The percutaneous closure of patent ductus arteriosus (PDA) has been considered the treatment of choice by most authors and several devices with different structural characteristics have been used. The initial experience with the novel Cera™ PDA Occluder is reported.
METHODSFrom March 2010 through December 2011, patients weighing over 5kg, with PDA diagnosed by transthoracic echocardiograms (TTE) with color Doppler flow mapping, and no associated defects, were submitted to the procedure. Follow-up was performed by TTE within one, three and six months after the procedure and yearly thereafter.
RESULTSOverall, 18 patients were referred for percutaneous occlusion, of which 61.2% were female. Mean age and weight were, respectively, 13.7+9.3years and 42.9+20.1kg. Regarding morphology, 11 were type A, 6 were type E and 1 was a residual postoperative defect. Mean diameter was 4.2mm. Implant was possible in all patients. Ten 6–4mm, one 8–6mm, three 10–8mm and four 12–10mm devices were used. All defects were completely closed by the first follow-up TTE. There were no deaths or complications in this series.
CONCLUSIONSThe Cera™ prosthesis may be used for the occlusion of small or large defects with excellent results in children and adults. The procedure is ease, safe, has a high efficacy and low morbidity and may be an excellent option for the percutaneous closure of PDA. Due to its flexibility, oversized devices greater than the 2mm usually recommended should be used.
Fechamento de Canais Arteriais com o Dispositivo Cera™ PDA Occluder:Mais uma Boa Opção na Caixa de Ferramentas
IntroduçãoO fechamento percutâneo de persistência dos canais arteriais (PCA) tem sido considerado tratamento de escolha pela maioria dos autores, e diversos dispositivos com diferentes características estruturais têm sido utilizados. Apresentamos a experiência inicial do grupo com a nova prótese Cera™ PDA Occluder.
MétodosEntre março de 2010 e dezembro de 2011 foram submetidos ao procedimento pacientes com mais de 5kg de peso, com PCA diagnosticada por meio de ecocardiograma transtorácico com mapeamento de fluxo em cores (ETT), sem defeitos associados. O seguimento foi feito com ETT no primeiro, no terceiro e no sexto meses subsequentes, e, a seguir, anualmente.
ResultadosNo total, 18 pacientes foram encaminhados para oclusão percutânea, dos quais 61,2% eram do sexo feminino. As médias das idades e dos pesos foram, respectivamente, de 13,7 ± 9,3 anos e 42,9 ± 20,1kg. Quanto à morfologia, 11 canais foram do tipo A, 6 foram do tipo E, e 1 pertuito residual após cirurgia. A média dos diâmetros foi de 4,2mm. O implante foi possível em todos os casos. Foram utilizadas 10 próteses 6–4mm, 1 prótese 8–6mm, 3 próteses 10–8mm e 4 próteses 12–10mm. Todos os canais estavam completamente fechados por ocasião do primeiro ETT de controle. Não houve óbitos ou complicações nesta casuística.
ConclusõesA prótese Cera™ pode ser utilizada para o fechamento de canais de pequeno ou grande calibres com excelente resultado, em crianças e adultos. O procedimento é fácil, seguro, com alta eficácia e baixa morbidade, e pode ser excelente opção para o fechamento percutâneo de PCA. Suas características de flexibilidade sugerem que sejam utilizadas próteses superdimensionadas acima dos 2mm habitualmente recomendados.
The percutaneous closure of a patent ductus arteriosus (PDA) represents an established alternative to surgical ligation and has been defined as the treatment of choice by most authors. To this end, several devices have been used.1–15
At the end of the 1990s, the first metal mesh prosthesis, the Amplatzer® Duct Occluder I (ADO I), capable of occluding larger diameter channels was developed as an alternative to embolisation coils.16 Universally used, it has occlusion rates of nearly 100%, with very low short- and long-term complication rates.17–20 Despite the success, new devices with different structural characteristics have been produced.
The objective of the present study was to present an initial experience with a new prosthesis, the Cera™ PDA Occluder (Lifetech Scientific Co. Ltd., Shenzhen, China), and to examine its role as another option for the occlusion of medium and large calibre PDA.
METHODSStudy designThis was a prospective, single-arm study performed at two centres. All patients underwent closure of PDA with the Cera™ prosthesis between March 2010 and December 2011. Characteristics of the device and the immediate results are described.
Selection criteriaAll consecutive patients weighing more than 5kg with PDA, and without any other associated defects that required surgical correction, were included in this study. Cases were chosen by transthoracic echocardiograms (TTEs) with colour flow mapping. The dimensions and morphology of the defects did not constitute exclusion criteria.
The prosthesisThe Cera™ occluder is a self-expansible prosthesis with a nitinol fragmented cone and ceramic coating. The proximal (pulmonary) extremity has a female thread, measuring 2mm less than the distal (aortic) extremity of the cone, and connects to the delivery system. There is a retention disc in the aortic extremity that measures 4mm more than the distal extremity (Figure 1).
– Two details of the Cera™ PDA Occluder. The retention disc can be observed in the aortic extremity and the thread in the opposed extremity connected to the releaser cable. The presence of expanded polytetrafluorethylene inside the prosthesis to increase its occlusive capacity can be noticed.
The device is available in 2-mm increments from 6mm to 24mm in diameter (distal extremity). The central portion measures 7mm in 6- to 14-mm prostheses, 8mm in 16- and 18-mm prostheses, 9mm in 20- and 22-mm prostheses, and 10mm in 24-mm prostheses.
The delivery system is composed of a 5F to 12F long and flexible sheath, a small loader of compatible size, a haemostatic valve, and a distal extremity with a metallic cable with threads.
Implant techniqueThe implant and follow-up protocols have been previously described and are the same protocols used for the ADO I prosthesis study.20
Statistical analysisContinuous variables were expressed as mean and standard deviation, while categorical variables were expressed as numbers and percentages. The objective of this article was to present the initial experience with the new Cera™ prosthesis for the treatment of PDA in a single-arm registry; therefore, comparisons were not made.
RESULTSEighteen patients were referred for percutaneous closure with the Cera™ prosthesis; 61.2% were female. Their ages ranged from 1 to 33 years (13.7±9.3years), and their weights ranged from 10kg to 72kg (42.9±20.1kg).
Two patients had recent onset of exertional dyspnoea (cases 17 and 18). Regarding the morphology, 11 channels were type A, six were type E,21 and the other was a residual channel after surgical ligation.
The smaller channel diameters, measured at the pulmonary extremity, ranged from 1mm to 8.6mm (4.2±2.4mm) (Table).
Characterisation of the Study Population
| Case No. | ID | Gender | Age (years) | Weight (kg) | Morphologic type | Diameter (mm) | Size of the device* | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | JOC | M | 27 | 70 | A | 6 | CPO 12 | Closed |
| 2 | ASC | F | 17 | 56 | A | 5 | CPO 10 | Closed |
| 3 | JSO | F | 5 | 14 | A | 5 | CPO 10 | Closed |
| 4 | CGNA | F | 12 | 44 | E | 1 | CPO 6 | Closed |
| 5 | ACLO | F | 18 | 49 | E | 8.6 | CPO12 | Closed |
| 6 | GBB | M | 20 | 72 | E | 1 | CPO 6 | Closed |
| 7 | LHL | F | 20 | 55 | A | 1 | CPO 6 | Closed |
| 8 | JPAU | M | 1 | 10 | A | 1 | CPO 6 | Closed |
| 9 | DHDT | M | 14 | 73 | E | 1 | CPO 6 | Closed |
| 10 | KRC | F | 10 | 50 | A | 1 | CPO 6 | Closed |
| 11 | ACPO | F | 13 | 40 | A | 6 | CPO 10 | Closed |
| 12 | MRFS | M | 12 | 50 | E | 1 | CPO 6 | Closed |
| 13 | GRB | F | 8 | 30 | PO | 1 | CPO 6 | Closed |
| 14 | CVPS | F | 3 | 26 | E | 1 | CPO 8 | Closed |
| 15 | ALCA | F | 3 | 15 | A | 2.5 | CPO 6 | Closed |
| 16 | TAS | M | 2 | 12 | A | 2 | CPO 6 | Closed |
| 17 | SLRM | M | 33 | 57 | B | 8 | CPO 12 | Closed |
| 18 | LSG | F | 30 | 50 | A | 7 | CPO 12 | Closed |
CPO=CeraTM PDA Occluder; F=female; ID=identification; M=male; PO=post-operative.
The systolic pulmonary pressure was higher than 30mmHg in 50% (9/18) of patients and ranged from 18mmHg to 45mmHg (31±7.9mmHg).
Implantation of the device was possible in all cases. Ten 6-4mm, one 8-6mm, three 10-8mm, and four 12-10mm prostheses were used.
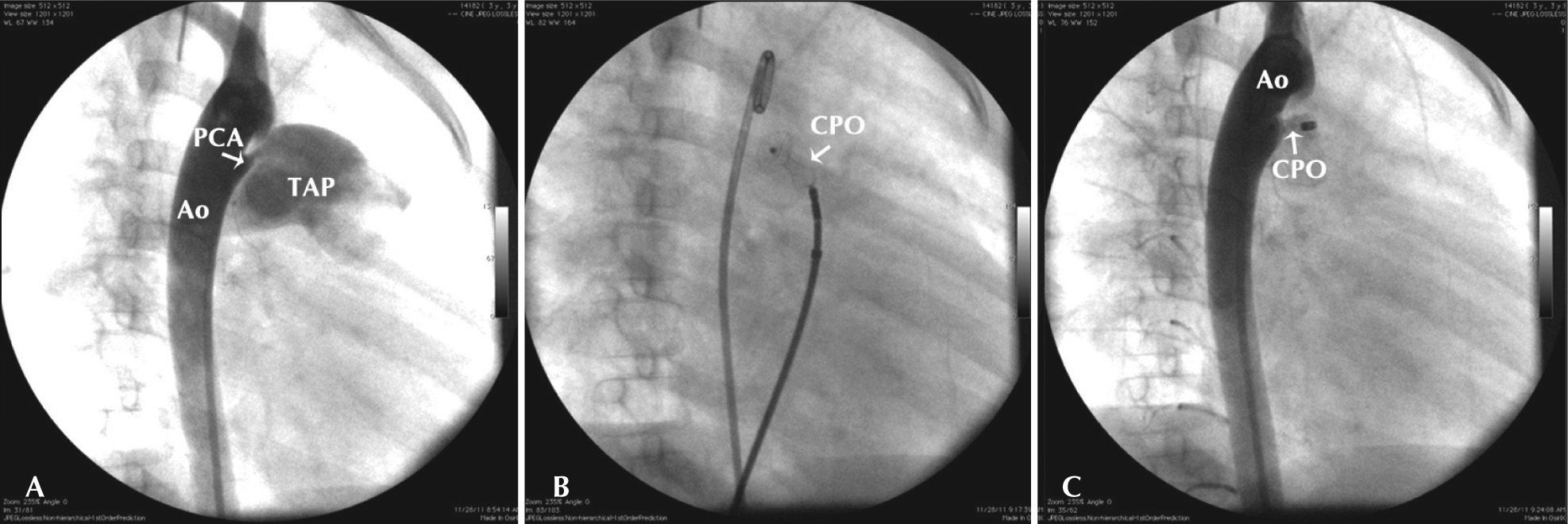
At the end of the procedure, two patients had minimal leakage of contrast inside the device; however, all defects were completely closed on the first control TTE, which was performed within the first week after the procedure (Figure 2). A gradient in the descending aorta or in the left branch of the pulmonary artery was not observed. In this initial series, complications were not observed.
– Steps of the procedure. In A, descending aortography in the right anterior oblique incidence showing a conic patent ductus arteriosus (type A) with 2.5mm in its smaller diameter, in the pulmonary entrance. In B, detail of the implant of a 6-mm (6–4mm) Cera™ PDA Occluder, with the retention disc in the aortic ampoule and the conic portion inside the channel. Note the significant constriction in the central portion of the cone, which stabilises the prosthesis. In C, control descending aortography showing the released prosthesis without evidence of contrast in the pulmonary artery. An approximately 90 degrees modification can be observed in the prosthesis angle after its release from the delivery system. Ao=aorta; CPO=Cera™ PDA Occluder; PCA=patent ductus arteriosus, TAP=pulmonary artery trunk.
The percutaneous closure of a PDA has been successfully performed through the use of several devices. The introduction of the ADO I prosthesis brought safety and ease of use, with excellent occlusion rates (99% to 100%) and a low incidence of complications (0% to 7%). This constituted an excellent alternative to percutaneous coil closure, despite the higher cost. The reported complications, in general, are minor and are usually observed in more severe patients and in patients with less body weight.22,23 There are no reports of late complications with this device.24
The Nit-Occlud® (PFM, Cologne, Germany) device was introduced as an intermediate device between the ADO I and coils. It utilises a controlled-release premolded coil that is shaped as an inverted double cone. It is capable of occluding intermediate size channels (< 6mm) and costs less. With an occlusion rate of 91% to 100% and few minor complications (0% to 9%), it has been used in some centres, including in Brazil.25–28
The Amplatzer® Duct Occluder II (ADO II) was developed to occlude channels in smaller children by reducing the introducer profile. It maintained the high occlusion rate of existing devices. With two articulated disks at the extremities and a central portion, it closes channels of less than 12mm in length and less than 5.5mm in diameter. Occlusion rates were maintained at nearly 100%, without reports of significant complications. 29–33 The report of a case in which residual flow appeared 24 hours after the successful closure of a 3mm channel due to kinking of the left disc in the ductal ampoule should be highlighted.34
The design of the Cera™ prosthesis is very similar to that of the ADO I, but it has a more flexible nitinol mesh and a ceramic coating. This characteristic gives the Cera™ prosthesis the theoretical advantage of less nickel release on the days after the implant, although this has not been a problem reported with the ADO I.
Its great flexibility allows for the supersizing of the prosthesis according to the channel diameter without damaging neighbouring structures. Hence, the prosthesis is more stable (greater constriction in its central portion), and, as a consequence, it has a lower embolisation risk.
The long introducer allows passage through accentuated curves without kinks or breaks. Another advantage is the presence of a radiopaque mark in the distal end, which allows the surgeon to safely know the position of the long sheath during all the steps of the procedure.
CONCLUSIONSIn this initial experience, the Cera™ prosthesis could be used in small or large calibre channels. This device was easy to use, was extremely safe, had a low risk of morbidity, and was highly effective. The implant procedure is very similar to that of other existing nitinol mesh prostheses.
The author believe that its greater flexibility allows its diameter to be supersized more than 2mm above the smaller channel diameter.
CONFLICTS OF INTERESTFrancisco Chamié is a Boynton technical consultant. The other authors declare no conflicts of interest.