Coronary perforation is currently a rare, but potentially catastrophic complication. The aim of the study was to evaluate the incidence, predictors, management and prognosis of coronary perforations at a hospital with a large number of percutaneous coronary interventions (PCIs).
MethodsClinical, angiographic, procedural and in-hospital outcomes of patients with or without coronary perforations were compared. Univariate analysis was performed to determine the predictors of this complication.
ResultsFrom December 2007 to January 2012, 5,585 consecutive patients were submitted to PCI and 18 had coronary perforation (0.32%), of whom 55.5% were female and 38.9% were diabetic. In this group, the left anterior descending artery was the most frequently treated vessel (61.1%) as well as type C lesion (61.1%) and chronic occlusions were approached in 27.8% of these cases. Most of the coronary perforations (11/18) had a lower complexity according to the modified Ellis classification, whereas the remaining perforations were classified as grades III (6/18) or IV (1/18). The balloon-catheter device was responsible for perforation in 61.1% of the cases. Prolonged inflation with a balloon-catheter and heparin reversal with protamine was performed in 72.2% and 88.9% of the cases, respectively. Only 1 patient (5.6%) required an emergency surgery due to cardiac tamponade. There were no deaths associated with coronary perforation. According to the univariate analysis, coronary perforation predictors were: female gender (P=0.03), chronic obstructive pulmonary disease (P=0.006) and chronic occlusion (P<0.01).
ConclusionsIn our experience, coronary perforation was a rare event, which was managed conservatively in most of the cases and was associated with a good in-hospital outcome.
Incidência, Manejo e Prognóstico de Perfurações Coronárias
IntroduçãoA perfuração coronária na atualidade é complicação rara, mas potencialmente catastrófica. Nosso objetivo foi avaliar a incidência, os preditores, o manejo e o prognóstico das perfurações coronárias na experiência de um serviço de cardiologia intervencionista com grande volume de intervenções coronárias percutâneas (ICPs).
MétodosComparamos as características clínicas, angiográficas e do procedimento e a evolução intra-hospitalar de pacientes que apresentaram ou não perfuração coronária. Análise univariada foi realizada para determinar os preditores dessa complicação.
ResultadosNo período de dezembro de 2007 a janeiro de 2012, 5.585 pacientes consecutivos foram submetidos a ICP e 18 apresentaram perfuração coronária (0,32%), dos quais 55,5% eram do sexo feminino e 38,9% eram diabéticos. Nesse grupo, a artéria descendente anterior foi o vaso mais frequentemente tratado (61,1%), assim como a lesão do tipo C (61,1%), e as oclusões crônicas foram abordadas em 27,8% desses casos. A maioria das perfurações coronárias (11/18) apresentou menor complexidade de acordo com a classificação de Ellis modificada, enquanto as demais foram qualificadas como graus III (6/18) ou IV (1/18). O cateter-balão foi o dispositivo responsável pela perfuração em 61,1% dos casos. Realizou-se insuflação prolongada com cateter-balão e inativação da heparina com protamina em 72,2% e 88,9% dos casos, respectivamente. Apenas 1 paciente (5,6%) necessitou de abordagem cirúrgica de emergência em decorrência de tamponamento cardíaco. Não houve óbito associado à perfuração coronária. Na análise univariada, os preditores de perfuração coronária foram: sexo feminino (P=0,03), doença pulmonar obstrutiva crônica (P=0,006) e oclusão crônica (P<0,01).
ConclusõesEm nossa experiência, a perfuração coronária foi evento raro, controlada conservadoramente na maioria dos casos e com evolução hospitalar satisfatória.
Coronary perforation is a rare complication of the percutaneous coronary intervention (PCI) procedure that is characterised by a rupture of the arterial lumen, resulting in the leakage of its contents. This complication can be caused by several percutaneous devices used during PCI, such as guidewires, atherotomes (directional or rotational), balloon catheters, and metallic stents. 1 Even with the large increase in the number of PCIs around the world, coronary perforation currently remains an infrequent phenomenon, albeit a potentially catastrophic event that may lead to the occurrence of acute myocardial infarction (AMI), cardiac tamponade, emergency surgery and death. In general, coronary perforation occurs in 0.1% to 0.6% of coronary angioplasty procedures, and its occurrence has historically been associated with PCIs in lesions with complex morphology, including calcification and severe chronic occlusion, the use of atherectomy devices and hydrophilic guidewires. 2−12 Moreover, previous studies have reported mortality due to coronary perforation in approximately 5% to 10% of cases, with advanced age, type (or severity) of perforation, and the occurrence of cardiac tamponade requiring emergency surgery as the commonly reported predictors. 3,4
When treating coronary perforation, it is necessary to institute immediate therapy aimed at containing the coronary leak and decompression of the pericardial cavity. Such measures include, but are not limited to, inactivation of antithrombin agents, prolonged insufflation with a balloon catheter, coated stent implantation, coil embolisation, pericardiocentesis or even emergency heart surgery. 3,4
This study aimed to report the incidence, management, prognosis and predictors of coronary perforation occurring in patients undergoing PCI in the daily practice of an interventional cardiology service with a high number of PCIs.
METHODSStudy design and populationA retrospective analysis of data from patients treated at the Department of Invasive Cardiology of the Instituto Dante Pazzanese de Cardiologia (São Paulo, SP, Brazil) from December of 2007 to January of 2012 was performed. During this period, all patients undergoing elective or emergency PCI had their information related to clinical, angiographic and procedural characteristics and the in-hospital clinical evolution prospectively collected and stored in a dedicated electronic database following a pre-established protocol. We identified patients who had coronary perforation during PCI. Patients who had coronary perforation during PCI were identified, and then compared to patients who did not suffer this complication.
Coronary perforation classification and definitionsThe coronary perforations followed the modified Ellis classification: 2
- –
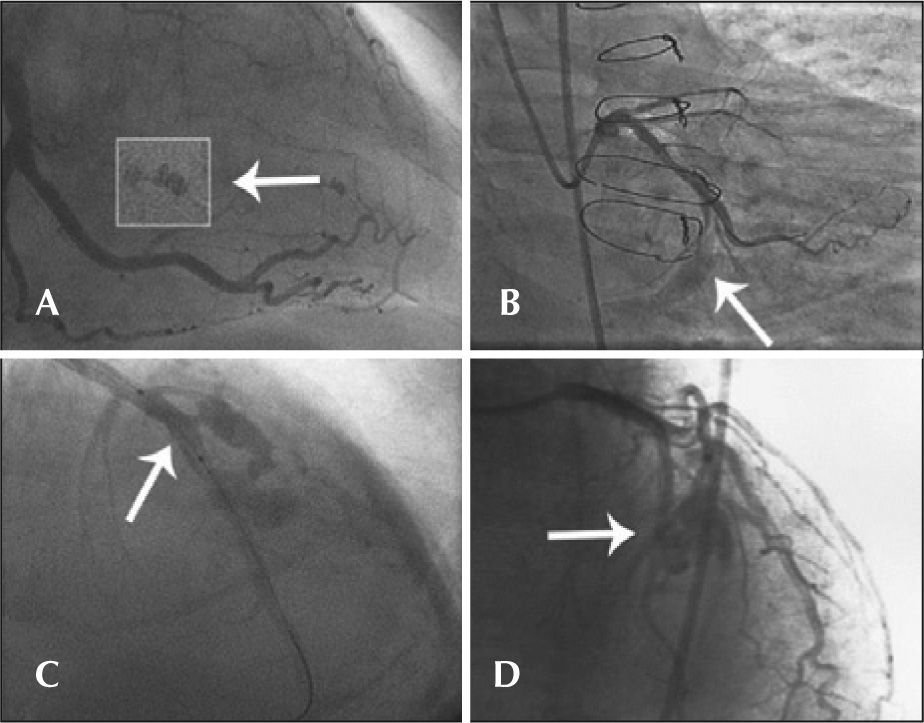
Type I: extraluminal crater with no linear contrast extravasation that suggests dissection (Figure 1A);
Figure 1.– In A, the left circumflex artery with an extraluminal crater (type I Ellis perforation). In B, the left circumflex artery with contrast extravasation into the pericardium with an orifice<1mm diameter (type II Ellis perforation). In C, left anterior descending artery showing rupture in its middle third, with diameter>1mm and significant extravasation of contrast outside the arterial lumen (type III Ellis perforation). In D, rupture of an important septal branch (left anterior descending artery branch) showing extravasation of contrast to the septum, with no extravasation of contrast to the pericardium (type IV Ellis perforation).
(0.13MB). - –
Type II: myocardial or pericardial blushing with an orifice<1mm (Figure 1B);
- –
Type III: frank contrast medium extravasation into the pericardium through an orifice>1mm in diameter (Figure 1C) and
- –
Type IV: perforation with contrast extravasation directly into the left ventricle, to the coronary sinus or other vascular chamber, excluding the pericardium (Figure 1D).
Cardiac tamponade was defined by the presence of one or more of the following characteristics: a) systemic hypotension (systolic blood pressure<90mmHg), with evidence of paradoxical pulse by clinical assessment or invasive method; b) evidence of pericardial effusion by angiography; and c) echocardiographic evidence of significant respiratory variation of the transmitral Doppler velocity, dilated inferior vena cava with collapse during inspiration, and/or diastolic collapse of the right ventricular free wall. 13 Periprocedural AMI was defined by the elevation of the biomarker creatine kinase MB fraction>three times the upper limit of normal values on examination performed up to 24 hours after the procedure. Major adverse clinical events (MACE) were defined by the combined outcomes of death from any cause, AMI, and emergency heart surgery. Angiographic success was defined by the following criteria: a) obtaining flow a Thrombolysis In Myocardial Infarction (TIMI) grade of 3; b) the absence of thrombus, dissection, or perforation with active contrast extravasation; and c) residual stenosis<30% (quantitative coronary angiography) in the treated segment after the procedure. Procedural success was defined as angiographic success plus the absence of MACE during the index hospitalisation.
ProcedureThe PCI procedures were performed according to current guidelines, aimed at optimal angiographic results after coronary device implantation. 14 The route of arterial access, the choice of material to perform the PCI (guidewire, balloon catheter, etc.), the type of stent used, the stent implantation technique, and the adjunctive medical therapy were at the discretion of the surgeon. Regarding antithrombotic therapy, the pretreatment included acetylsalicylic acid at a dose of 100mg/day in cases of chronic use (> seven days) or 200mg given>24 hours before PCI and clopidogrel at a loading dose of 300mg>24 hours before surgery or 600mg before the procedure (preferably>two hours) in patients with acute coronary syndrome. After the procedure, patients were instructed to maintain dual antiplatelet therapy (acetylsalicylic acid 100mg plus clopidogrel 75mg) for at least one month in case of bare-metal stent implantation and one year for drugeluting stents. As for antithrombin therapy administered during the procedure, heparin was administered intravenously at a dose of 70 U/kg to 100 U/kg body weight to maintain an activated clotting time>250 seconds or>200 seconds in cases of concomitant administration of glycoprotein IIb/IIIa inhibitors, at the discretion of the surgeon.
Complementary examinations were conducted according to the institution protocol and included 12-lead ECG before, immediately after, and daily after the procedure until hospital discharge. The laboratory tests included cardiac biomarkers pre-procedure, in the first 24 hours after the procedure, and daily until discharge.
Angiographic analysisQualitative and quantitative angiographic analyses were performed before and after the procedure. A qualitative assessment was performed according to the criteria used for the classification of the American College of Cardiology and the American Heart Association (ACC/ AHA), 15 where lesions were considered type C when at least one of the following was present: a) length>20mm (diffuse lesion), b) significant tortuosity (three or more angles≥75 degrees in the segment proximal to the lesion), c) significant angulation of the lesion (≥90 degrees), d) bifurcated lesion with an incapacity to protect the side branch with the guidewire, e) lesion in degenerate saphenous vein and f) chronic occlusion (≥three months). The analysis of quantitative coronary angiography was performed offline by professionals who had experience with the method, through a validated and commercially available program (QAngio XA−version 7.3−Medis Medical Imaging Systems bv−Leiden, the Netherlands). Lesion extension was delimited by the distance between points immediately before and after the target stenosis considered free of atheromatous disease, that is, the transition between the stenotic segment and the normal references. The minimal lumen diameter (MLD) and the reference diameter (RD) were used to calculate the diameter stenosis (DS) through the following formula: DS (%)=[1−(MLD/RD)] x 100. Immediate gain was defined as the pre-and post-procedure MLD difference (post-procedure MLD−pre-procedural MLD). The balloon:artery ratio was determined by the maximum diameter of the inflated catheter-balloon divided by the RD. Quantitative variables were reported for intrastent segment and intrasegment, which incorporate the intrastent segment plus the 5-mm borders in the persistent regions, according to previously described methodology.
Statistical analysisQualitative variables were expressed as absolute frequencies and percentages and compared by chi-squared test or Fisher's exact test, as appropriate. Quantitative variables were expressed as the mean and standard deviation and compared using Student’s t-test. A value of P<0.05 was considered significant.
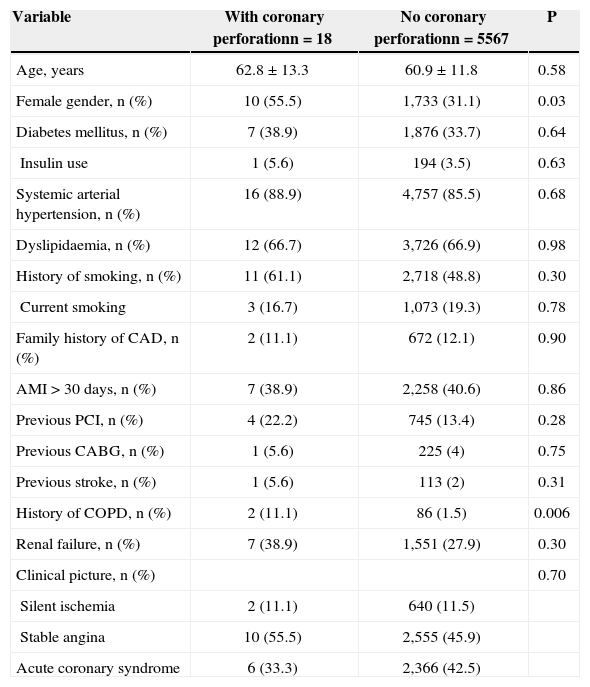
RESULTSPCIs were performed in 5,585 patients, of whom 18 (0.32%) had coronary perforation; 55.5% were females, with a mean age of 62.8±13.3years, and 38.9% were diabetics (Table 1). The group of coronary perforation patients had a higher prevalence of females (P=0.03) and history of chronic obstructive pulmonary disease (P=0.006).
Baseline clinical characteristics
| Variable | With coronary perforationn=18 | No coronary perforationn=5567 | P |
|---|---|---|---|
| Age, years | 62.8±13.3 | 60.9±11.8 | 0.58 |
| Female gender, n (%) | 10 (55.5) | 1,733 (31.1) | 0.03 |
| Diabetes mellitus, n (%) | 7 (38.9) | 1,876 (33.7) | 0.64 |
| Insulin use | 1 (5.6) | 194 (3.5) | 0.63 |
| Systemic arterial hypertension, n (%) | 16 (88.9) | 4,757 (85.5) | 0.68 |
| Dyslipidaemia, n (%) | 12 (66.7) | 3,726 (66.9) | 0.98 |
| History of smoking, n (%) | 11 (61.1) | 2,718 (48.8) | 0.30 |
| Current smoking | 3 (16.7) | 1,073 (19.3) | 0.78 |
| Family history of CAD, n (%) | 2 (11.1) | 672 (12.1) | 0.90 |
| AMI>30 days, n (%) | 7 (38.9) | 2,258 (40.6) | 0.86 |
| Previous PCI, n (%) | 4 (22.2) | 745 (13.4) | 0.28 |
| Previous CABG, n (%) | 1 (5.6) | 225 (4) | 0.75 |
| Previous stroke, n (%) | 1 (5.6) | 113 (2) | 0.31 |
| History of COPD, n (%) | 2 (11.1) | 86 (1.5) | 0.006 |
| Renal failure, n (%) | 7 (38.9) | 1,551 (27.9) | 0.30 |
| Clinical picture, n (%) | 0.70 | ||
| Silent ischemia | 2 (11.1) | 640 (11.5) | |
| Stable angina | 10 (55.5) | 2,555 (45.9) | |
| Acute coronary syndrome | 6 (33.3) | 2,366 (42.5) |
CAD=coronary artery disease; COPD=chronic obstructive pulmonary disease; AMI=acute myocardial infarction; PCI=percutaneous coronary intervention; n=number of patients; CABG=coronary artery bypass graft surgery.
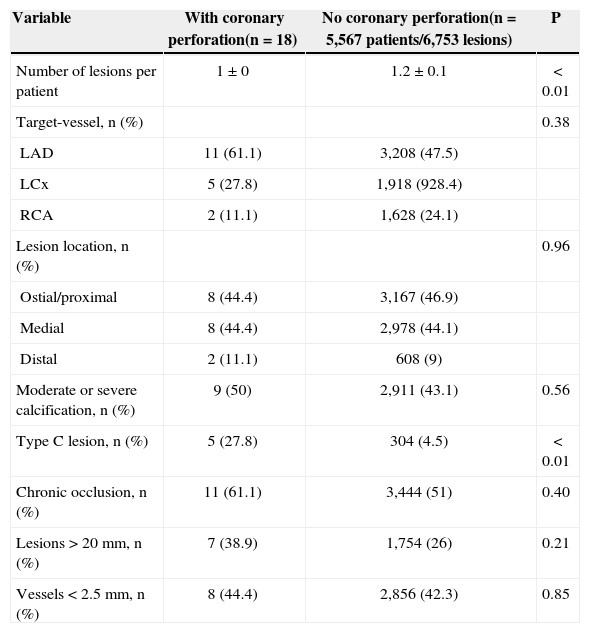
The pre-procedural angiographic data are shown in Table 2. In the group of patients with coronary perforation, the left anterior descending artery was the most frequently treated vessel (61.1%). The chronic occlusions were more frequently treated (27.8% vs. 4.5%; P<0.01), but the prevalence of lesions with moderate /severe calcifications (50% vs. 43.1%; P=0.56), lesions>20mm (38.9% vs. 26%; P=0.21), C-type morphology (61.1% vs. 51%; P=0.40), and vessels≤2.5mm (44.4% vs. 42.3%; P=0.85) did not differ between groups.
Angiographic characteristics
| Variable | With coronary perforation(n=18) | No coronary perforation(n=5,567 patients/6,753 lesions) | P |
|---|---|---|---|
| Number of lesions per patient | 1±0 | 1.2±0.1 | < 0.01 |
| Target-vessel, n (%) | 0.38 | ||
| LAD | 11 (61.1) | 3,208 (47.5) | |
| LCx | 5 (27.8) | 1,918 (928.4) | |
| RCA | 2 (11.1) | 1,628 (24.1) | |
| Lesion location, n (%) | 0.96 | ||
| Ostial/proximal | 8 (44.4) | 3,167 (46.9) | |
| Medial | 8 (44.4) | 2,978 (44.1) | |
| Distal | 2 (11.1) | 608 (9) | |
| Moderate or severe calcification, n (%) | 9 (50) | 2,911 (43.1) | 0.56 |
| Type C lesion, n (%) | 5 (27.8) | 304 (4.5) | < 0.01 |
| Chronic occlusion, n (%) | 11 (61.1) | 3,444 (51) | 0.40 |
| Lesions>20mm, n (%) | 7 (38.9) | 1,754 (26) | 0.21 |
| Vessels<2.5mm, n (%) | 8 (44.4) | 2,856 (42.3) | 0.85 |
RCA=right coronary artery; LCx=left circumflex artery; LAD=left=anterior descending artery.
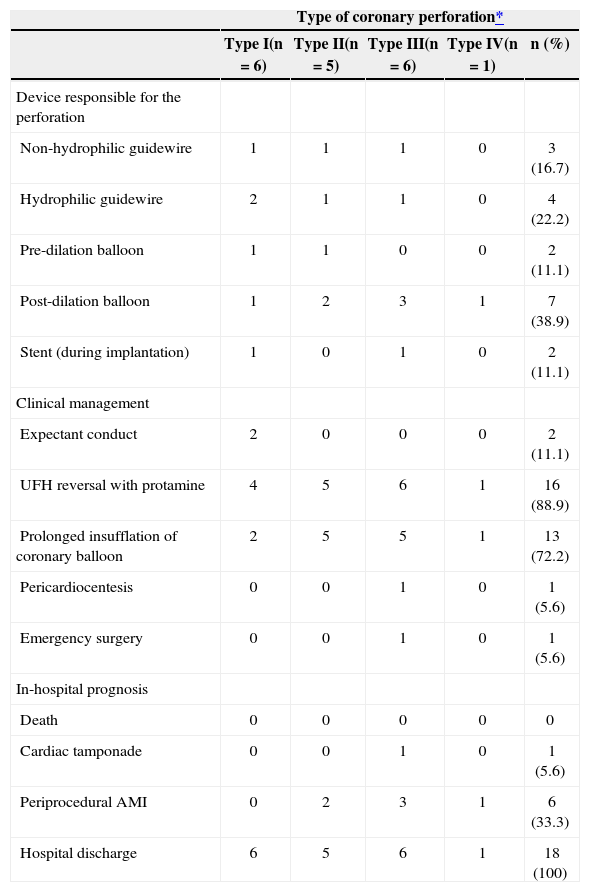
During the procedure, a balloon catheter device was the cause of coronary perforation in 61.1% (11/18) of cases, including, two ruptures during the pre-dilation, two during stenting, and seven while conducting post-dilation (Table 3). In the remainder (7/18), a guidewire was responsible for coronary perforation, with a hydrophilic guidewire accounting for 22.2% (4/18) of cases and a non-hydrophilic guidewire accounting for 16.7% (3/18) of cases.
Types of perforation-related devices, management and prognosis of patients with coronary perforation (n=18)
| Type of coronary perforation* | |||||
|---|---|---|---|---|---|
| Type I(n=6) | Type II(n=5) | Type III(n=6) | Type IV(n=1) | n (%) | |
| Device responsible for the perforation | |||||
| Non-hydrophilic guidewire | 1 | 1 | 1 | 0 | 3 (16.7) |
| Hydrophilic guidewire | 2 | 1 | 1 | 0 | 4 (22.2) |
| Pre-dilation balloon | 1 | 1 | 0 | 0 | 2 (11.1) |
| Post-dilation balloon | 1 | 2 | 3 | 1 | 7 (38.9) |
| Stent (during implantation) | 1 | 0 | 1 | 0 | 2 (11.1) |
| Clinical management | |||||
| Expectant conduct | 2 | 0 | 0 | 0 | 2 (11.1) |
| UFH reversal with protamine | 4 | 5 | 6 | 1 | 16 (88.9) |
| Prolonged insufflation of coronary balloon | 2 | 5 | 5 | 1 | 13 (72.2) |
| Pericardiocentesis | 0 | 0 | 1 | 0 | 1 (5.6) |
| Emergency surgery | 0 | 0 | 1 | 0 | 1 (5.6) |
| In-hospital prognosis | |||||
| Death | 0 | 0 | 0 | 0 | 0 |
| Cardiac tamponade | 0 | 0 | 1 | 0 | 1 (5.6) |
| Periprocedural AMI | 0 | 2 | 3 | 1 | 6 (33.3) |
| Hospital discharge | 6 | 5 | 6 | 1 | 18 (100) |
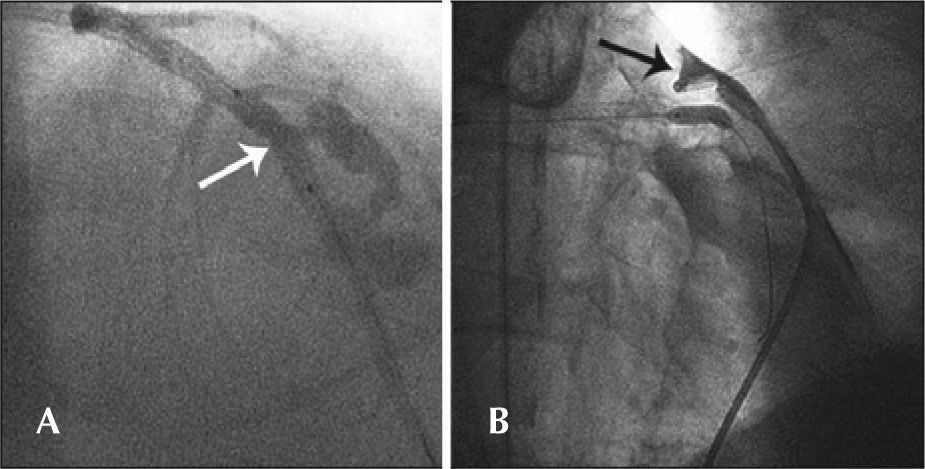
Simple perforations (type I) occurred in 33.3% (6/18) of patients, and their management included expectant and/or conservative treatment in four of six cases. Type II coronary perforations (27.8%, 5/18) were associated with guidewires (n=2) or balloon-catheter dilation (n=3). In these cases, the treatment included the administration of protamine for heparin inactivation associated with prolonged balloon inflation, and there was in-hospital periprocedural AMI in two cases. The type III coronary perforations were more often associated with ruptures caused by balloon catheters (50%), and one case had cardiac tamponade, requiring emergency surgery. Figure 2 shows the case with pericardial extravasation treated by prolonged inflation. Type IV perforation occurred in one patient post-dilation. There were no deaths associated with coronary perforation in any of the 18 cases, and the success rate of the procedure was 77.8% (n=14), as there was failed attempted occlusion recanalisation in three cases and suboptimal angiographic results (residual stenosis>30%) in one case. The median length of hospitalisation was 3.1days (range 2-58 days), and one patient who underwent emergency surgery presented a number of postoperative complications, including renal failure, requiring hemodialysis.
– In A, arterial lumen rupture with diameter>1mm and contrast extravasation through an orifice (type III Ellis perforation). Note the position of the balloon catheter in place to achieve prolonged inflation. In B, the balloon catheter inflated at the site of perforation and positioning of a pigtail catheter into the pericardial cavity after performing the pericardiocentesis.
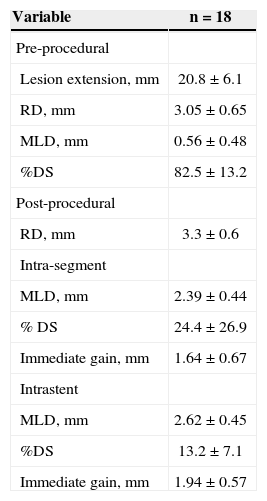
Table 4 shows the results of the quantitative coronary angiography analysis in patients with coronary perforation. The balloon-artery ratio was 1.2±0.1, as determined when measuring the maximum balloon diameter during inflation resulting in coronary perforation.
Quantitative coronary angiography cases with coronary perforation
| Variable | n=18 |
|---|---|
| Pre-procedural | |
| Lesion extension, mm | 20.8±6.1 |
| RD, mm | 3.05±0.65 |
| MLD, mm | 0.56±0.48 |
| %DS | 82.5±13.2 |
| Post-procedural | |
| RD, mm | 3.3±0.6 |
| Intra-segment | |
| MLD, mm | 2.39±0.44 |
| % DS | 24.4±26.9 |
| Immediate gain, mm | 1.64±0.67 |
| Intrastent | |
| MLD, mm | 2.62±0.45 |
| %DS | 13.2±7.1 |
| Immediate gain, mm | 1.94±0.57 |
MLD=minimal luminal diameter; RD=reference diameter; DS=diameter stenosis; n=number of patients.
The main findings of the current study, which included a retrospective analysis of a large cohort of patients (n=5,585) undergoing PCI in the daily practice of a tertiary public hospital were: a) coronary perforation was a rare phenomenon (0.32%), with an incidence similar to that reported in the literature (0.1%-0.6%); 2−12 b) most of the perforations were caused by balloon-catheter dilation; c) the clinical management was successfull and included a conservative approach in cases of simple coronary perforation and invasive approach in cases of marked extraluminal contrast extravasation; and d) in the univariate analysis, the predictors included female gender, history of chronic obstructive pulmonary disease and chronic occlusion.
Coronary perforations have different presentations and are classically classified into types I-III or, more recently, types I-IV (modified Ellis classification) according to the degree and direction of contrast extravasation. 3 It is noteworthy that the incidence of subsequent complications varies according to the perforation severity. For types I, II and III-IV, the rates of adverse events were as follows: AMI, 0% to 29%, 13% to 29%, and 0% to 30%, respectively; cardiac tamponade, 6% to 8%, 5% to 13%, and 20% to 63%, respectively; emergency surgery, 15% to 24%, 0% to 24%, and 50% to 60%, respectively, and death, 0% to 6%, 0% to 6%, and 19% to 21%, respectively. 3,4,11
The same trend was observed in the present study in relation to AMI (0%, 40%, 43%), cardiac tamponade (0%, 0%, 14%) and emergency surgery (0%, 0%, 14%) for coronary perforations types I, II and III-IV, respectively; however, there were no fatal cases associated with this complication. This finding may be associated with the low incidence and the resulting small sample included in the present series. Regarding the devices that cause coronary perforation, previous studies suggest that guidewires (mainly the hydrophilic type) are the most often responsible for coronary perforations types I and II. However, types III and IV occur, in general, due to balloon-catheters or atheroablative devices.7 It is interesting to note that of the 11 cases of coronary perforation types I and II observed in the present analysis, 46% (n=5) were caused by guidewires (hydrophilic and non-hydrophilic), and the remainder were caused by balloon-catheters.
Regarding the clinical and angiographic predictors, a significant association was observed in the univariate analysis between the occurrence of coronary perforation and female gender, history of chronic obstructive pulmonary disease, and chronic occlusion. Hendry et al. 16 demonstrated that female gender, advanced age, coronary calcification, use of a cutting balloon and rotational atherectomy, and treatment of chronic occlusion were predictors of coronary perforation; however, coronary perforation was not associated with the use of high-inflation pressures in balloons, suggesting the safety of such a practice, which aims to provide an optimal angiographic result with adequate stent expansion, which could, theoretically, minimize late complications such as restenosis and stent thrombosis. In general, it is recommended that a balloon:artery ratio between 1 and 1.1 is used for optimal stent implantation, with an angiographic result goal of residual stenosis<30%. 14 However, the feasibility of this procedure is often limited based on the degree of rigidity and resistance of the atherosclerotic plaque, especially in calcified lesions. Thus, suboptimal results often lead to the use of balloon-catheters of greater calibre, which can be inflated at very high pressures to optimize stent expansion. Consequently, such procedure may lead to coronary rupture. In the present study, half the patients had moderate or severe lesion calcification and the mean balloon:artery ratio of cases of coronary perforation caused by balloon-catheters (either pre- or post-dilatation or during stent implantation) was 1.2 (greater than that recommended in clinical practice).
Moreover, the studies by Fasseas et al. 17 and by Gruberg et al. 4 identified the use of atheroablative devices and female gender in coronary perforation. In the multivariate analysis of Shimony et al., 18 the treatment of total chronic occlusion was the strongest independent predictor of coronary perforation; the other independent variables included were lesion calcification and AMI. Regarding female gender, previous studies suggest that body surface area, and not gender, was the determining factor of the association commonly found between females and coronary perforation, as women have, on average, lower weight and smaller body surface area when compared to men. 19 It is noteworthy that the small-calibre coronary vessels have been systematically identified as an important predictor of PCI failure, including acute complications, such as coronary perforation, as well as late complications, such as restenosis and stent thrombosis. 18 In the present analysis, 44% of patients with coronary perforation had small vessels (< 2.5mm); however, the difference was not significant.
Regarding the management and treatment of coronary perforations, several authors have demonstrated that most types I and II Ellis perforations can be treated conservatively with inactivation of unfractionated heparin with protamine (suggested dose: 1mg per 100 units of unfractionated heparin), prolonged inflation with a balloon-catheter, and the use of coils and coated stents. Type III and IV perforations may have more severe complications, often requiring more invasive measures such as surgery and emergency pericardiocentesis. 4,16−18,20 In the present series with 18 patients, only one patient with type III coronary perforation required such procedures. In general, the present population had a highrisk profile, either due to their clinical characteristics or the lesion complexity, as shown in Tables 1 and 2. However, the incidence of coronary perforation is in agreement with the literature. 2−12 When this complication occurs, it must be treated immediately, with reversal of unfractionated heparin with protamine and prolonged inflation of a balloon-catheter for a minimum period between 15 and 30 minutes, using control injections to confirm that extravasation of contrast medium through the arterial lumen has stopped. In addition, it is recommended that a further transthoracic echocardiogram in the catheterisation laboratory is performed, and seriate echocardiography should be performed in the intensive care unit, due to the possibility of the later formation of massive pericardial effusion, which may cause future hemodynamic damage. Pericardiocentesis or emergency surgery is reserved for cases with cardiac tamponade and/or significant hemodynamic damage. Nonetheless, specific cases may require individual approaches; the surgeon’s experience and service infrastructure are extremely important. Embolisation with coils or coated stents is an alternative approach; however, logistical and availability issues may hinder its routine use.
CONCLUSIONSIn daily practice complex patients, the incidence of coronary perforation was rare and was significantly associated with the female gender, history of chronic obstructive pulmonary disease and chronic occlusion coronary lesions. These patients were successfully treated through conservative conduct (without pericardiocentesis and/or emergency surgery) in most cases, with satisfactory in-hospital outcomes.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.