The anatomy of the coronary arteries is well known, but there is a wide variety in their origin and distribution. The dual left anterior descending artery is defined as the presence of two left anterior descending arteries within the anterior interventricular sulcus and is classified into four types. It is a benign anatomical variant that should be recognized, especially before interventional procedures. We report a patient with type I dual left anterior descending artery, with acute anterior wall ST elevation myocardial infarction, referred for primary percutaneous coronary intervention.
A anatomia das artérias coronárias é bem conhecida, mas há grande variedade em sua origem e distribuição. A artéria descendente anterior dupla é definida como a presença de duas artérias descendentes anteriores dentro do sulco interventricular anterior, sendo classificada em quatro tipos. É uma variante anatômica benigna que deve ser reconhecida, especialmente antes de procedimentos intervencionistas. Relatamos o caso de um paciente com artéria descendente anterior dupla tipo I, com apresentação clínica de infarto agudo do miocárdio com supradesnivelamento de segmento ST em parede anterior, encaminhado para a realização de intervenção coronária percutânea primária.
Left anterior descending artery (LAD) is an artery with a more consistent pattern in its course and distribution in coronary circulation and, usually, originates in the left main coronary artery, runs through the anterior interventricular sulcus and issues septal and diagonal branches, which ensure irrigation of anterior, septal and lateral walls of left ventricle.
The anomalies of coronary origin and course are widely described and classified in the literature. However, cases of dual LAD are rarely described, in which a short segment ends at the upper portion of the anterior interventricular sulcus and a long-branch segment reaches the apex.
The correct angiographic recognition of anatomical variations is very important during revascularization procedures, whether by percutaneous or surgical route, especially in cases of primary percutaneous coronary intervention (PCI).
Case reportThis is a male patient, aged 71 years, transferred from the Basic Health Unit to cardiologic emergency unit of Santa Casa de Ribeirão Preto, due to chest pain with 8 hours’ duration and an electrocardiogram with an extensive anterior ST-segment elevation. At admission, the patient was in Killip I functional class, the blood pressure was 170 90mmHg, heart rate 94 bpm, peripheral oxygen saturation 96%, and receiving oxygen through a nasal cannula at 2 L/min. The heart and lung auscultations were normal.
With the clinical diagnosis of Killip's class I ST-segment elevation myocardial infarction, pharmacological measures have been implemented (acetylsalicylic acid - ASA 200mg, clopidogrel 600mg, and intravenous nitroglycerin), and the patient was referred to primary PCI.
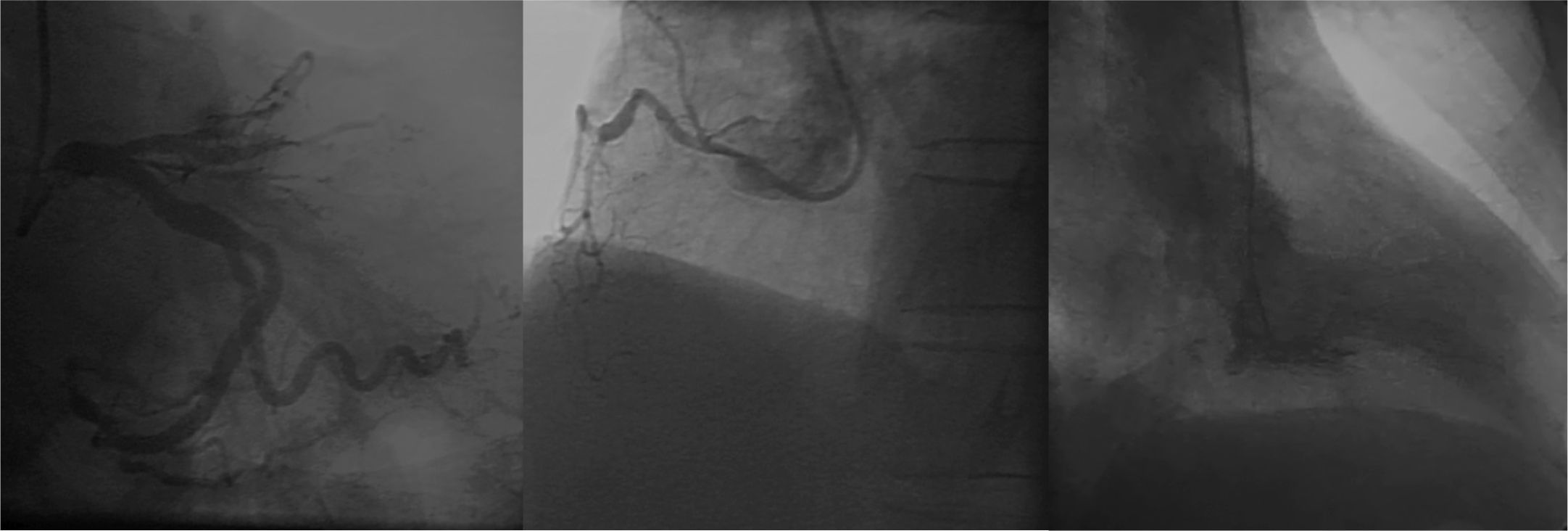
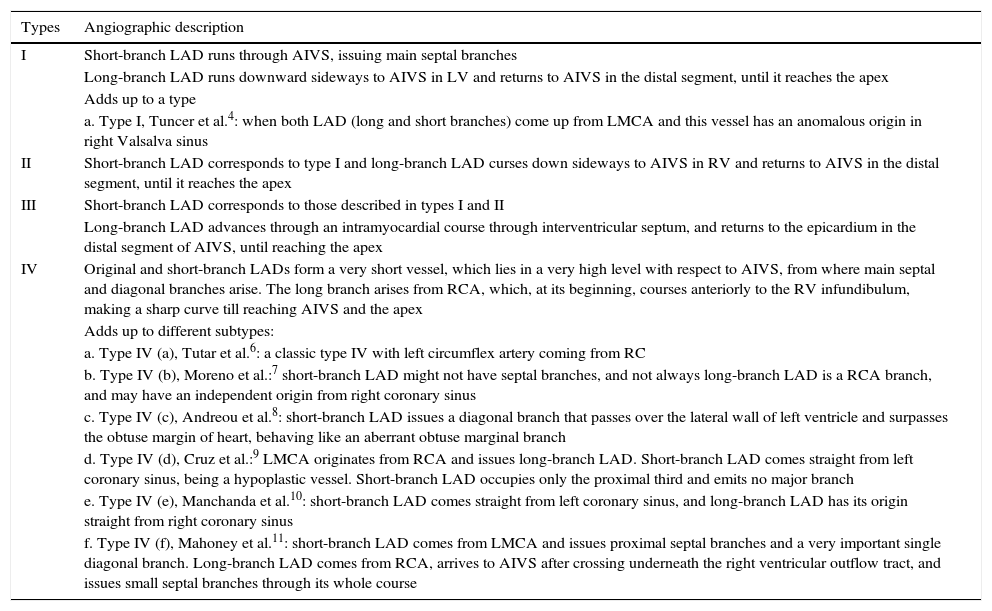
Coronary angiography was performed by brachial artery puncture with a 6 F sheath, due to excessive tortuosity, spasm and no guide wire progression by radial approach. The examination showed proximal occlusion of LAD; absence of obstructive lesions in left circumflex and right coronary arteries; and left ventricular anteroapical akinesis (Fig. 1).
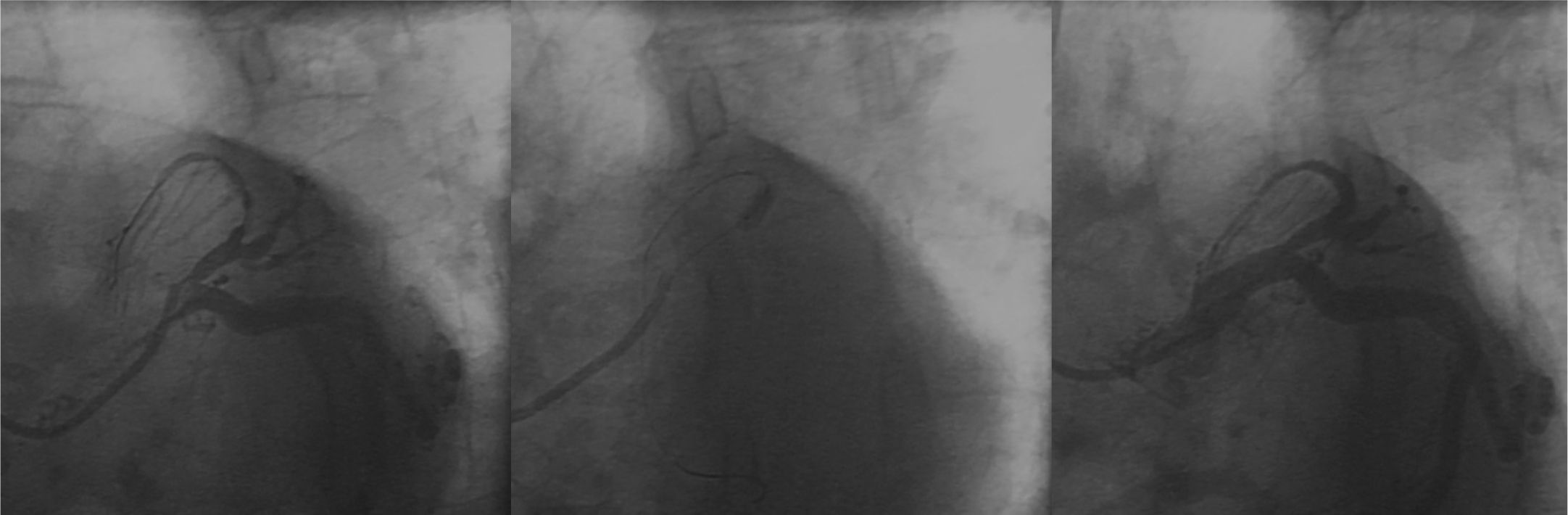
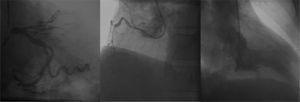
The patient underwent primary PCI, with administration of 100 IU/kg of unfractionated heparin and passage of a 0.014-inch intermediate ChoiceTM guide wire (Boston Scientific Corporation, Natick, USA). Then, pre-dilation with a 2.5 10mm Pantera LuxTM semi-compliant balloon catheter (Biotronik, Bulach, Switzerland) up to 8 atm was performed, followed by implantation of a 2.75 15mm MultilinkTM stent (Abbott Vascular, Santa Clara, USA) up to 14 atm. A control coronary angiography showed dissection of distal border of stent, which was treated with implantation of a new 2.5 18mm MultilinkTM stent (up to 12 atm), with good angiographic result and TIMI 3 distal runoff (Fig. 2).
As the patient remained with residual pain and with persistence of ST-segment elevation after leaving the catheterization laboratory, coronary angiography was revised. An anatomical variant was hypothesized, considering that the treated LAD did not reach the apex; thus, the patient was restudied by femoral approach, with imaging findings suggestive of contrast retention at the first diagonal branch.
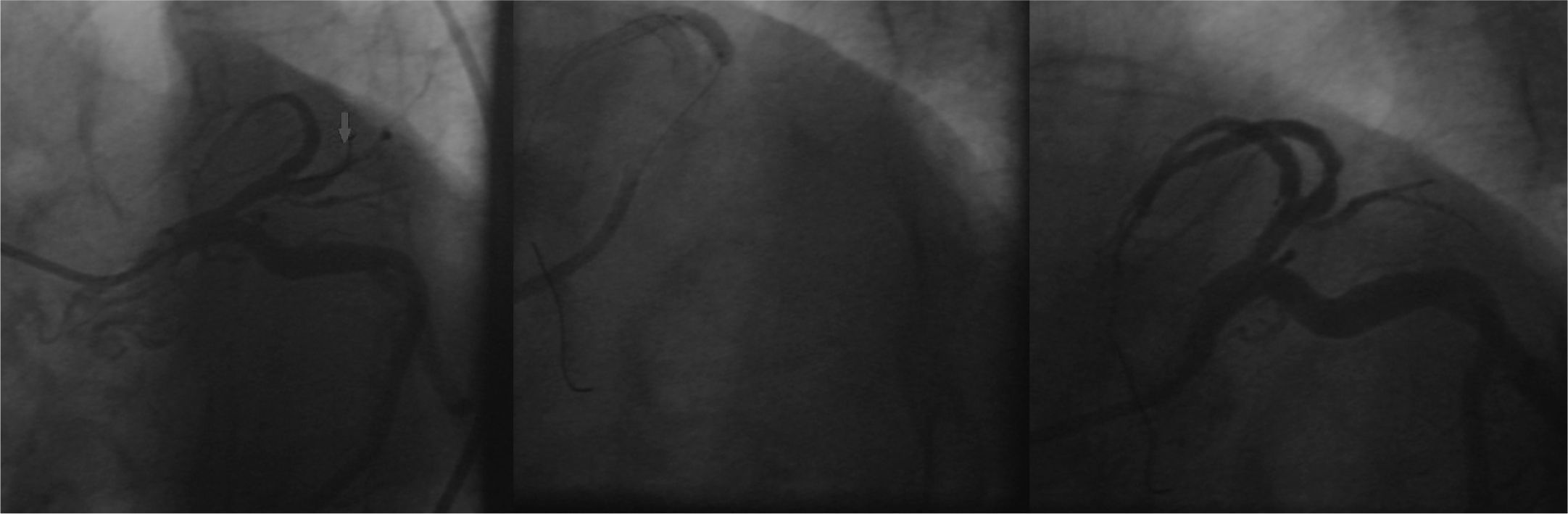
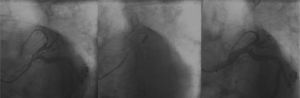
A new procedure was performed by right femoral route with a 6-F sheath; 100 IU/kg of unfractionated heparin was administered. The previously implanted stents were patent; we opted for the passage of a 0.014-inch GaleoTM guide-wire (Biotronik, Berlin, Germany) at the occlusion point with pre-dilation with a 2.0 10mm PanteraTM semi-compliant balloon catheter (Biotronik, Berlin, Germany) up to 6 atm. On that occasion, a new LAD bed was visualized, with occlusion at the proximal segment. A 2.5 38mm MultilinkTM stent was implanted at 12 atm in the middle segment, and a 2.57 23mm MultilinkTM stent was implanted at 12 atm in the proximal segment, with overlapping of its borders. The final angiographic result was satisfactory, with a TIMI 3 distal runoff (Fig. 3).
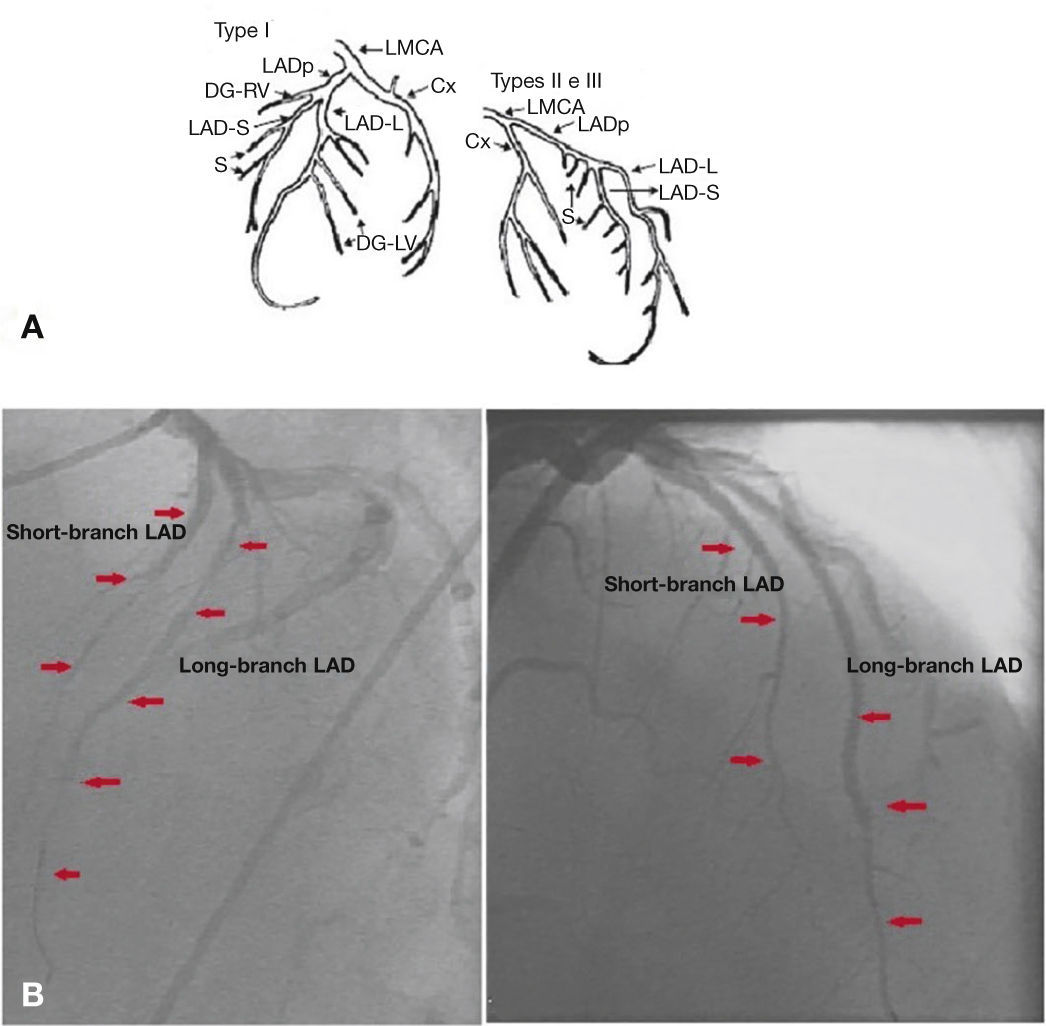
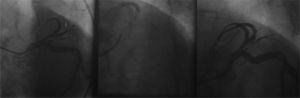
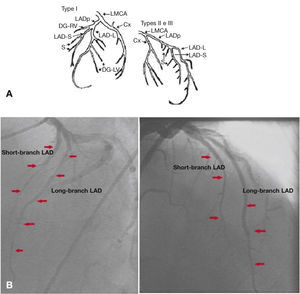
A final control angiogram showed presence of a type I dual LAD (Spindola-Franco), with a short branch that issued septal branches and a long-branch branch that issued diagonal and distal septal branches (Fig. 4).
Spindola-Franco diagram showing a type I dual left anterior descending artery (A). Left coronary artery in left and right anterior oblique projections (B). LAD: left anterior descending artery. Source: Spindola-Franco et al.1
The patient progressed with disappearance of chest pain and regression of ST-segment elevation and also with clinical improvement, being discharged after 7 days with prescription of adjuvant medication (carvedilol, enalapril, simvastatin, aspirin and clopidogrel).
DiscussionThe anatomical variants of origin, course and distribution and branching pattern of coronary arteries are uncommon, with an incidence from 0.13 1 to 1.38%2,3 in patients undergoing coronary arteriography. These variants may be associated with congenital malformations, such as a complete transposition of the great arteries and tetralogy of Fallot. Tuncer et al.,4 in an analysis of 70,850 coronary angiography procedures in adults, found 171 cases with coronary anomalies, of which 0.017% were dual LAD. Although relatively rare, Spindola-Franco et al.2 describe and classify an unusual pattern (1%) called dual LAD, stressing the surgical implications of its correct diagnosis. Later, this classification was modified by Moreno-Martínez et al.5 (Table 1).
Dual left anterior descendent artery (LAD), modified by Moreno-Martínez et al.5
| Types | Angiographic description |
|---|---|
| I | Short-branch LAD runs through AIVS, issuing main septal branches |
| Long-branch LAD runs downward sideways to AIVS in LV and returns to AIVS in the distal segment, until it reaches the apex | |
| Adds up to a type | |
| a. Type I, Tuncer et al.4: when both LAD (long and short branches) come up from LMCA and this vessel has an anomalous origin in right Valsalva sinus | |
| II | Short-branch LAD corresponds to type I and long-branch LAD curses down sideways to AIVS in RV and returns to AIVS in the distal segment, until it reaches the apex |
| III | Short-branch LAD corresponds to those described in types I and II |
| Long-branch LAD advances through an intramyocardial course through interventricular septum, and returns to the epicardium in the distal segment of AIVS, until reaching the apex | |
| IV | Original and short-branch LADs form a very short vessel, which lies in a very high level with respect to AIVS, from where main septal and diagonal branches arise. The long branch arises from RCA, which, at its beginning, courses anteriorly to the RV infundibulum, making a sharp curve till reaching AIVS and the apex |
| Adds up to different subtypes: | |
| a. Type IV (a), Tutar et al.6: a classic type IV with left circumflex artery coming from RC | |
| b. Type IV (b), Moreno et al.:7 short-branch LAD might not have septal branches, and not always long-branch LAD is a RCA branch, and may have an independent origin from right coronary sinus | |
| c. Type IV (c), Andreou et al.8: short-branch LAD issues a diagonal branch that passes over the lateral wall of left ventricle and surpasses the obtuse margin of heart, behaving like an aberrant obtuse marginal branch | |
| d. Type IV (d), Cruz et al.:9 LMCA originates from RCA and issues long-branch LAD. Short-branch LAD comes straight from left coronary sinus, being a hypoplastic vessel. Short-branch LAD occupies only the proximal third and emits no major branch | |
| e. Type IV (e), Manchanda et al.10: short-branch LAD comes straight from left coronary sinus, and long-branch LAD has its origin straight from right coronary sinus | |
| f. Type IV (f), Mahoney et al.11: short-branch LAD comes from LMCA and issues proximal septal branches and a very important single diagonal branch. Long-branch LAD comes from RCA, arrives to AIVS after crossing underneath the right ventricular outflow tract, and issues small septal branches through its whole course |
AIVS: anterior interventricular septum; LV: left ventricle; LMCA: left main coronary artery; RV: right ventricle; RCA: right coronary artery.
The presence of dual LAD have great clinical importance, and an accurate identification of its short and long-branch segments is critical for a proper planning of surgical grafting, or during percutaneous revascularization procedures.12 In addition, the knowledge of dual LAD variants help us understand some discrepancies among the location of coronary lesions and segmental contractility changes of left ventricle, and one should think of a short segment when facing a change in septal wall, normal motility in the anterior wall, or long-branch segment occlusion in a patient with anteroapical akinesis or dyskinesia with a normal septal wall.
In this case report, corresponding to type I of Spindola-Franco classification, we point out the distinguishing feature of an atherothrombotic involvement in both branches. With the unfavorable evolution after the first PCI and considering the non-immediate recognition of this coronary anatomical variation that led to a persistence of ischemic manifestations, a coronary angiography review was in order, in anticipation of the possible presence of a dual LAD, given that the course of the first branch approached did not reach the distal portions.
We emphasize the need for interventional cardiologists to become familiar with anatomical types of dual LAD, because their angiographic characterization has important implications in the process of decision-making, in face of revascularization procedures.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.