Intravascular ultrasound (IVUS) provides a sensitive and reproducible measurement of the coronary artery dimensions, atherosclerotic plaque, and arterial lumen. Advances in IVUS technology now allow for the characterisation of the composition and morphology of atherosclerotic plaques. Although previous studies have reported data using IVUS with radiofrequency analysis, little is known regarding the use of a new modality (iMap®, Boston Scientific – Santa Clara, CA, USA) of atherosclerotic plaque characterisation. This study aims to analyse the morphological, phenotypic, and tissue characteristics of ‘culprit’ and ‘non-culprit’ atherosclerotic plaques determined by angiography in patients undergoing coronary angiography due to acute myocardial infarction (AMI).
MethodsThis will be a prospective, cross-sectional, single centre study (Hospital São Paulo – Escola Paulista de Medicina – Universidade Federal de São Paulo/UNIFESP, São Paulo, SP, Brazil). Fifty patients will be selected for the ultrasound analysis according to the following inclusion criteria: age<75years, non-ST-segment elevation AMI or recent ST-segment elevation AMI, and with or without previous fibrinolytic agents.
ConclusionsThis study aims to obtain the morphological, phenotypical, and tissue characteristics of the atherosclerotic plaque using an imaging modality that has not been extensively evaluated in patients with AMI.
Estudo iWONDER(Imaging WhOle vessel coroNary tree with intravascular ultrasounD and iMap® in patiEnts with acute myocaRdial infarction): Racional e Desenho do Estudo
IntroduçãoO ultrassom intracoronário (USIC) é um método capaz de fornecer medida sensível e reprodutível das dimensões da artéria coronária, da placa aterosclerótica e do lúmen arterial. Avanços em sua tecnologia permitem agora a caracterização da composição e da morfologia das placas ateroscleróticas. Embora estudos prévios tenham reportado dados utilizando USIC com análise de radiofrequência, o uso de uma nova modalidade (iMap®, Boston Scientific, Santa Clara, Estados Unidos) de caracterização da placa aterosclerótica é muito pouco conhecida. Nosso objetivo será analisar as características morfológicas, teciduais e fenotípicas das placas ateroscleróticas consideradas angiograficamente “culpadas” e “não culpadas” em pacientes submetidos a angiografia coronária decorrente de infarto agudo do miocárdio (IAM).
MétodosEstudo prospectivo, transversal, em único centro (Hospital São Paulo – Escola Paulista de Medicina – Universidade Federal de São Paulo/ UNIFESP, São Paulo, SP, Brasil). Serão selecionados 50 pacientes para análise ultrassonográfica, de acordo com os seguintes critérios de inclusão: idade<75 anos, IAM sem supradesnivelamento do segmento ST ou IAM com supradesnivelamento do segmento ST recente, com ou sem uso de fibrinolítico prévio.
ConclusõesO presente estudo objetivará a caracterização morfológica, tecidual e fenotípica da placa aterosclerótica utilizando uma nova modalidade de imagem ainda pouco estudada em pacientes com IAM.
The angiogram of the coronary artery is a two-dimensional image of the silhouette of the arterial lumen from a vascular, three-dimensional, complex structure. This test has many well-documented limitations,1,2 including the shortening of the vessel, overlapping images, and intra-and interobserver variabilities during the analysis of the injury severity; it also has considerable limitations in the diagnosis of the atherosclerotic plaque composition. However, an intravascular ultrasound (IVUS) provides a sensitive and reproducible measurement of the dimensions of the vessel, atherosclerotic plaque, and arterial lumen. The usefulness of greyscale IVUS as a prognostic tool and as a guide in percutaneous coronary intervention (PCI) is already well established.3,4 More recently, IVUS has also generated new insights into the safety and efficacy of drug-eluting stents. Advances in IVUS technology now allow for the characterisation of the composition and morphology of atherosclerotic plaques. Specifically, the analysis of the frequency of IVUS waves, in addition to the interpretation of the wavelengths used in greyscale IVUS, allows for the in vivo analysis of the atherosclerotic plaque composition with high levels of sensitivity and specificity.5 Although previous studies have reported data using IVUS with radiofrequency analysis,6 little is known about the use of a new modality (iMap®, Boston Scientific – Santa Clara, CA, USA) for the characterisation of the atherosclerotic plaque. Furthermore, the analysis and comparison of the lesions known as ‘culprit’ and ‘non-culprit’ using iMap® in the same patient has also been little studied.7–9
This study aims to analyse the morphological, tissue, and phenotypical characteristics of the atherosclerotic plaques angiographically considered to be ‘culprit’ and ‘not-culprit’ in patients undergoing coronary angiography due to acute myocardial infarction (AMI) with or without ST segment elevation.
METHODSStudy design and patient selectionThe study will be conducted as a prospective cross-sectional, single centre (Hospital São Paulo – Escola Paulista de Medicina – Universidade Federal de São Paulo/UNIFESP, São Paulo, SP, Brazil) study. A total of 50 patients will be consecutively selected for ultrasound assessment according to the following inclusion criteria: age<75years, AMI without ST-segment elevation and AMI with recent ST-segment elevation, and with or without the prior use of fibrinolytics. This selection is based on the coronary anatomy and the presence of at least one lesion characterised by angiography as an ‘acute plaque’ or ‘culprit lesion’ by symptoms in one of the major epicardial arteries, as well as the presence of at least one ‘non-culprit’ lesion (≥30% by visual estimation) in the other two major epicardial arteries unrelated to the event. The exclusion criteria will be the presence of haemodynamic instability, ventricular dysfunction after myocardial infarction (Killip class III/IV), lesion in the left main coronary artery, angiographic findings of a coronary anatomy with significant tortuosity or significant proximal calcification, very severe coronary obstruction blocking the passage of the IVUS catheter, total occlusion of any of the three epicardial coronary arteries, and patients previously submitted to coronary artery bypass graft (CABG). The procedures will be performed according to the guidelines of the Brazilian Society of Cardiology (Sociedade Brasileira de Cardiologia – SBC) and the American Heart Association (AHA).10,11 All patients or legal guardians will have to read and sign an informed consent. The study was approved by the Ethics Committee of UNIFESP (protocol #0889/11) and is registered at ClinicalTrial.org (protocol #NCT01437553). The schedule provides for the inclusion of patients up to the first semester of 2012.
Diagnostic or therapeutic procedureInitially, an angiography will be performed after the intracoronary injection of 200–300 μcg of nitroglycerine. When considered appropriate, the IVUS of the three epicardial coronary arteries (left anterior descending artery, circumflex artery, and right coronary artery) will be performed using a 40MHz IVUS catheter (Atlantis SR Pro, Boston Scientific – Santa Clara, CA, USA), with greyscale analysis and morphological tissue characterisation using the iMap® software. Automatic retreats of the ultrasound catheter will be performed at a velocity of 0.5mm/s, starting at a point>10mm distal to the ‘culprit’ lesion toward the ostium of the analysed artery. In the ‘non-culprit’ arteries, the same routine will be performed for the analysis of the plaques unrelated to the event. Finally, according to the ultrasonographic and angiographic findings and based on the clinical and electrocardiographic data, the surgeons will decide, together with the cardiologist responsible for the patient, on the treatment option to be adopted (clinical treatment, surgical revascularisation, or percutaneous revascularisation).
A ‘culprit’ lesion will be defined as the coronary obstruction that the angiography shows as having characteristics of instability, such as ulceration, irregular borders and eccentricity, luminal thrombus, blurred density of the atherosclerotic plaque or significant obstruction of the diameter in a compatible artery, and related to the ischaemic area in the electrocardiogram or echocardiogram.
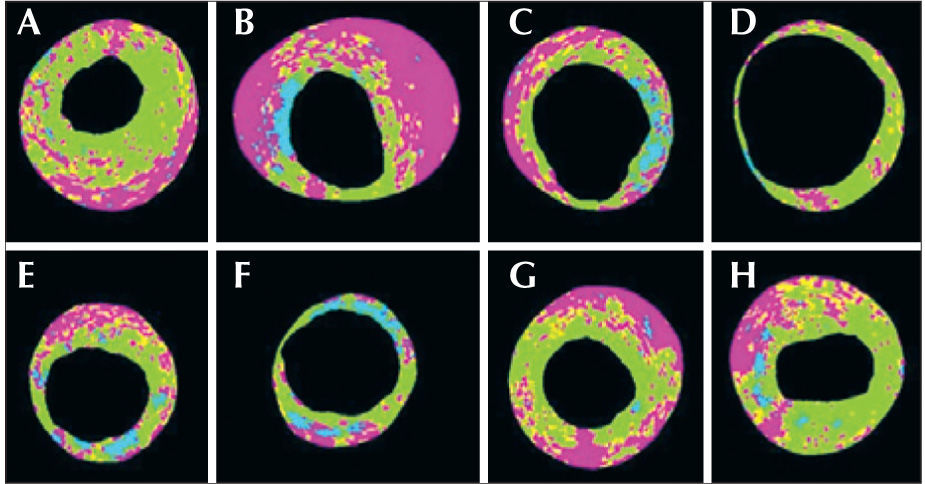
Analysis by greyscale IVUS and by iMap®The ultrasound images obtained will be recorded for later analysis and offline interpretation through QIvus® software (release 2.1, Medis Medical Imaging Systems – Leiden, the Netherlands) in the research laboratory of invasive imaging analysis of the Hospital Albert Einstein (São Paulo, SP, Brazil). The ‘culprit’ and ‘non-culprit’ atherosclerotic lesions will be identified. The lesion definition is based on the finding of three consecutive frames at the IVUS with a plaque burden≥40%. The lesion will be considered a single lesion if the interval between two or more lesions (plaque burden≥40%) is<10mm. The proximal and distal references will be set from the finding of the coronary segment with a plaque burden<40% in the 5mm proximal and distal to the lesion. ‘Culprit’ and ‘non-culprit’ lesions will be those identified by angiography. For these lesions to be identified on the IVUS, reproducible landmarks will be adopted both in the angiography and IVUS (e.g., ostium vessel, side branch, calcium, and pericardium) to help with the analysis reproduction. The following measurements will be performed with the greyscale IVUS: area of the external elastic membrane, arterial lumen area, vessel diameter, vessel lumen diameter, plaque burden, lesion length, minimal luminal area, and total volume of the vessel of the lumen and of the atheroma. The remodelling index is calculated by the lesion external elastic membrane area divided by the area of the external elastic membrane of the main reference. Positive remodelling will be defined as a remodelling index>1.05, negative remodelling as a remodelling index<0.95, and intermediate remodelling as a remodelling index between 0.95 and 1.05.12 The plaque burden is defined as the plaque area normalised to the vessel area. Then, the quantification of the percentage of the total plaque area and cross-sectional area of the following histological characteristics by iMap® will be performed (Figure 1): 1) fibrous tissue (light green), 2) fibroadipose tissue (yellow), 3) necrotic core (red), and 4) dense calcium (blue). After the quantification of the plaque composition, the lesions will be phenotypically classified as follows: 1) pathological intimal thickening, 2) fibrotic, 3) fibrolipidic, 4) thick-cap fibroatheroma, and 5) thin-cap fibroatheroma (vulnerable plaque by iMap®).13,14 This phenotypic classification will be performed in a hierarchical manner. In lesions with multiple phenotypic patterns, the most severe will be selected according to the following hierarchical relationship: thin-cap fibroatheroma, thick-cap fibroatheroma, fibrotic plaque, fibrocalcified plaque, and pathological intimal thickening.
– Examples of several types of atherosclerotic plaque compositions. In A, the predominance of fibrous and necrotic tissue (fibroatheroma with a thick fibrous cap, in which the necrotic core occupies 27% of the total plaque area). In B, the predominance of necrotic tissue with a significant calcification area between the 8 and 10 o’clock positions. Between the 11 and 7 o’clock positions, the plaque is mostly occupied by necrotic tissue in contact with the vessel lumen (fibrous thin-cap fibroatheroma). In C, the fibronecrotic tissue with calcification is observed at the 3 o’clock position. In D, predominantly fibrotic tissue. In E, fibronecrotic tissue with calcification spots. In F, fibrotic tissue with significant calcification between the 12 and 6 o’clock positions. In G and H, the predominance of fibronecrotic tissue (fibroatheromas).
The main cause of AMI is the rupture or erosion of an atherosclerotic plaque with superimposed thrombus formation.15 Histopathologically, the type of plaque more prone to rupture is the thin-cap fibroatheroma,13 characterised by a thin fibrous cap richly infiltrated by macrophages and covering a large necrotic core with neovascularisation. This fibroatheroma in the pre-rupture phase is called a vulnerable plaque. Alternatively, a clinical definition of a vulnerable plaque would be an atherosclerotic lesion that places the patient at greater risk for future adverse cardiovascular events. The identification of a vulnerable plaque before its clinical presentation is a great challenge, but it offers the potential benefit of guiding preventive therapy in a large number of patients who develop acute coronary syndrome. Notably, most vulnerable plaques that trigger future events have an obstruction<50%, both on angiography and histopathology assessments.16
The greyscale IVUS derived from the analysis of the intravascular ultrasound signal amplitude is commonly used for the quantitative analysis of the atheroma, such as lesion length, vessel diameter, plaque burden, stenosis severity, and lumen dimensions, and is a useful tool to guide PCIs with stent implanting. The morphologic characteristics of ‘culprit’ lesions on the greyscale IVUS are variable and may include an echolucent core, eccentricity, positive remodelling, ulceration, thrombosis, and calcification.17 Recently, the presence of an attenuated plaque on the greyscale image has been correlated with microcalcification, thrombus, or cholesterol crystals.18,19 This situation has been more predominantly observed in patients with acute coronary syndrome than in patients with stable angina,20 and in situations that trigger the no-reflow phenomenon (absence or marked reduction in coronary flow after angioplasty, in the absence of a residual stenotic lesion) or increase in the creatine kinase MB fraction after PCI.21,22 However, the greyscale IVUS is unable to identify the histomorphological characteristics associated with plaque rupture and vulnerability, including thin-cap fibroatheroma.
Variations in the traditional greyscale IVUS technique will allow for a more detailed characterisation of the atherosclerotic plaque tissue. Among the available options, the most widespread are virtual histology (VH® IVUS, Volcano Therapeutics, Inc. – Rancho Cordova, CA, USA) and, more recently, the characterisation of the atherosclerotic plaque using the iMap® software. The technology using VH® IVUS has been studied in patients with acute coronary syndrome6,7 and in analyses evaluating the regression and progression of atherosclerotic plaques associated with various therapeutics, such as statins.23,24
The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study6 is the largest prospective study evaluating the natural history of coronary artery disease to date. This study, involving 697 patients undergoing VH® IVUS, was designed to correlate the histological characteristics of the lesions, patient risk factors, and other measurements (clinical and laboratory) with subsequent cardiac events. After a mean follow-up of 3.4years, lesions considered to be ‘culprit’ (those initially treated after the initial acute coronary syndrome) and ‘non-culprit’ lesions (lesions considered mild to moderate on angiography, which were not treated initially) were responsible for similar rates of the primary composite endpoint of cardiac death, AMI, and unstable or progressive angina (12.9% vs. 11.6%, respectively). The main predictors correlating ‘non-culprit’ lesions to the primary endpoint were diabetes with insulin use, plaque burden≥70%, presence of a plaque morphologically classified as a thin-cap fibroatheroma, and a minimal lumen area≤4mm2. Thus, the authors were able to conclude that the prospective identification and characterisation of ‘non-culprit’ lesions in the coronary arteries with virtual histology are predictors of adverse cardiovascular events over the subsequent 3 years.
In the ‘VH-IVUS in Vulnerable Atherosclerosis (VIVA)’ study,25 using the VH® IVUS technology, 170 patients with stable angina or acute coronary syndrome referred for PCI were prospectively enrolled and submitted to preintervention IVUS of the three epicardial coronary vessels. In total, 30,372mm of IVUS were analysed with virtual histology. A total of 18 major cardiovascular events (i.e., death, AMI, or non-planned revascularisation) occurred in 16 patients after a mean follow-up of 625 days; 1,096 lesions were identified, with 19 resulting in events (13 ‘non-culprit’ and six ‘culprit’ lesions). The risk factors associated with ‘non-culprit’ lesions that resulted in major cardiovascular events were, as in the PROSPECT study, the presence of a thin-cap fibroatheroma, plaque burden≥70%, and minimal lumen area≤4mm2.
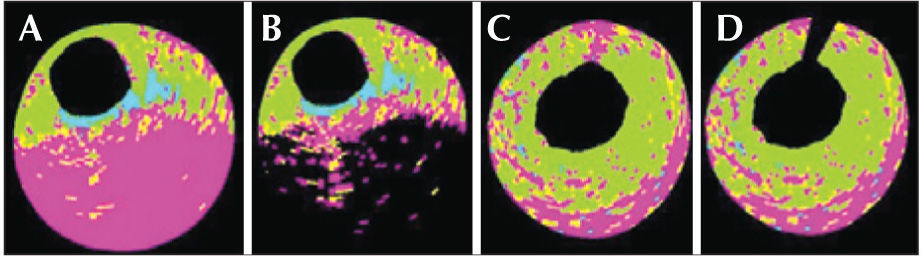
Detailed studies of the phenotypic and tissue composition of the atherosclerotic plaques in patients with acute coronary syndrome using the iMap® mapping are still scarce. Offline analysis with the iMap® software allows for the interference of calcium or guidewire artefacts mistakenly classified as calcium (pseudocalcification) to be attenuated or even negated (Figure 2).
– Examples of two types of pseudocalcification determined by the presence of calcium (A and B) and a guidewire (C and D). In A, the presence of a calcium arc between the 3 and 7 o’clock positions prevents the passage of ultrasound waves; the tissue interpretation is thus wrongly classified as necrotic tissue. In C, the artefact of the guidewire can be noticed within 12 hours. In B and D, the offline analysis software allows for the minimization of the calcium and guidewire artefacts.
Thus, mapping by iMap® has potential advantages compared with other technologies available for the characterisation of atherosclerotic plaques and for the better understanding of the physiopathology of AMI.
CONCLUSIONSThis study will aim to perform the morphologic, tissue, and phenotypic characterisation of the atherosclerotic plaque using a new imaging modality, which has been scarcely studied in patients with AMI.
CONFLICTS OF INTERESTBoston Scientific (Santa Clara, CA, USA) donated the IVUS catheters used in the study. Dr. Juan Rigla is a physician employed by Boston Scientific. The other authors declare no conflicts of interest.