Renal sympathetic denervation (RSD) has emerged as an adjunct strategy in the treatment of resistant hypertension. Several other clinical conditions are characterized by sympathetic hyperactivity and could theoretically benefit from RSD. We report the first case of RSD performed in Brazil in a patient with Chagas’ disease and refractory arrhythmia, treated by the EnligHTN® multi-electrode catheter.
Denervação Simpática Renal: um Novo Cateter em um Novo Cenário
A denervação simpática renal (DSR) surgiu como uma estratégia terapêutica adjunta no tratamento da hipertensão arterial sistêmica resistente. Diversas outras condições clínicas cursam com hiperatividade simpática, às quais, teoricamente, a DSR seria benéfica. Relatamos o primeiro caso realizado no Brasil de DSR em paciente com doença de Chagas e arritmia refratária, tratada por meio do cateter multieletrodo EnligHTN®.
Renal sympathetic denervation (RSD) has emerged as an adjunct, safe, and effective therapeutic strategy in the treatment of resistant systemic arterial hypertension (SAH). Several other clinical conditions occur with sympathetic hyperactivity, to which, theoretically, RSD would be beneficial, which is a matter under investigation. There is little evidence of the effects of this procedure in the context of cardiac arrhythmias.
Sympathetic hyperactivity significantly contributes to the development of ventricular arrhythmias.1 RSD has shown to reduce the spillover of norepinephrine by 42% and neuromuscular efferent sympathetic activity by 66%.2
The authors report the first case treated in Brazil with a dedicated system for RSD in a patient with Chagas disease and refractory ventricular arrhythmia, aiming to reduce episodes of ventricular tachycardia/ fibrillation (VT/VF).
CASE REPORTA 56-year-old female patient, from the state of Bahia and living in São Paulo, with SAH, started receiving medical care at this service in 1992, with complaints of fatigue and palpitations. The diagnosis of Chagas disease was based on the results of indirect immunofluorescence. The electrocardiogram (ECG) showed sinus rhythm, low QRS voltage in the frontal plane, and isolated ventricular extrasystoles. The outpatient follow-up disclosed progressive increase in density of ventricular extrasystoles and several episodes of nonsustained VT, in spite of the use of amiodarone and diltiazem. In 2007, during an investigation of syncope, at the 24-hour Holter she had 636 VT episodes, with the longest being 53 beats at a rate of 200 systoles per minute. In 2009, an electrophysiological study was conducted, and ablation of the arrhythmogenic focus was performed successfully. However, she had two other outbreaks of sustained VT, and an implantable cardioverter defibrillator (ICD) was indicated. Despite optimization of the antiarrhythmic therapy (amiodarone 400mg/day and beta-blockers at maximum tolerated dose), the patient had three episodes of sustained VT in a three-month period, all refractory to the rapid programmed stimulation therapy, requiring defibrillation by the device. Ablation of the renal arteries was then indicated, in an attempt to reduce the arrhythmic burden.
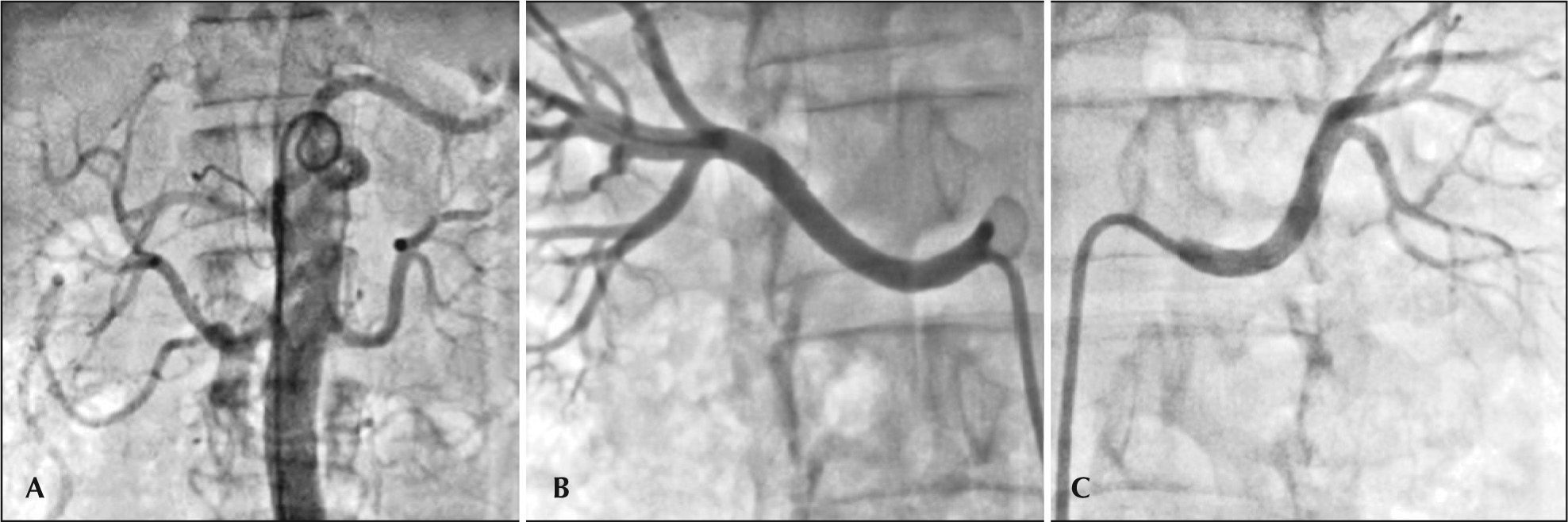
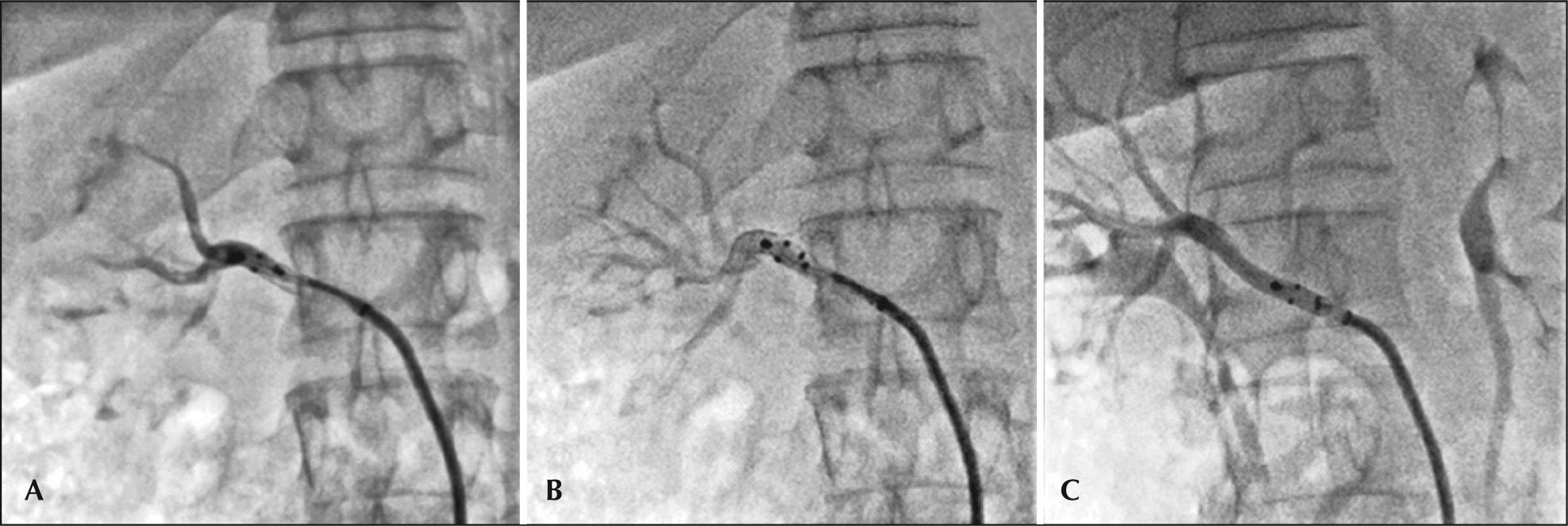
ProcedureThe procedure was approved by the local research ethics committee and the patient signed the informed consent form. The right femoral artery was punctured after local anesthesia with 2% lidocaine and sedation/analgesia with narcotics and opioids, followed by introduction of a 55cm 8F sheath, equipped with the EnligHTN® (St. Jude Medical) ablation system, with the aid of a 0.035-inch guide wire. Unfractionated heparin was administered intravenously (100IU/kg). An abdominal aortography was performed with a 6F pigtail catheter to check for possible accessory renal arteries, using low-osmolarity ionic contrast medium (Figure 1A). Selective renal angiography (Figures 1B and 1C) was performed through the sheath after intraarterial administration of nitroglycerin (200 mcg), followed by the measurement of diameters and extensions of renal arteries by online quantitative angiography. The right renal artery (RRA) measured 4.7/36mm and the left (LRA), 5.2/ 50mm. A 16-mm multielectrode ablation catheter was then selected and positioned in the distal RRA through the sheath (Figures 2A and 2B). Four applications of radiofrequency (RF) were performed sequentially and then the catheter was removed and rotated at approximately 45° for other four applications, as recommended by the manufacturer (Figure 2C). Finally, a control angiography was performed after administration of nitroglycerin (200 mcg), for investigation of vascular integrity. The same procedure was performed in the LRA. There were no complications. The total fluoroscopy time was 8 minutes and 90mL of contrast medium were used.
– (A) Abdominal aortography with pig-tail catheter to assess the presence of possible accessory renal arteries; (B) Selective angiography of the right renal artery, and (C) Selective angiography of the left renal artery after intra-arterial administration of nitroglycerin (200 mcg).
– (A) A 16-mm multielectrode ablation catheter positioned at the distal right renal artery through the sheath, with the basket still closed. (B) The basket is open, promoting proper contact of the electrodes with the vascular wall for effective ablation of renal nerves. (C) After four sequential RF applications, the catheter was withdrawn and rotated at about 45° for four other applications.
The sheath was removed when the activated clotting time (ACT) reached < 200/s, followed by hemostasis with manual compression for 20 minutes. Ambulation was allowed after a four-hour period of rest. The patient was discharged on the following day, without complications at the puncture site nor any others.
Clinical follow-up involved the periodic assessment of the ICD, which showed no sustained ventricular arrhythmias or need for therapy by the device during the two-month follow-up to this date.
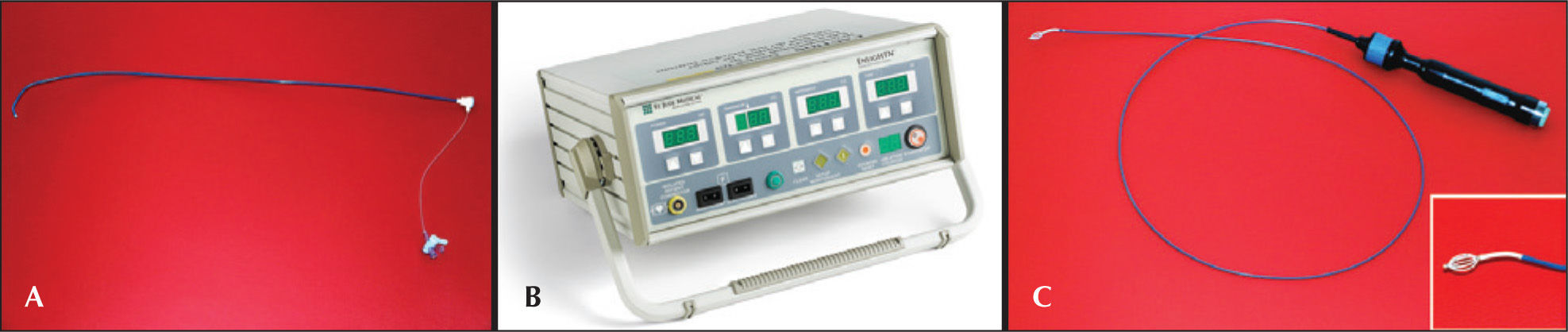
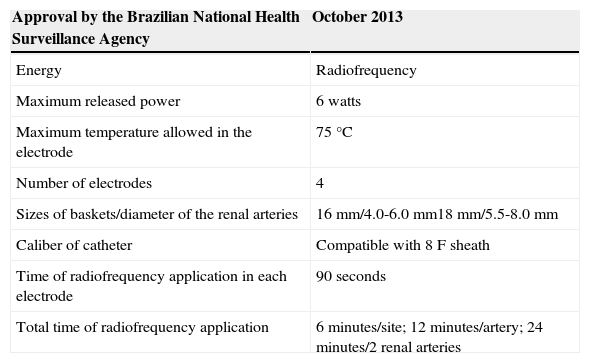
The EnligHTN® systemConsisting of a sheath, RF generator, and catheter (Figure 3), the EnligHTN® is the first dedicated system for RSD approved by the Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) for clinical use in Brazil. It is convenient system, as it has a multielectrode catheter, which greatly facilitates the procedure, requiring less catheter manipulation and allowing the RF applications to be more evenly distributed, when compared to a single electrode catheter. The technical specifications for the EnligHTN® system are shown in Table 1.
Technical specifications of the EnligHTN® system for renal sympathetic denervation
| Approval by the Brazilian National Health Surveillance Agency | October 2013 |
|---|---|
| Energy | Radiofrequency |
| Maximum released power | 6 watts |
| Maximum temperature allowed in the electrode | 75°C |
| Number of electrodes | 4 |
| Sizes of baskets/diameter of the renal arteries | 16mm/4.0-6.0mm18mm/5.5-8.0mm |
| Caliber of catheter | Compatible with 8F sheath |
| Time of radiofrequency application in each electrode | 90seconds |
| Total time of radiofrequency application | 6minutes/site; 12 minutes/artery; 24 minutes/2 renal arteries |
The sheath has an 8F caliber, is 55cm long, and includes two appropriate distal curvatures to facilitate selective catheterization of the renal arteries and ablation of the catheter passage (Figure 3A).
The RF generator has a digital display to control power, temperature, impedance, and time of application, in addition to displaying the number of the treatment being performed (Figure 3B). Per protocol, two treatments are performed at distinct sites in each renal artery, one distal and one more proximal. Each treatment consists of the sequential release of RF by one of the four electrodes, each lasting 90 seconds; thus, six minutes are required for each treatment, 12 minutes for each renal artery, and 24 minutes is the total time of energy application.
The ablation catheter has a compatible caliber with the 8F sheath and the four electrodes at its distal end are arranged in the shape of a basket (Figure 3C). There are two sizes of baskets: one larger, of 18mm, for the treatment of arteries between 4.0mm and 6.0mm of diameter, and a smaller basket, of 16mm, for the treatment of arteries between 5.5mm and 8.0mm in diameter. Its tip is flexible and designed to be non-traumatic. The knob controls flexion (with longitudinal movement) and the opening/closing of the basket (with clockwise/counterclockwise rotational motion). The basket must be introduced closed into renal artery, and promptly opened as soon as it is positioned precisely at the site of RF application, providing adequate contact with the vascular wall for appropriate delivery of energy and consequent ablation of renal nerves. After the first treatment, the basket is closed, and the catheter is pulled for 10mm and rotated at 45° for the second sequence of RF application. After being positioned in the appropriate location, using fluoroscopy and radiological contrast, the basket is opened once more. After the second treatment, the basket is closed and the catheter is removed. Angiographic control after RSD is performed to assess vascular integrity. The same procedure is repeated in the contralateral renal artery. After the procedure, the sheath should be withdrawn with routine care.
The second generation of the EnligHTN® system, now available in Europe, has significantly shortened the total duration of RF application: from 24 to 4 minutes, as the four electrodes are used simultaneously for 60 seconds, totaling 2 minutes for each artery; thus, there is a total of 4 minutes of treatment for two renal arteries.
DiscussionSympathetic hyperactivity plays a relevant role in the development, maintenance, and worsening of ventricular arrhythmias.1 RSD appeared as a safe and effective adjunctive therapy in the treatment of resistant hypertension, and is currently under investigation in other clinical conditions associated with increased sympathetic tone, such as cardiac arrhythmias. This report shows the first case of refractory arrhythmia in a patient with Chagas disease, treated with RSD using a dedicated system.
In the atrium, the autonomic nervous system influences chronotropism and dromotropism. It has been well established that sympathetic activation increases the heart rate and facilitates atrioventricular conduction. Conversely, parasympathetic activity has opposite effects.3 In the ventricles, increased sympathetic tone reduces ventricular effective refractory period, increases automaticity, and decreases the threshold for ventricular arrhythmias.4
Despite the implementation of socioeconomic measures and development of drugs that allow for the treatment of the acute phase of the disease, chronic Chagas cardiomyopathy remains a major public health problem in many Latin American countries. This disease affects approximately 15 to 16 million individuals and is responsible for about 20,000 deaths a year worldwide.5 Approximately two-thirds of patients with chronic symptoms develop heart lesions, including ventricular dilation and severe ventricular dysfunction, tachyarrhythmia, bradyarrhythmia, and often, sudden death.6 The arrhythmogenic nature of Chagas disease is related to the presence of fibrous tissue intertwined with areas of preserved myocardium and dyskinetic regions, creating a territory with high propensity to complex ventricular arrhythmias. Sustained VT/VF is the leading cause of death from this disease. Rates of sudden death are responsible for approximately 55% to 65% of the total mortality in patients with Chagas’ disease, superior to mortality rates due to heart failure.7
Ukena et al.8 reported the first experience with RSD in electrical storm in two patients with heart failure: a patient with hypertrophic cardiomyopathy and monomorphic VT, despite the use of antiarrhythmics and unsuccessfully performed endocardial and epicardial cardiac ablation; and another with dilated cardiomyopathy and frequent episodes of VF/polymorphic VT, who refused to undergo cardiac ablation. In both cases, the RSD resulted in significant reduction in the arrhythmic burden.
Staico et al.9 recently reported a substantial reduction in VT and appropriate ICD therapies in a patient with dilated cardiomyopathy and contraindication to cardiac ablation (left ventricular thrombus) submitted to RSD. Notably, this patient had three accessory renal arteries and, due to the small diameter, only one was treated, in addition to the main renal arteries.
It is possible that in addition to its action on the autonomic tone, the RSD could result in effects secondary to the reduction in excess volume and hormonal activation involved in heart failure. The pilot study Olomouc-1, presented at the Congress of the European Society of Cardiology in 2012, compared the effects of RSD to optimal medical therapy in 51 patients with advanced heart failure, and demonstrated that in addition to reducing the left ventricular dimensions, the RSD reduced the autonomic tone and mean heart rate. However, in the present case, the patient had no ventricular dysfunction, suggesting that the reduction of arrhythmia was related to autonomic tone modulation.
Even though there are yet no definitive recommendations, the importance of sympathetic hyperactivity in patients with ventricular arrhythmias is clear. The study illustrates the feasibility and potential effectiveness of RSD in this scenario. Larger clinical trials on the impact of RSD on arrhythmias are in progress. If its safety and efficacy are demonstrated in the control of ventricular arrhythmias, RSD can become an interesting strategy for the treatment these patients.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.