (%) |
|---|---|---|---|
| Gonads (70 cm) | |||
| 50 cm | –79.19 | –85.56 | –90.96 |
| 100 cm | –55.23 | –85.76 | –65.18 |
| 150 cm | –50.54 | –85.30 | –59.50 |
| 200 cm | –49.08 | –85.89 | –59.51 |
| 250 cm | –47.06 | –84.31 | –61.76 |
| 300 cm | –48.61 | –84.72 | –62.50 |
| Thorax (120 cm) | |||
| 50 cm | –0.69 | –93.43 | –94.35 |
| 100 cm | –49.54 | –80.40 | –50.00 |
| 150 cm | –55.59 | –87.46 | –82.71 |
| 200 cm | –56.11 | –86.67 | –86.67 |
| 250 cm | –52.68 | –84.82 | –84.82 |
| 300 cm | –52.63 | –85.53 | –84.21 |
| Crystalline lens (160 cm) | |||
| 50 cm | 1.36 | –89.31 | –93.06 |
| 100 cm | 0.90 | –84.84 | –89.17 |
| 150 cm | –44.29 | –77.50 | –34.29 |
| 200 cm | –53.53 | –85.88 | –61.18 |
| 250 cm | –52.78 | –84.26 | –71.30 |
| 300 cm | –51.90 | –84.81 | –87.34 |
The reduction of the radiation accumulated in the three points of interest is shown in Figures 4 and 5, according to the distance from the source. The median radiation reduction was 50.6% (IQ: 39.42% to 51.05%) with the use of the lower lead shield, 71.3% (IQ: 67.66% to 77.05%) with addition of an upper angulated shield, and 84.7% (IQ: 83.75% to 85.87%) with addition of the upper shield in line with the lower shield.
Compared with studies evaluating patient exposure, there is relatively little literature focusing on interventionist exposure; most of the radiation studies are expressed in fluoroscopy time, dose area product (DAP), or air kerma (AK).
Currently, both the medical community and regulatory agencies are aware of the oncological effect of low-dose ionizing radiation, assuming uncertainties due to the lack of direct scientific evidence for risk calculations, which may be two- to three-fold higher or lower than estimated; generally, the acceptable risk threshold for occupational exposure is 50 mSv.1 However, a study that evaluated interventional cardiologists exposed to a mean radiation dose of 4 mSv per year showed a two-fold increase in the presence of lymphocytes with chromosomal aberrations and the presence of micronuclei, surrogate markers for cancer risk.13
Certainly, scientific evidence, albeit not conclusive, is a warning for practical purposes of recommendations aimed at the protection against ionizing radiation. The risk of the average exposure should be considered even from the perspective of highly-exposed professionals and not only the mean exposure, which can be quite heterogeneous within the same environment. With an exposure of 5 mSv per year, the incidence of cancer after 20 years of professional activities is estimated as 1 in 100, which, even within the limits of occupational safety, cannot be considered a negligible risk.1
In the present study, the use of protection devices effectively reduced scattered radiation, the main risk focus for the interventionist. When analyzed individually, the lower shield had a significant impact on reducing radiation at the gonad level, as expected, but it was observed that the upper lead glass shield, positioned above, meant additional reduction, especially when rectified in accordance with the skirt and at a distance of 50 to 100cm. This finding is logical, considering that the main source of scattered radiation is the phantom, rather than the X-ray tube, positioned in the lower portion of the fluoroscopy station, and source of the primary beam. In relation to the levels of thoracic radiation, the results demonstrate effective radiation reduction after adding the upper shield, which also occurs in relation to the simulated crystalline lens height of 160cm.
The interventionist's position is particularly important. It is noteworthy that there is a reduction in the interventionist's protection when he or she moves away from the upper shield, as observed when we analyze the distance between 50 to 100cm. Additionally, it is important to observe that, when the upper shield is angulated, a quite common habit in clinical practice, an escape route is created, reducing the protection efficiency at 100cm in both gonads and thorax, and at 150cm in the crystalline lens. Since this is the usual distance from the interventionist to the source, it potentially reflects a reduction in protection provided by the devices in case of improper positioning, even supplanting the expected reduction by increasing the distance between the interventionist and the phantom.
With the correct use of both shields, the total accumulated radiation between 100 and 150cm was approximately 2μSv/min, that is, a rate quite similar to the open field beyond 250 to 300cm. When comparing this dose to the usual amount of radiation from a chest X-ray (20μSv),3 the exposure would be equivalent to one radiography every 10minutes of work, with adequate protection, and one radiography per minute, in the absence of protection devices at the fluoroscopy station.
Between 50 and 150cm, the greatest reduction in scattered radiation (85%) was provided by the concomitant use of the upper and lower shields. As a theoretical exercise, the threshold of 20 mSv per year for the crystalline lens would be reached after just over 3,600minutes of radiation at 100cm from the source. In 2016, with just over 250 working days, a busy interventionist performing 8 procedures a day with less than 2minutes of fluoroscopy per examination would reach this threshold if he or she did not use the radiation protection devices. While this calculation is certainly only hypothetical, as it does not consider the use of radiation protection glasses, it is useful to demonstrate the importance of the correct use of the room's protection equipment, which must be complemented with the individual radiation protection equipment.
Finally, and surprisingly, the association between the total accumulated radiation in relation to the thorax, where the standard thermoluminescent dosimeter is positioned, oscillated between 2.5 and 3.0 times in the open field simulations, raising the possibility that the usual estimate of correction for individual dosimetry in homogeneous fields (with a correction factor of 1.14, derived from radiological studies) may be underestimated in relation to the reality of interventional cardiology procedures, especially considering angulations and directional escape in relation to the primary beam.
The evaluation of radiological risks has been of growing interest in scenarios where increasingly complex procedures are performed, with a longer time of radiological exposure for both the patient and the interventionist. In this aspect, to the best of the authors’ knowledge, this is the first study in which several aspects of radiological impact of clinical importance, such as simulation of exposure points for the occupationally exposed physician and evaluation of the effectiveness of local radiation protection devices, were simultaneously evaluated.
LimitationsThis is a pilot study and has important limitations, such as not analyzing the effects of angulations of different projections, using a standard shield positioning (which may not reflect daily practice), and not taking into account variations from different types of fluoroscopy equipment. However, the findings regarding the impact of total radiation accumulated at the different measurement points and the divergence found in relation to the correction factor usually used as a supposition for occupational risk calculations certainly raise questions that should be analyzed in other specific studies.
ConclusionsThe use of local radioprotection devices showed to be effective in the overall reduction of radiological impact to the interventionist; however, there are radiation escape routes, especially with non-ideal positioning, demonstrating not only a potential field of research but also the importance of additional use of individual protection devices.
Sources of fundingThe analysis was funded through the use of radiological measurement services from an outsourced company, as informed in the conflicts of interest. Supri Artigos Médico Hospitalares Ltda. sponsored the study.
Conflicts of interestThe analysis of the ionizing radiation was carried out in a public-private partnership in the Catheterization Laboratory of Faculdade Estadual de Medicina de Marília. The main author is a consultant on ionizing radiation, and the analysis was sponsored by a private radioprotection equipment company. The sponsoring was blinded, unrestricted, and had no influence on the observed results. The other authors have no conflicts of interest related to the study.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.