In patients considered inoperable or at high risk for surgical aortic valve replacement, transcatheter aortic valve replacement (TAVR) has been established as the treatment of choice.1
Coronary obstruction following TAVR, while uncommon, is an acute life-threatening complication. The incidence of this catastrophic complication is described as less than 1% in most of the current registries.2,3 The acute and late mortality rate post-coronary obstruction is very high. After successful percutaneous coronary intervention (PCI), it is 22%, and after successful coronary artery bypass surgery, 50%, while unsuccessful PCI correlates with 100% mortality. Overall, the 30-day mortality approaches 40%.3
Clinical presentation includes severe profound refractory hypotension or immediate cardiac arrest, electrocardiographic changes such as ST elevation, ST depression, and ventricular arrhythmias with hemodynamic collapse, as well as segmental wall motion abnormalities shown echocardiographically. Unfortunately, the sole presence of hypotension without electrocardiographic changes is also not unusual. The fact that on some occasions the coronary flow is not completely obstructed may explain the less dramatic initial presentations.
It has been shown that coronary obstruction after TAVR is, in most of the cases, related to displacement of a bulky calcified native leaflet towards/over a coronary ostium during valve implantation.4 Theoretically, the complication can be also the consequence of a high valve implantation, with the sealing cuff placed against the coronary ostium. A dislodged native valve calculus could migrate and occlude a coronary artery, even hours after the procedure, as recently reported.5
The risk of coronary obstruction is difficult to assess, since there is no single measurement to consider, but rather a constellation of clinical, anatomical, and procedural factors that play a different role in each procedure. A recent registry has provided further insight into the baseline characteristics, identifying advanced age, female sex, prior valve surgery, and higher EuroSCORE as clinical predictors, while showing that the use of balloon-expandable valves doubles the risk as a procedural factor.3 The fact that self-expanding valve anatomical requirements are much more conservative than balloon expandable valve requirements has generated a selection bias that may, at least partially, explain this difference. However, self-expanding valves pose their own unique challenges: it is more difficult to perform coronary protection with a guiding catheter trapped behind the metallic struts (ascending aorta level) of this valve type. Also, coronary access through the metal cage of a self-expanding valve can be particularly challenging or impossible. A duplicate implant of a self-expanding valve may extend the covered stent strut area to the higher level in a particularly unfavorable configuration.
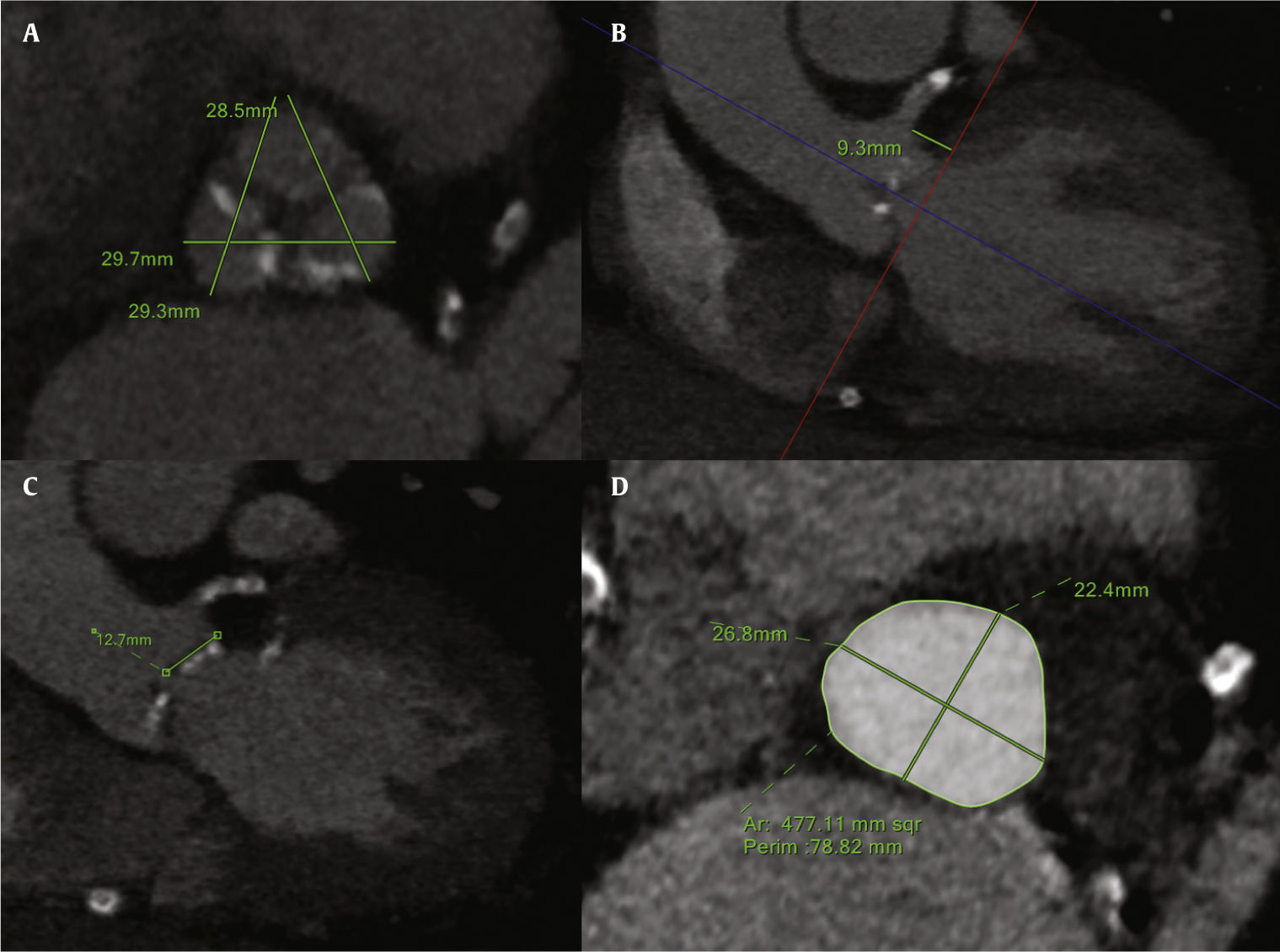
The major anatomical predictors of coronary obstruction are coronary height, coronary sinus, and aortic root size (Fig. 1). Measuring the coronary height perpendicular to the annular plane is more conservative and has been shown to be more reproducible. However, it could also be measured from the leaflet insertion to the lower edge of the coronary ostia, in an oblique fashion. The left main coronary artery (LMCA; 89%) is more commonly affected in comparison to the right coronary artery (RCA; 4%), or bilateral obstruction (7%),3 most likely due to the generally higher level of RCA ostia take-off. The cut-off for LMCA height is < 12mm (mean of 11mm), while for RCA it is unknown, given the low number of patients reported. A narrow aortic “tube-like” root together with shallow sinuses of Valsalva is also strongly associated to coronary obstruction. The cutoff for the sinuses of Valsalva width is < 30mm. It is paramount to stress that the sinuses of Valsalva measure must be taken in relation to the annulus, since the difference between them will represent the space where the leaflet will be accommodated after valve deployment. A sinuses of Valsalva/annulus ratio under 1.26±0.04 has been demonstrated to be highly correlated with this complication (odds ratio=20; 95% of confidence interval=1.28–333).3,6
Multidetector computed tomography analysis of the aortic valvar complex. (A) Sinuses of Valsalva dimensions. (B) Left main coronary artery height, measured perpendicular from aortic annulus. (C) Left leaflet length. (D) Aortic annulus measurements (area, perimeter, maximal, and minimal diameters).
Other weaker predictors recognized in the computed tomography analysis are the degree of valve calcification, the presence of eccentric bulky calcific nodules in relation to the coronary ostia, and the leaflet length.6 The leaflet length should be measured in an oblique coronal view, from the leaflet insertion to the tip of the leaflet. Its absolute value is useless unless associated to the coronary height. In fact, it has been postulated that the risk of obstruction increases as the ratio LMCA ostium height/leaflet length falls below 1/1.
Only the integration of these factors, together with the pre-procedural considerations such as a proper valve over/under-sizing, can aid in obtaining a reliable and predictable result, often accepting minor paravalvular leaks in order to avert catastrophic complications such as coronary obstruction and annular rupture.
In these cases, preventive measures should be instituted only if the final risk/benefit evaluation after the 3-D imaging screening favors TAVR over surgical aortic valve replacement/medical treatment.
After identifying a high-risk patient, the interventional team must prepare to protect the coronary artery at risk. These patients should be treated in a fully equipped hybrid operating room, under general anesthesia and with expert 3-D echo guidance.
In uncertain cases, in which computed tomography and 3-D echo have poor correlation or the risk is thought to be moderate, an aortogram in left anterior oblique/cranial projection during balloon aortic valvuloplasty may simulate the final result after valve deployment.7
In this issue of the Revista Brasileira de Cardiologia Invasiva, Furini et al.8 elegantly present “Coronary occlusion after TAVI: safety strategy report.” The authors describe in detail the steps taken in order to prevent coronary obstruction in a high-risk case. Of note, this group performed an aortogram during balloon aortic valvuloplasty to assess coronary patency. This technique is well known and appears to be somewhat useful, particularly if it confirms absent coronary flow with balloon inflation in the valve location. However, the best subsequent step is not clear in cases with patent coronary arteries during balloon inflation.
Several factors may preclude the technique to properly simulate the actual valve implantation. First, the aortogram must be performed only when the balloon is fully expanded. Moreover, the balloon's nominal size should theoretically equal the final outer size of the implanted valve. The latter is obviously very difficult to accomplish, due to limited balloon size availability and the fact that final oversizing or undersizing is not easy to predict, especially in cases that require post-dilatation due to para-valvular leak (notably, this should be rarely undertaken if a possible coronary obstruction is suspected). For safety reasons, there will obviously be a general tendency to use smaller valvuloplasty balloons than needed.
In this case report, despite no coronary compromise in the aortogram, the operators still decided to protect the RCA with a wire and a stent.
It is important to stress the fact that in this frail population, minimizing the use of contrast and valve manipulation could make a difference in term of contrast induced nephropathy and stroke. Also, balloon aortic valvuloplasty alone was responsible for about 10% of the coronary occlusion during TAVR in a recent registry,3 and several groups are avoiding the valvuloplasty step prior to TAVR. Thus, wiring the at-risk coronary artery could also be considered prior to this maneuver.
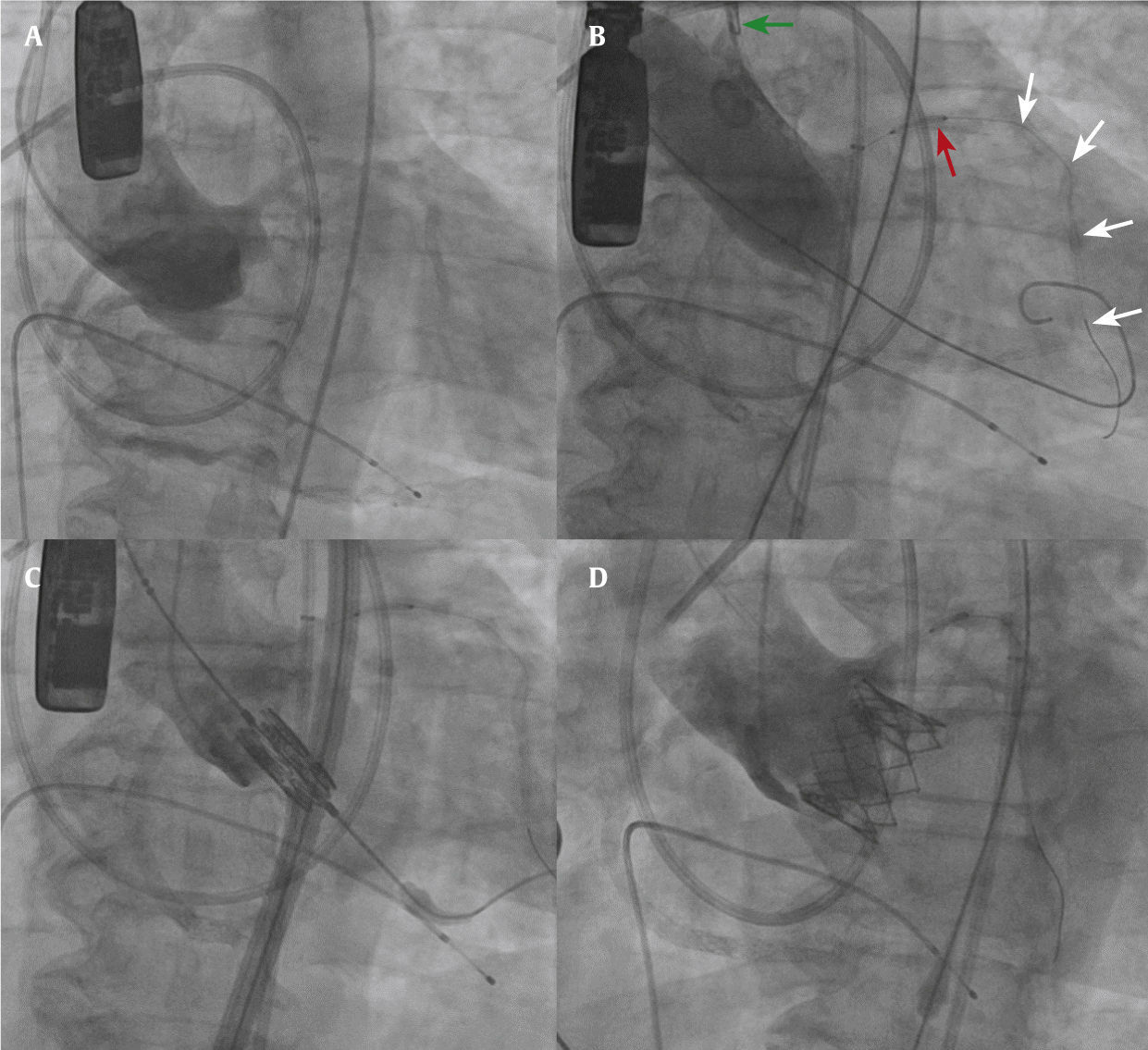
Due to the difficulty in delivering a stent once coronary obstruction is established, we suggest protecting the coronary at risk with a wire and a stent (Fig. 2). As Furini et al.8 described, after documenting absence or impaired flow in the coronary artery, pulling the stent and deploying it at the ostium would be much easier than trying to advance it against a calcified obstructive leaflet. Also, it is believed that the stent shaft itself may contribute to avoid the coronary occlusion by preventing the valve tissue from displacement over the ostia. Therefore, after valve deployment, if no intervention is needed, the stent should be retrieved gently and a re-assessment should be done before pulling the coronary wire.
Fluoroscopic images of a coronary protection case. (A) Aortogram in a coplanar view demonstrating the three aligned aortic valve cusps, and the low origin of the left main coronary artery. (B) Balloon aortic valvuloplasty. The guiding catheter is pulled into the ascending aorta (green arrow). Note the undeployed stent (red arrow) and the coronary wire (white arrows). (C) Balloon-expandable valve in position prior to implantation. (D) Aortogram after valve deployment, demonstrating good position, without evidence of coronary obstruction.
Of note, some groups have started to protect the coronaries with a deflated balloon. Unfortunately, in some cases, after ostia balloon dilatation, the instant recoil of the “squashed” leaflet between the valve struts and the coronary ostia precludes the advancement of the stents. Interestingly, the Mayo Clinic group recently described the use of temporary balloon inflation in LMCA ostium during valvuloplasty and TAVR in order to avoid coronary embolization, after having identified mobile debris in the left coronary cusp by 3-D trans-thoracic echocardiography.9 This must certainly be considered an exceptional measure, driven by highly-individualized particular measurements of low coronary ostium height, narrow sinuses of Valsalva and root diameters, and an eccentric unfavorably-situated calcification.
In order to avoid the need of an extra arterial access for coronary protection, one may consider the use of a 6 F guiding catheter with side-holes that will replace the pigtail for the aortogram. The economy of arterial access may become critical in the next few years, if the ongoing clinical trials favor the use of embolic protection devices (which will require a dedicated arterial access site).
After valve deployment, the trained echocardiographer must certify the appropriate global left ventricular function, the presence of laminar flow in the coronary arteries, and the absence of new segmental wall motion abnormalities. The identification of turbulent flow together with the previously described clinical scenario will confirm the complication on course, and should alert the interventionalist to perform an emergent angioplasty to re-establish the coronary flow.
Since stent compression after deployment is not rare, identification of laminar coronary flow using 3-D transesophageal echocardiography, or evaluation of stent patency by intravascular ultrasound or angiography, should be carefully performed after coronary angioplasty/stent. Not infrequently, a second stent needs to be deployed to achieve enough radial force and improve the minimal lumen area. It is important to emphasize that the stent should protrude from the true ostium, creating a tunnel through the compressed leaflets towards the aorta. A proximal “flaring” technique to complete the intervention may facilitate a re-intervention if needed in the future.
The treatment of this complication mandates immediate action in order to re-establish coronary perfusion. Even when the coronary artery was not previously protected, nearly 80% of patients can be rescued by successful PCI. However, most of them will require hemodynamic support or conversion to open heart surgery. In some situations, the rapid initiation of temporary extracorporeal circulation may be of assistance if coronary bypass grafting might solve the problem.
Retrograde left ventricular support devices, such as Impella™ were reported to be beneficial in the setting of hemodynamic instability due to coronary obstruction.10 This device has arisen as an expeditious alternative to cardiopulmonary bypass for the treatment of shock during TAVR, given the already available large-bore arterial access. Nevertheless, this option may be limited by the difficulty of recrossing the newly implanted valve (this is especially true if a double self-expanding implant has been used); therefore, maintaining wire access in the left ventricle during echocardiographic post-TAVR evaluation of possible coronary obstruction may be important. Theoretically, an intraortic balloon pump would be of little help if the coronary artery is occluded, but it may be considered if there is a partial occlusion.
The decline in catastrophic complications after TAVR could be explained by the improvement in valve prostheses, the decreasing diameter of the delivery sheaths, the improved imaging methods, and the operators’ experience. Furthermore, advances in careful pre-procedural screening and planning utilizing 3-D imaging, such as multidetector computer tomography and echo, have contributed enormously to the identification of high-risk patients.
Awareness of high-risk features for coronary obstruction, prophylactic measures, and prompt recognition and management of this complication are paramount for a successful TAVR. A well-established and well-functioning heart team composed not only by cardiac surgeons and interventional cardiologists, but also by imaging specialists in cardiovascular computed tomography and interventional echocardiographers and cardiac anesthesiologists, as well as a fully trained technician and nurse team, plays a critical role in the final outcome of this procedure.
Conflicts of interestThe authors declare no conflicts of interest.