Percutaneous Mitral Valvotomy in a Patient with Lutembacher Syndrome as a Bridge to Definitive Surgical Intervention A 45-year-old female patient, Jehovah’s Witness, was diagnosed with Lutembacher syndrome, New York Heart Association (NYHA) class IV congestive heart failure (CHF) and severe malnutrition. Echocardiogram showed an ostium secundum atrial septal defect, 38mm in diameter, mitral valve area of 0.5cm2, Wilkins score of 10, severe pulmonary hypertension, estimated systolic pressure of 96mmHg, right ventricle with significant dilation and severe dysfunction and severe tricuspid valve insufficiency. Despite optimal clinical treatment, there was no improvement of CHF or the patient’s overall condition, which led to a change in the initial conduct of surgical treatment to a two-stage therapy, starting with balloon mitral valvotomy, as a bridge to surgery. Postoperative mitral valve area increased to 1.34cm2. The patient evolved with significant clinical improvement, and surgery was performed 120 days later with mitral valve replacement by a mechanical valve and atrioseptoplasty using a bovine pericardial patch in addition to tricuspid valve cerclage. Patient was discharged 11 days after the surgery and is currently on the sixth postoperative month, evolving with clinical stability and improvement in quality of life.
Valvotomia Mitral Percutânea em Paciente com Síndrome de Lutembacher como Ponte para Intervenção Cirúrgica Definitiva
Paciente com 45 anos de idade, do sexo feminino, testemunha de Jeová, portadora de síndrome de Lutembacher, com insuficiência cardíaca congestiva (ICC) grau funcional IV da New York Heart Association (NYHA) e desnutrição grave. O ecocardiograma revelou comunicação interatrial tipo ostium secundum, com 38mm de diâmetro; área valva mitral de 0,5cm2, com escore de Wilkins de 10; hipertensão pulmonar grave, com pressão sistólica estimada em 96 mmHg; ventrículo direito com significativa dilatação e disfunção grave; e insuficiência valvar tricúspide grave. Apesar do tratamento clínico otimizado, não houve melhora do quadro de ICC nem do estado geral, motivando a mudança da conduta de tratamento cirúrgico inicial para tratamento em dois tempos, primeiramente por meio de valvotomia mitral por balão, como ponte para a cirurgia. A área valvar mitral pós-procedimento aumentou para 1,34cm2. A paciente evoluiu com significativa melhora clínica, sendo realizada cirurgia 120 dias após, com substituição da válvula mitral por uma prótese mecânica e atriosseptoplastia com patch de pericárdio bovino, além de cerclagem da válvula tricúspide. A alta hospitalar ocorreu 11 dias após a cirurgia. Atualmente, encontra-se no sexto mês pós-procedimento cirúrgico, evoluindo com estabilidade clínica e melhora da qualidade de vida.
In 1916, René Lutembacher1 was the first to describe the combination of mitral stenosis and interatrial communication (IAC). The resulting haemodynamic repercussion of this association depends on the size of the communication and severity of the valvular stenosis, which can cause varying degrees of pulmonary hypertension, dilation of the right chambers, and tricuspid regurgitation. Currently, this condition can be treated using percutaneous intervention (balloon mitral valvotomy and occlusion of the defect using a prosthesis) or a standard surgical treatment.2–7
The case of a patient with a wide ostium secundum type IAC associated with mitral stenosis is reported; she was admitted in poor general state with New York Heart Association (NYHA) class IV severe congestive heart failure (CHF) refractory to optimal clinical treatment. Performing balloon valvotomy as the initial treatment resulted in haemodynamic and overall improvement of her condition, allowing the subsequent surgery to proceed with the patient in better condition and at lower risk.
CASE REPORTThe patient had a history of dyspnea on exertion since childhood, when she was diagnosed with congenital cardiopathy, for which follow-up was recommended. She had led a normal life until her second pregnancy when, at five months, her gynaecologist referred her to a cardiologist. At that time, the patient had developed dyspnea on minimal exertion, she felt fullness when eating, and she had edema in the lower limbs.
She did not keep a regular follow-up until she was referred to the Cardiology Outpatient Clinic at the Cardiac Surgery Service of the Hospital Santa Izabel da Santa Casa de Misericórdia da Bahia (Salvador, BA, Brazil). She was immediately admitted due to her precarious state, anaemia, and NYHA class IV CHF.
A physical exam on admission demonstrated that the patient was malnourished, with pale 2+/4+ mucous membranes, jugular veins distended at 45 degrees, and palpable and filiform peripheral pulses.
- –
Examination of the cardiovascular system showed that the precordial region was active with a right precordial impulsion.
- –
Upon palpation, the presence of a systolic thrill was found on the tricuspid region, and diastolic thrill was found on the mitral region. In addition, the apex impulse was decreased and palpable on the left mid-axillary line, and a second heart sound was palpable on the pulmonic region.
- –
Upon auscultation, the heart rate was 100 beats/ minute, with a regular rhythm and accentuation of the second heart sound, which presented as a fixed splitting. A IV/VI systolic murmur on the high left sternal border, a IV/VI diastolic murmur on the mitral region and a IV/VI systolic murmur on the tricuspid region that intensified with deep inspiration were also observed.
An increased abdominal volume secondary to a large ascites was present. On palpation, the liver was enlarged and the patient reported pain. Pulmonary auscultation revealed the presence of rales on the inferior third of both lungs. The presence of 2+/4+ lower limb edema was noticed as well.
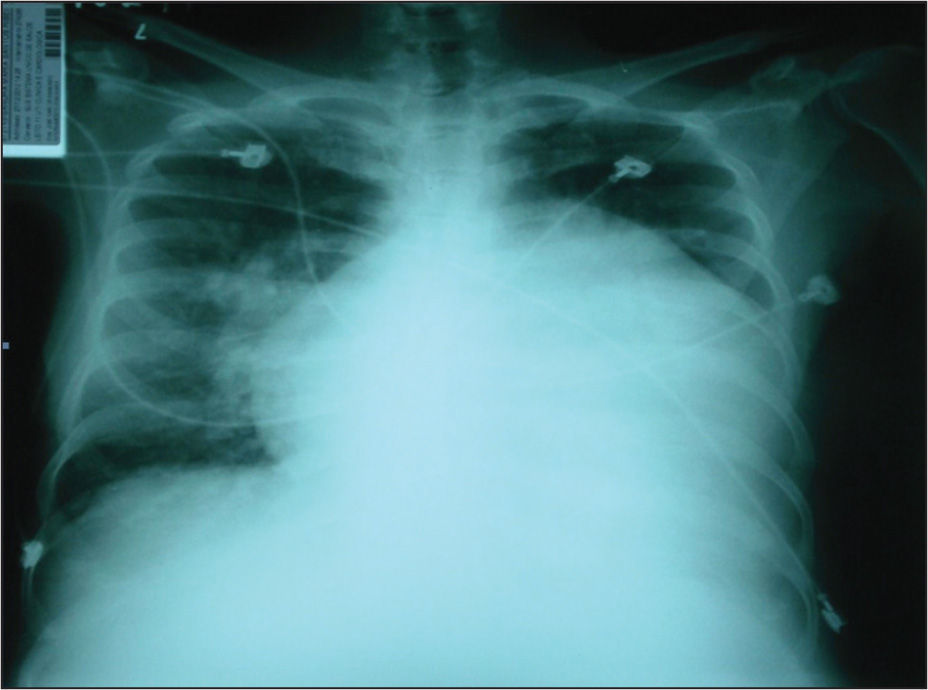
An electrocardiogram showed bi-atrial and right ventricular enlargement. A chest X-ray showed increased pulmonary flow and an increased heart area with predominance of the right chambers and dilation of the medium arch.
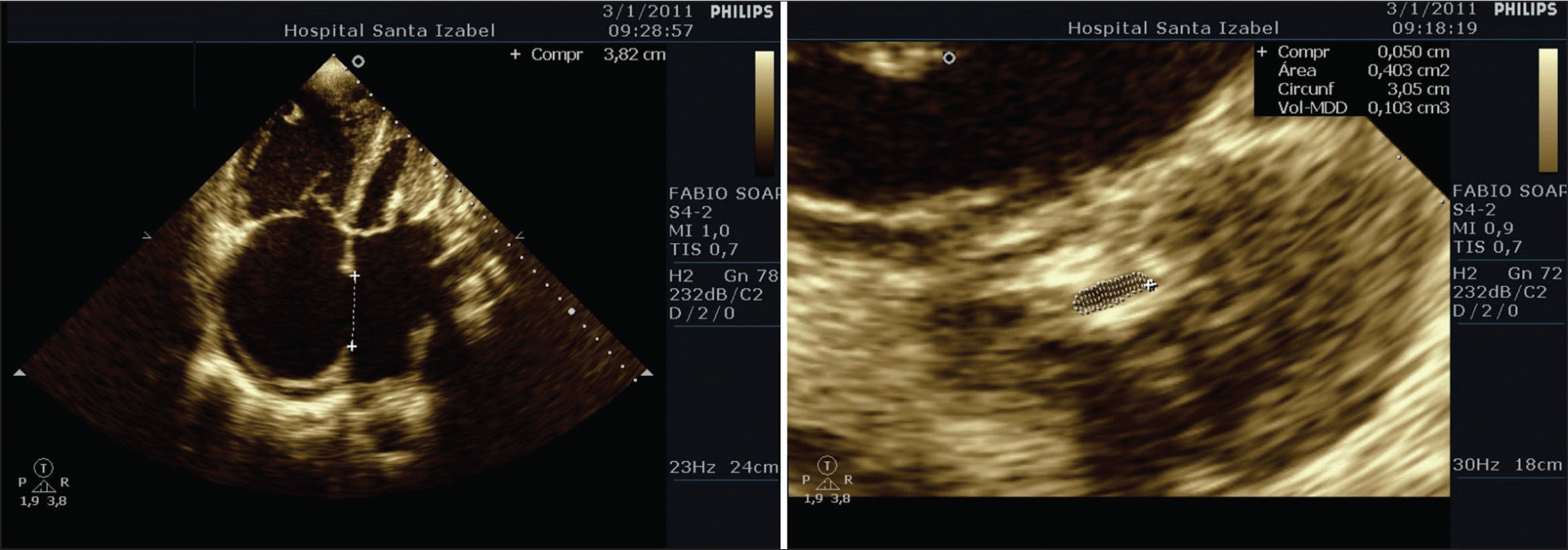
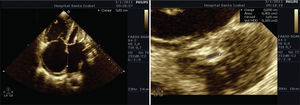
A transthoracic echocardiogram showed the presence of a large ostium secundum type IAC measuring 38mm, with severe mitral stenosis at a mitral valve area of 0.5cm2 and a Wilkins8 score of 10. Severe pulmonary hypertension with an estimated systolic pressure of 96mmHg, a right ventricle with significant dilation and severe dysfunction, an increase in the size of the atria, severe tricuspid valve regurgitation, and normal left ventricular systolic function with an ejection fraction of 61% (Figure 2) were also observed.
Treatment consisted of absolute bed rest with the head of the bed elevated, and optimised pharmacologic measures to treat the CHF. A significant improvement was not observed despite increasing doses of intravenous furosemide. The case was discussed in a panel whose members included her clinician, the intensive care unit (ICU) physician, and her surgeon. A two-part approach to treatment was selected. Initially, a balloon mitral valvotomy would be performed as a palliative treatment to relieve the mitral stenosis and improve the patient’s haemodynamics and symptoms. Then, definitive surgery would be performed.
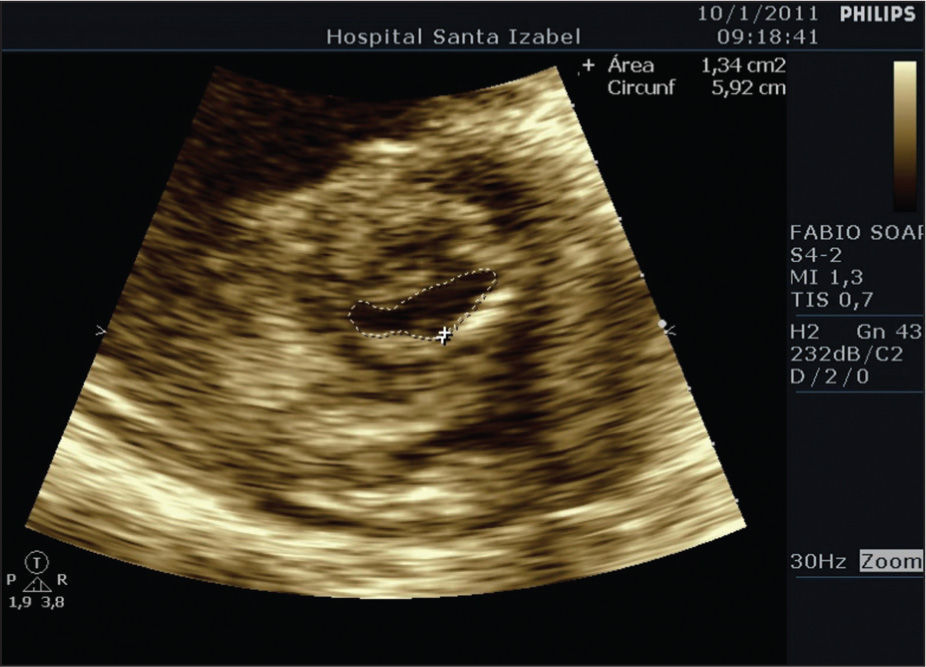
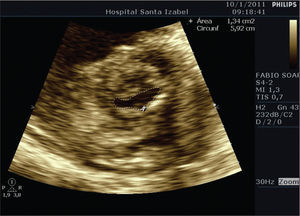
Balloon mitral valvotomy was preceded by cardiac catheterisation, which did not show obstructive coronary disease. Dilation was performed with a 26-mm balloon catheter using the Inoue technique. The balloon was initially inflated to 24mm, and then a second dilation to 26mm was performed. Left ventriculography after the dilation showed mild mitral regurgitation. The procedure was performed without intercurrences. The area of the mitral valve was 1.34cm2 as measured on the following day by planimetry (Figure 3).
The development of atrial flutter on the first week post-valvotomy required cardioversion, which was successful and occurred without intercurrences. Despite the suboptimal results from the balloon mitral valvotomy, the patient showed progressive improvement after the percutaneous intervention. After hospitalisation for slightly over two weeks, the patient was discharged with NYHA class II and was prescribed iron and folic acid supplements to increase haemoglobin levels (under strict orders from the surgical team) since she refused to use blood products during surgery for religious reasons.
The patient was readmitted to the hospital on May 19, 2011 and underwent mitral valve replacement surgery using a St. Jude 29M mechanical prosthesis (St. Jude Medical – St. Paul, MN, United States). The IAC was closed with a bovine pericardial patch (atrioseptoplasty), and cerclage of the tricuspid valve was performed using the De Vega technique. The patient was discharged from the ICU three days after surgery. In the infirmary, the patient’s condition continued to stabilise, and she was discharged from the hospital on May 30, 2011.
DISCUSSIONLutembacher syndrome is characterised by the association of IAC and mitral stenosis. The prevalence of this syndrome is uncertain. It is more common in females, partly due to the greater incidence of both mitral stenosis and IAC in females. Haemodynamic repercussions and clinical manifestations of Lutembacher syndrome depend on the size of the IAC and on the severity of the mitral stenosis.
Currently, Lutembacher syndrome can be treated by percutaneous intervention or standard surgery. In very experienced centres, and in select cases, percutaneous occlusion of IAC using different devices associated with balloon mitral valvotomy can be an option. The selection criteria for percutaneous intervention are the same as those used for the individual diseases. Regarding balloon mitral valvotomy, a Wilkins score≤8 represents a good expectation of immediate results and a good prognosis. With respect to the IAC, an ostium secundum or oval fossa type can be treated percutaneously, for which a defect≤35mm and a≥5mm distance of the borders (in its more rigid portion) from the coronary sinus, mitral valve, and superior vena cava are required. This information can be obtained with a transesophageal echocardiogram.
In the present case, because the patient was from the Sistema Único de Saúde (Brazilian Unified Health System) and did not have medical coverage for IAC occluder prosthesis, initial percutaneous treatment was not considered. In addition, a Wilkins echocardiographic score of 10 was the limiting factor for balloon mitral valvotomy. The option for balloon mitral valvotomy was determined to be viable after a serious discussion among clinical cardiologists, surgeons, and ICU physicians. The initial treatment with high doses of diuretics was not effective and did not provide adequate clinical control of the patient’s systemic congestion symptoms. There was concern that if she remained refractory to treatment, she would not survive the surgery due to her poor general state and the complications inherent to the postoperative period that are experienced by patients with right ventricular dysfunction. Her religion was another complicating factor, since she was a Jehovah’s Witness and refused blood products.
The patient and her family were informed about the risks of the proposed procedures, including those inherent to balloon mitral valvotomy. Erythropoietin, iron, and folic acid were then administered to progressively increase haemoglobin levels, and the administration of cardiovascular drugs was maintained.
The success of balloon mitral valvotomy is characterised by an increased valvular area > 25% of the initial area and a mitral valvular area≥1.5cm2 in the absence of mitral regurgitation > 2+, according to angiographic criteria.9,10 The patient had an echocardiographic score of 10, subvalvular stenosis (3), thickening (2), mobility (3), and calcification (2). On radioscopy, she had a calcification of 2++. The suboptimal result obtained with balloon mitral valvotomy, with a final mitral valvular area of 1.34cm2, resulted in a significant clinical improvement, and the patient was discharged from the hospital with NYHA class II/IV CHF. She returned later for surgery with increased haematocrit levels as required by the cardiovascular surgeon.
Surgery consisted of mitral valve replacement using a St. Jude 29M mechanical prosthesis and closure of the IAC with a bovine pericardial patch (atrioseptoplasty), as well as tricuspid valve cerclage using the De Vega technique to correct the tricuspid regurgitation. The choice of valvular substitute for atrioventricular orifices has been the subject of many discussions among those who defend biological or mechanical prostheses; thrombosis and longevity are the most relevant factors in this context. Although biological prostheses have a low incidence of thrombosis and do not require anticoagulation, they do not last as long as mechanical prostheses. In the present report, it was decided to use a mechanical prosthesis in order to offer a more definitive treatment, which implied the indefinite use of anticoagulants.
The postoperative evolution of the patient was surprisingly good; she maintained a satisfactory cardiac output and stable haemodynamics, and she did not experience significant bleeding. She was discharged from the hospital 11 days after the surgery.
She is now in the sixth postoperative month. She is stable, and her quality of life has improved.
This report confirms the importance of collaborative, interdisciplinary work among clinical cardiologists, ICU physicians, and cardiovascular surgeons in defining the best individual strategy to be used with the devices that are available at a particular institution.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.