The authors report their initial experience with the Memopart™ device (Shanghai Shape Memory Alloy Co Ltd, Shanghai, China) for the occlusion of secundum atrial septal defect (ASD).
MethodsThis was a prospective observational study of a series of patients undergoing percutaneous occlusion of ASD with right ventricle volume overload and favorable anatomic characteristics. The procedure was performed by percutaneous femoral approach. The mean follow-up was 10.3±5 months, with clinical and 24-hour echocardiographic evaluations (or before hospital discharge), 1, 3, 6, and 12 months after implantation.
ResultsFrom February/2012 to April/2013, 21 patients, 16 females, mean age 33.1±18.7years, were submitted to percutaneous occlusion of an ASD. The average diameter of the defect was 19.04±6.25mm and the device size was 21.42±6.73mm (8 to 34mm). Total occlusion of the defect was observed in all cases before hospital discharge. During follow-up, all patients were asymptomatic and without residual shunt. There was no deaths or any other complications in the series.
ConclusionsThe percutaneous closure of ASD using a Memopart™ device is an effective and safe procedure within the limits of this investigation. The device is user-friendly and has a high rate of immediate occlusion, even in large defects.
Oclusão Percutânea de Comunicação InteratrialTipo Ostium Secundum com Prótese Memopart®
IntroduçãoOs autores relatam a experiência inicial da oclusão da comunicação interatrial ostium secundum (CIA) com a utilização da prótese Memopart® (Shanghai Shape Memory Alloy Co Ltd, Shanghai, China).
MétodosEstudo prospectivo observacional, no qual uma série de pacientes portadores de defeitos com significativa repercussão hemodinâmica e características anatômicas favoráveis ao implante foi submetida à oclusão percutânea de CIA. O procedimento foi realizado por via femoral percutânea, pela técnica habitual. O período de seguimento foi de 10,3±5 meses, com controles clínicos e ecocardiográficos 24 horas (ou antes da alta hospitalar), 1, 3, 6 e 12 meses após o implante.
ResultadosNo período de fevereiro de 2012 a abril de 2013, foram submetidos à oclusão percutânea de CIA 21 pacientes, sendo 16 do sexo feminino, com idade média de 33,1±18,7 anos. O diâmetro médio do defeito foi de 19,04±6,25mm e o tamanho da prótese foi de 21,42±6,73mm (8 a 34mm). O implante foi realizado com êxito em todos os casos, verificando-se oclusão total no controle antes da alta hospitalar. No seguimento, todos os pacientes estiveram assintomáticos e comprovou-se a persistência da oclusão total do defeito. Não houve mortalidade e nem outras complicações na série.
ConclusõesA oclusão percutânea da CIA utilizando-se prótese Memopart® é um procedimento eficaz e seguro, dentro dos limites desta investigação. O implante da prótese é simples e apresenta alto índice de oclusão imediata, inclusive de defeitos de grandes dimensões.
Ostium secundum atrial septal defect (ASD), located in the oval fossa, constitutes approximately 80% of ASDs.1 Percutaneous occlusion should be performed in cases in which the anatomy is favorable, when there are hemodynamic repercussions (class I indication, level of evidence B), in association with orthodeoxia platypnea syndrome (class IIa indication, level of evidence B), or when there is a history of paradoxical embolism (class IIa indication, level of evidence C).2 Approximately 85% of the ASDs have essential structural factors that determine favorable anatomy for percutaneous occlusion, which are: stretched defect diameter<42mm, which is the maximum diameter of the available prostheses, and borders with dimensions≥5mm, with the exception of the aortic or anterosuperior border.
The most commonly used devices for percutaneous closure are double-disc nitinol occlusion devices, which are self-centering, retrievable, and repositionable; easy to handle; and have high success rates in ASD occlusion. These prostheses consist of numerous filaments of nitinol (a nickel and titanium alloy), welded at their ends and molded to form a central waist, which acts as a stent in the communication orifice of two discs, a larger left disc and a smaller right disc. The different types of this prosthesis vary slightly in relation to the nitinol filament thickness, the flaps inserted into the discs, their dimensions in relation to the central waist, and the device fixation system to the delivery cable.
This study aimed to report the results of a prospective series of patients undergoing percutaneous closure of ASD with the Memopart® device (Shanghai Shape Memory Alloy Co. Ltda., Shanghai, China).
METHODSStudy designThis was a prospective, longitudinal, and observational cohort study, performed in a single center, from February 2012 to April 2013, of patients submitted to percutaneous ASD occlusion using Memopart® device. The study was performed at the Hemodynamics and Interventional Cardiology Laboratory of Hospital das Clínicas, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo (HCFMRP-UPS), Brazil and was approved by the institution’s ethics committee. All patients or their legal guardians signed an informed consent after being informed about the purposes of the study.
Inclusion criteriaPatients with ASD that participated in the study were referred to HCFMRP-USP with the following clinical, anatomical, and hemodynamic characteristics: weight>25kg; dilation of the right cardiac chambers visualized during echocardiographic evaluation; QP/ QS ratio>1.5; pulmonary arterial pressure≤2/3 of the systemic level; pulmonary vascular resistance<5 UW; defect diameter<42mm; defect borders>5mm, except the retroaortic border; signing of the informed consent approved by the Institutional Ethics Committee.
Exclusion criteriaPatients with other associated congenital or acquired heart diseases, with indication of surgical correction; with thin, hypermobile, or deficient ASD borders; those with active infection of any kind or infectious process in the previous month; patients with hypercoagulable syndrome, with contraindication to antiplatelet medication, were excluded from the study.
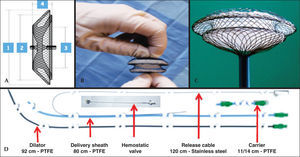
PROSTHESISMemopart® (Figure 1) is a prosthetic device manufactured with filaments of nitinol, a nickel and titanium metal alloy at a ratio (in relation to atomic weight) of 55% and 45%, respectively. The set of filaments is welded together at the ends, forming a bundle that has pins at both ends. The bundle is then molded, at high temperature, as a double disc of different sizes, with a waist in the middle, which acts as a centering mechanism and positions itself in the defect as a stent. The pin of the smaller disk, which is the right one, has a bolt and nut system, which is screwed into the delivery cable. The prosthesis is fixed in delivery cable and stretched, to be introduced in the carrier and transferred to the delivery sheath. Due to the thermal memory of nitinol, the device, when externalized, regains its original shape.
– Prosthesis, and set of delivery and release. (A) Schematic representation of the prosthesis: (1) Left disc diameter, (2) Right disc diameter, (3) Central disc diameter, (4) Total thickness of the device. (B) Prosthesis discreetly stretched at the proximal and distal ends, showing both the upper left and lower right discs, in addition to the central disc. (C) Prosthesis mounted on the delivery system, partially externalized, with the right disk still not fully exposed. (D) The different components of the delivery and release system.
The diameter of the central disk determines the size of the device, which ranges from 6mm to 42mm, with 1-mm increments between the sizes from 6mm to 20mm and 2-mm increments between sizes 22mm to 42mm. The technical characteristics of the prostheses and releasing sheaths are shown in Table 1.
Clinical, echocardiographic, and hemodynamic parameters of patients
| Patient | (years) | Weight (Kg) | Gender | Atrial septal defect diameter | QP-QS | Prosthesis (mm) | Residual flow | ||
|---|---|---|---|---|---|---|---|---|---|
| Transesophageal echocardiogram | Cardiac catheterization | Immediate | 24hours | ||||||
| 1 | 8 | 35 | F | 7 | 7 | 1.5 | 8 | No | No |
| 2 | 45 | 67 | F | 8 | 14 | 1.9 | 16 | No | No |
| 3 | 35 | 55 | F | 18 | 23 | 3.3 | 24 | Minimum | No |
| 4 | 3 | 16 | F | 14 | 18 | 4.8 | 20 | No | No |
| 5 | 15 | 83 | F | 13 | 16 | 2.6 | 18 | No | No |
| 6 | 20 | 87 | M | 12 | 11 | 1.7 | 12 | No | No |
| 7 | 75 | 52 | F | 6 | 14 | 1.9 | 16 | No | No |
| 8 | 37 | 94 | F | 24 | 32 | 1.9 | 34 | No | No |
| 9 | 47 | 66 | F | 15 | 17 | 3.0 | 20 | No | No |
| 10 | 9 | 29 | M | 10 | 17 | 2.9 | 18 | No | No |
| 11 | 54 | 54 | M | 20 | 26 | 4.6 | 30 | No | No |
| 12 | 41 | 69 | F | 15 | 18 | 2.7 | 22 | No | No |
| 13 | 57 | 79 | F | 17 | 21 | 1.9 | 22 | No | No |
| 14 | 9 | 25 | F | 16 | 18 | 1.8 | 24 | No | No |
| 15 | 27 | 55 | F | 15 | 24 | 3.0 | 26 | No | No |
| 16 | 29 | 78 | F | 20 | 24 | 2.6 | 28 | Minimum | No |
| 17 | 39 | 105 | F | 10 | 18 | 2.0 | 20 | No | No |
| 18 | 44 | 84 | F | 21 | 27 | 2.5 | 30 | Minimum | No |
| 19 | 51 | 60 | F | 10 | 10 | 1.6 | 12 | No | No |
| 20 | 12 | 31 | M | 13 | 16 | 1.7 | 18 | Minimum | No |
| 21 | 38 | 99 | M | 24 | 29 | 2.7 | 32 | Minimum | No |
QP/QS=pulmonary flow/systemic flow; F=female; M=male.
The procedures were performed by femoral approach under general anesthesia, with transesophageal echocardiography. Unfractionated heparin was administered at a dose of 100IU/kg, as well as antimicrobial prophylaxis with first-generation cephalosporin. The ASD size was determined by static balloon catheter technique, and the diameter at which there was flow cessation at color Doppler was measured by fluoroscopy and echocardiography. The implantation technique of the double-disc nitinol prostheses has been often described by other authors.3,4 Briefly, the prosthesis is soaked in saline solution to remove air from the mesh and patches; the right disc pin is screwed onto the delivery cable; the prosthesis is then inserted into the carrier and transferred to the delivery sheath, previously positioned in the left superior pulmonary vein. The left disk is externalized in the left atrium, near the mouth of the pulmonary vein, the delivery sheath is removed from the pulmonary vein and the set is subsequently retracted, until the disc is anchored in the interatrial septum. Keeping the release cable fixed, the sheath is withdrawn until the externalization of the right disk. After confirming the appropriate position of the device in three basic echocardiographic views (short axis, bicaval, and four-chamber) and the absence of significant residual flow, the prosthesis is released, unscrewing the delivery cable by clockwise rotation (Figures 2 and 3).
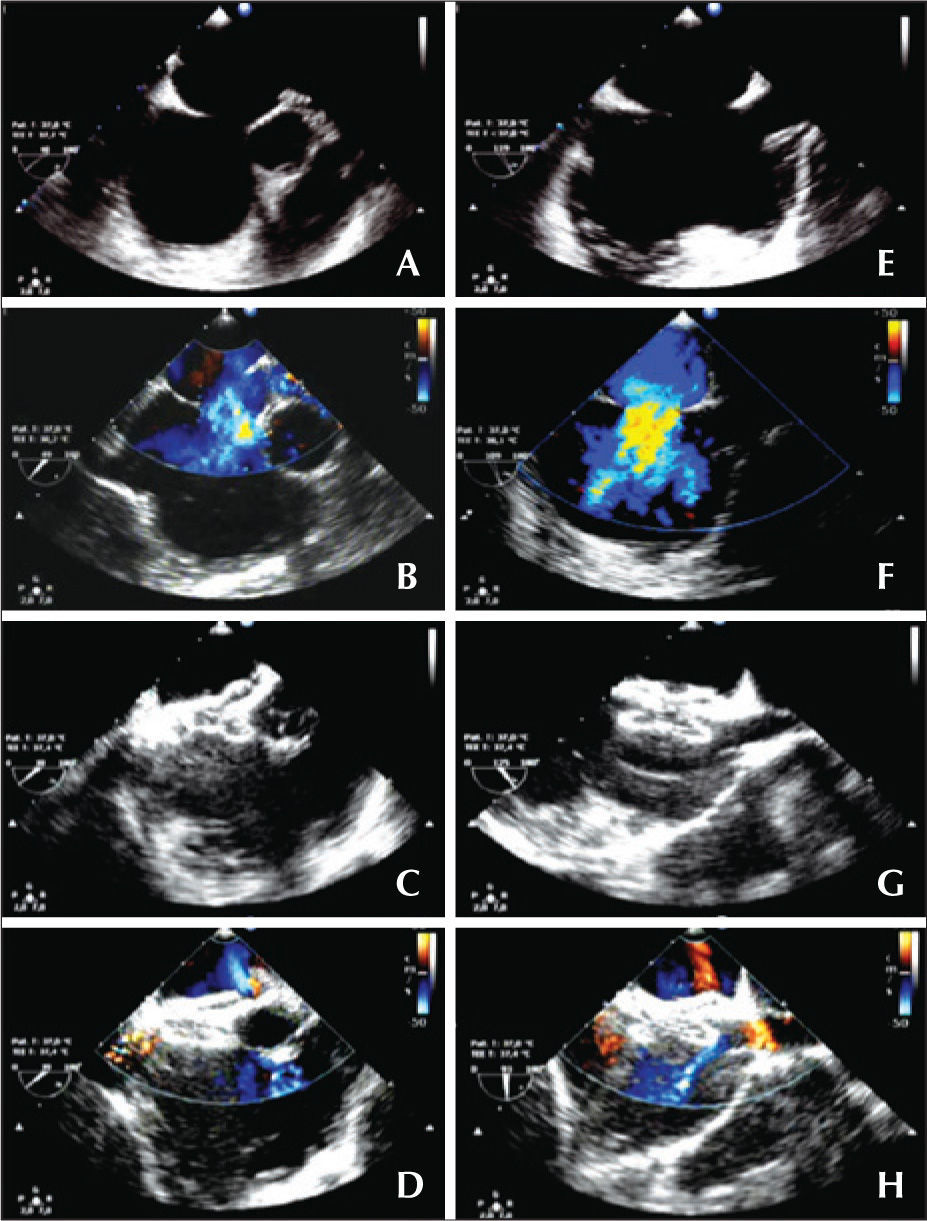
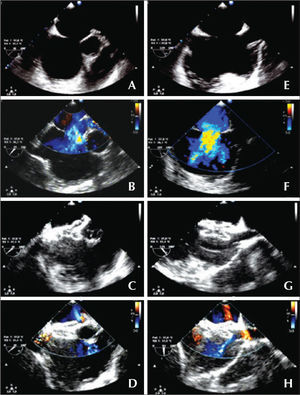
– Atrial septal defect of 16mm without aortic border: short axis (A, B, C and D) and Bicaval or long axis (E, F, G and H). (A and E) Diameter of the defect. (B and F) Left-right flow in color Doppler. (C and G) Adequate positioning of the prosthesis, embracing the aorta in the short axis and the borders of both cavas in the long axis. (D and H) No residual flow.
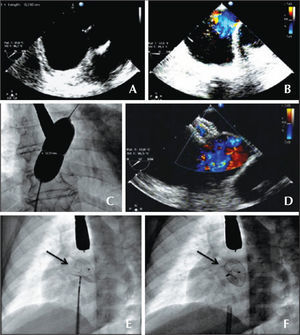
– Atrial septal defect (ASD) with no aortic border. (A) Echocardiogram in the short axis. (B) Color Doppler showing left-right flow. (C) Measuring balloon with discreet waist, determining an ASD diameter of 19.5mm. (D) Color Doppler with the implanted device and the absence of residual flow. (E and F) Fluoroscopy in the left anterior oblique view of the implanted prosthesis, still attached to the delivery cable and under mild tension (C), and released prosthesis showing a more vertical position (D). The arrows show the greater separation of the disks at the top, where the prosthesis embraces the aorta.
Several variations of this basic technique are sometimes implemented for proper positioning of the prosthesis according to the different anatomical ASD characteristics. These include the partial opening of the waist, before anchoring the left disk; the initial approach from the right or left superior pulmonary vein; and the partial externalization of the left disk in the pulmonary vein, followed by sheath withdrawal to the right atrium, in order to impact the right disk first in the interatrial septum.5,6
Procedural success was defined when the prosthesis was implanted with proper placement and no residual shunt or<2mm on transesophageal echocardiogram performed during the procedure.
Follow-upPatients were evaluated clinically and with transthoracic echocardiography before hospital discharge and after one, three, six, and 12 months. They were treated during this period with acetylsalicylic acid in a single daily dose of 5mg/kg (maximum dose of 100mg/day) for six months. In adult patients, clopidogrel was associated, at a dose of 75mg/day for six months. Antibacterial prophylaxis was prescribed, in the event of surgical or interventional procedures during the first six months of evolution.
Statistical analysisThe continuous variables were described as maximum and minimum values, as well as means and standard deviations. Categorical variables were described as frequencies or percentages.
RESULTSA total of 21 patients underwent the procedure, of whom 16 were females, with mean age of 33.1±18.7years and mean weight of 63.0±24.81kg. Transesophageal echocardiography disclosed the presence of single septal defect in all patients, with maximum diameter between 7 and 24mm (14.66±5.1mm). All treated patients had borders>5mm, but eight patients had deficient or absent aortic border. Clinical, echocardiographic and hemodynamic parameters are shown in Table 1.
Cardiac showed revealed a mean pulmonary-tosystemic flow ratio (QP/QS) of 2.47±1.14, systolic pulmonary artery pressure (SPAP) of 35.2±9.5mmHg, and pulmonary arteriolar resistance of 0.9±0.5 UW. The ASD diameter measured by balloon-catheter ranged between 7 and 32mm (19.04±6.25mm) and determined the selection of the prosthesis; a total of 21 devices were used, varying in size between 8 and 34mm (21.42±6.73mm).
Implantation was possible in all patients. In two patients, it was necessary to position the sheath in the right superior pulmonary vein; in one patient, the distal portion of the left disc was released inside the pulmonary vein and the sheath was withdrawn up to the right disk opening, in the right atrium; the set was then pushed and anchored in the interatrial septum, and the left disc was released from the pulmonary vein, impacting on the left side of the septum (30-mm prosthesis).
In all cases, the prosthesis was implanted without technical difficulties. In five patients (24%), there was minimum residual flow immediately after implantation, through the central portion of the prosthesis, which disappeared at the control Doppler echocardiography prior to discharge. None of the patients needed intensive care and all were discharged on the day after the implant. There were no deaths or other periprocedural complications in this series.
During the mean follow-up period, which was 10.3±5 months, all patients were free of cardiovascular symptoms and the echocardiographic assessment showed absence of residual flow. In 18 cases (86%), there was normalization of the right ventricle size; in three patients, aged>50 years, there was persistent right ventricular dilation; however, with a significant reduction in its size. There were no late complications such as erosion, pericardial effusion, arrhythmias, thrombus formation, or infective endocarditis.
DISCUSSIONThe literature review disclosed a single series published with 73 patients undergoing percutaneous closure of ASD using this prosthesis, performed at the First People’s Hospital of Yanzhou (China).7
In this series, the rate of complete occlusion within the first 24 hours was 100%. In five patients, minimal central residual flow was observed through the implant immediately after implantation, which may be explained by one of the occlusion mechanisms: the thrombogenicity of polyester flaps and the fact that clotting time of patients is variable. This adds to the fact that, during the procedure, complete anticoagulation is achieved. Complete occlusion was verified in small, moderate, and large defects, similarly to cases from published studies using nitinol double-disc prostheses.8−13
It is believed that the absence of complications was due to the following factors: the prosthesis flexibility, ease of implantation, and accurate selection of patients. All patients had optimal anatomic characteristics for implant, and borders of appropriate thickness and size>5mm, with the exception of the anterosuperior border, where the graft can be anchored, embracing the posterior aspect of the aorta.
The follow-up period of this study is considered too short to exclude more severe and at times lateonset complications, i.e., the erosion of the atrial walls with cardiac tamponade or of aortic wall with formation of fistula at the left atrium.14−16 However, the following precautions were taken when selecting the device to reduce the likelihood of this complication: prevent prosthesis oversizing, do not implant a device with left disk size greater than the maximum length of the atrial septum, and even in cases with deficient anterosuperior border, anchor the device embracing the posterior portion of the aorta, so that the borders of the discs, which have a higher erosion potential, do not come into contact with the juxtaposition of the atrial and aortic walls.
CONCLUSIONSThe Memopart® prosthesis has adequate flexibility and low profile, and was easy to handle and implant. Its use in percutaneous closure of ostium secundum ASDs with favorable anatomy was shown to be a safe and effective procedure, even in large-size defects within the limits of this investigation. A larger series with longer follow-up is needed to assess the possible occurrence of late-onset complications.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
The Lepu Medical Systems material used in the procedures was donated to the USP-HCFMRP by Valflux Comércio de Materiais Hospitalares (Goiânia, GO, Brazil), the representative in Brazil of Lepu (Shanghai Shape Memory Alloy Co. Ltda., Shanghai, China).