Percutaneous closure of patent ductus arteriosus is a safe, effective and easily reproducible method. Adult patent ductus arteriosus may present degenerative changes that increase surgical risk and favor percutaneous closure. We report a single center experience with percutaneous closure of patent ductus arteriosus in adults and highlight specific technical aspects of this procedure.
MethodsThe records of patients≥20 years of age submitted to patent ductus arteriosus closure between March 2001 and December 2012 were evaluated. Cases were selected by transthoracic echocardiography.
ResultsWe analyzed 33 patients, most of them females (72.7%), with mean age of 30.9±12.8years and weight of 63.9±12.4kg. Only 3 patients had symptoms and 2 had associated congenital defects, treated in the same procedure. Implants were possible in all cases. One Flipper Coil, 19 Amplatzer™ Duct Occluders type I, 3 Amplatzer™ Duct Occluders type II, 8 Cera™ PDA Occluders and 2 Muscular VSD devices were used. Sizing balloons were used in 5 cases. Mean follow-up was 46.1±42.9months and was obtained in 84.9% of patients. Two cases presented residual shunts immediately after the procedure. There were no major complications or deaths.
ConclusionsPercutaneous closure of adult patent ductus arteriosus may be performed safely and effectively with the devices used in this study.
Aspectos Particulares da OclusãoPercutânea do Canal Arterial do Adulto
IntroduçãoA oclusão percutânea do canal arterial é um método seguro, eficaz e facilmente reproduzível. O canal arterial do adulto pode apresentar alterações degenerativas, que aumentam o risco da cirurgia e favorecem o procedimento percutâneo. Descrevemos a experiência de um centro único com a oclusão percutânea do canal arterial em adultos e destacamos aspectos técnicos particulares desse procedimento.
MétodosRevisamos os registros de todos os pacientes > 20 anos de idade submetidos a oclusão do canal arterial entre março de 2001 e dezembro de 2012. Os casos foram selecionados por ecocardiografia transtorácica.
ResultadosAnalisamos 33 pacientes, a maioria do sexo feminino (72,7%), com médias de idade de 30,9±12,8 anos e de peso de 63,9±12,4kg. Somente 3 pacientes tinham sintomas e 2 pacientes apresentaram defeitos associados, tratados no mesmo procedimento. Os implantes foram possíveis em todos os casos. Foram utilizadas 1 mola Flipper, 19 próteses Amplatzer™ Duct Occluder tipo I, 3 próteses Amplatzer™ Duct Occluder tipo II, 8 próteses Cera™ PDA Occluder e 2 próteses para comunicação interventricular muscular. O uso de balões medidores foi necessário em 5 casos. O seguimento médio foi de 46,1±42,9 meses e foi obtido em 84,9% dos pacientes. Dois casos apresentaram shunt residual imediatamente após o procedimento. Não ocorreram complicações maiores ou óbitos.
ConclusõesA oclusão percutânea dos canais dos adultos com os dispositivos empregados pode ser realizada com segurança e eficácia.
Percutaneous closure of ductus arteriosus is, currently, the therapeutic choice in all centers capable of performing procedures in interventional cardiology. 1-7 Although it is a relatively simple procedure when performed in children weighing > 5kg, some characteristic features are found in adult ductus arteriosus. Anatomical and morphological changes may be present, such as aneurysms, calcifications, diverticula, and friability of the ductal tissue, which increase the surgical risk and favour the option of transcatheter closure.8-14 Late complications are also reported in the evolution of untreated ductus arteriosus in adults, especially endarteritis, arrhythmias, ventricular dysfunction, and progressive pulmonary arterial hypertension.15-17
The transcatheter closure of adult patent ductus arteriosus (PDA) exhibits a few difficulties and requires specific care.3,8,18 This study reports the group’s experience with percutaneous closure of PDA in adults, discussing available options and techniques in detail.
METHODStudy designThis was a retrospective single-arm study, carried out in a single center (Interventional Cardiology – INTERCAT, Rio de Janeiro, RJ, Brazil), which included all adult patients undergoing PDA closure with different devices, between March 2001 and December 2012.
Patient selectionRecords of all patients≥20 years of age, with ductus arteriosus and indication for percutaneous closure without associated defects that required surgical correction were reviewed. The cases were selected by means of transthoracic echocardiography with colour flow mapping. The dimensions and morphology of the defects were not used as exclusion criteria.
Implantation techniquesAll patients received general anesthesia and underwent tracheal intubation. Sodium heparin at a dose of 5,000 U plus 2g of intravenous cefazolin, as antibiotic prophylaxis, were administered. The femoral artery and vein were punctured, and left and right catheterizations were performed with pressure recordings. Oximetry was performed only in cases where it was necessary to study the pulmonary vascular reactivity.
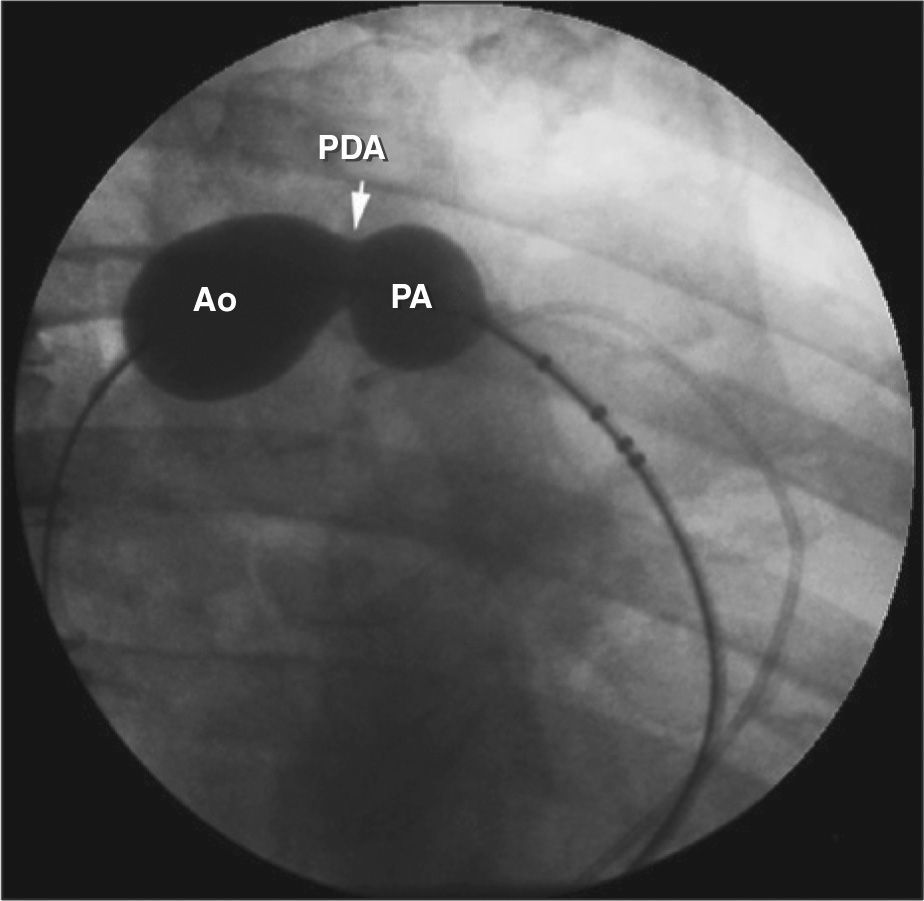
Descending thoracic aorta angiograms were obtained in the right anterior oblique and left lateral view using a 5F pigtail catheter. Angiograms were analyzed regarding shape, according to the classification of Krichenko et al.,19 and the dimensions of the ductus arteriosus, with special emphasis on its smallest diameter. Some ductae showed significant constriction in the pulmonary end, and it was decided to attribute the value of 1mm to its smallest diameter. In this case, when it was impossible to cross them by the antegrade route, they were accessed via arterial route, and the rigid exchange guide wire was captured with a 15mm or 20mm catheter loop in the main pulmonary artery and externalized through the venous sheath. Subsequently, the long sheath compatible with the selected device was advanced through the vein to the descending aorta, and the device was implanted in the usual way.20
In cases where the angiograms were unable to outline the ductus for morphological analysis and measurement, a partially insufflate sizing balloon, with contrast diluted in saline solution, was introduced intravenously into the descending aorta over the guidewire, to occlude the interatrial septal defect, allowing the contrast to be ejected into the pulmonary artery through the ductus arteriosus. The frames were reviewed in slow motion to verify the exact time of passage through the ductus, making it possible to outline its shape, safely measuring its smaller diameter.20 The different prostheses used were selected with diameters at least 2mm larger than the smallest diameter of the ductus arteriosus, according to the protocols of the respective implant manufacturers, as previously reported.20-23
Follow-up was performed by transthoracic echocardiography, seeking residual flows24 and obstructions in the descending aorta and left pulmonary branch on the day following implantation, one month, three months, and six months after the procedure, and annually thereafter. Prophylaxis for infective endocarditis was indicated for six months following the implant.
Statistical AnalysisContinuous variables were expressed as means and standard deviations, and categorical variables as numbers and percentages. This study aimed to demonstrate the group’s experience with the closure of the ductus arteriosus in adult patients, and thus, comparisons between devices were not performed.
RESULTSThe records of 33 patients submitted to percutaneous closure of patent ductus arteriosus between March 2001 and December 2012 were retrospectively analyzed, of whom 72.7% were females. Ages ranged from 20 to 66 years (30.9±12.8years) and weight from 38kg to 92kg (63.9±12.4kg). Three patients complained of dyspnoea on exertion (patients 5-RSS, 15-LEF, and 25-JOC), and two patients (10-SCFA and 30-LCMM) had severe pulmonary arterial hypertension and were categorized in New York Heart Association (NYHA) functional class III. One patient (33-GOR) also presented an atrial septal defect that was closed with an 18-mm Cera™ prosthesis (Lifetech – Shenzhen, China) in the same procedure. Another patient (13-NNFO) had mesocardia with left pulmonary sequestration, and one patient was deaf-mute (15-LEF).
The smallest ductus diameters varied from 1mm to 10mm (4.9±2.8mm). Regarding the morphology, the assessed ductae were type A in 76%, type C in 9%, type D in 6%, and type E in 9%. None showed significant tissue degeneration or calcification (Table 1). The implantation was feasible in all cases. One Flipper coil 5PDA5 (Cook Medical Inc., Bloomington, United States), 19 Amplatzer™ Duct Occluders type I (ADO I; AGA – Golden Valley, United States) (three 6-4 a Smallest diameter observed. PASP, pulmonary artery systolic pressure; F, Female; ADO, Amplatzer™ Duct Occluder; M, Male; AMVSD; CPO, Cera™ PDA Occluder; CMVSD, Cera™ Muscular VSD Occluder. prostheses, three 8-6 prostheses, five 10-8 prostheses, and eight 12-10 prostheses), three ADO II prostheses (two 5-4 prostheses and one 6-4 prosthesis), eight Cera™ PDA Occluders (CPO; two 6-4 prostheses, two 8-6 prostheses, and four 12-10 prostheses), one 12mm Amplatzer™ Muscular VSD prosthesis Occluder (AMVSD) and one 16-mm Cera™ Muscular VSD prosthesis Occluder (CMVSD). Angiography was not capable of clearly delineating the shape and size of the ductus arteriosus in five cases: four type A, measuring 3.3mm, 6mm, 6mm, and 8mm, and one type C, which measured 10mm. In these cases, 24-mm AGA sizing balloons were used in ductus type A and a 30–40mm PTS sizing balloon (Numed, Hopkinton, USA) was used in type C ductus, which is larger (Figure 1).
Study population
| Number | Identification | Gender | Age(years) | Morphologic type | Diametera (mm) | PSAP (mmHg) | Prosthesis | Immediate result |
|---|---|---|---|---|---|---|---|---|
| 1 | LER | F | 20 | E | 1 | 22 | Mola Flipper5PDA5 | Closed |
| 2 | RRMF | F | 38 | A | 4.5 | 28 | ADO I 8-6 | Closed |
| 3 | IRC | F | 22 | A | 3.5 | 32 | ADO I 10-8 | Closed |
| 4 | P8 | F | 21 | A | 2.5 | 21 | ADO I 6-4 | Closed |
| 5 | RSS | F | 24 | A | 2 | 24 | ADO I 8-6 | Closed |
| 6 | FBS | M | 42 | A | 8 | 22 | ADO I 12-10 | Closed |
| 7 | MBM | F | 22 | C | 4 | 22 | ADO I 10-8 | Closed |
| 8 | MGSS | F | 42 | A | 4 | 32 | ADO I 12-10 | Closed |
| 9 | PEPS | F | 30 | A | 5 | 33 | ADO I 10-8 | Closed |
| 10 | SCFA | F | 50 | A | 8 | 85 | AMVSD 12 | Closed |
| 11 | MNM | F | 21 | A | 2 | 36 | ADO I 6-4 | Closed |
| 12 | AOG | F | 20 | A | 10 | 31 | ADO I 12-10 | Closed |
| 13 | NNFO | F | 21 | A | 1 | 28 | ADO I 6-4 | Closed |
| 14 | VPO | M | 22 | E | 3.5 | 27 | ADO I 10-8 | Closed |
| 15 | LEF | M | 30 | A | 8 | 30 | ADO I 12-10 | Minimal shunt |
| 16 | FAAE | F | 23 | A | 6 | 17 | ADO I 12-10 | Minimal shunt |
| 17 | NMO | F | 66 | D | 6 | 18 | ADO I 12-10 | Closed |
| 18 | JU | F | 62 | A | 6 | 34 | ADO I 12-10 | Closed |
| 19 | JJMA | M | 24 | A | 6 | 43 | ADO I 12-10 | Closed |
| 20 | VS | F | 32 | A | 2.5 | 23 | ADO I 8-6 | Closed |
| 21 | IFP | M | 29 | A | 6 | 32 | ADO I 12-10 | Closed |
| 22 | AVA | F | 21 | A | 3 | 24 | ADO II 6-4 | Closed |
| 23 | APNS | F | 34 | A | 4 | 22 | ADO II 5-4 | Closed |
| 24 | ECAS | F | 44 | A | 3.5 | 35 | ADO II 5-4 | Closed |
| 25 | JOC | M | 27 | A | 6 | 42 | CPO 12-10 | Closed |
| 26 | G88 | M | 20 | E | 1 | 39 | CPO 6-4 | Closed |
| 27 | LHL | F | 20 | A | 1 | 39 | CPO 6-4 | Closed |
| 28 | SLRM | M | 33 | A | 8 | 20 | CPO 12-10 | Closed |
| 29 | LSG | F | 30 | C | 7 | 42 | CPO 12-10 | Closed |
| 30 | LCMM | F | 35 | C | 10 | 95 | CMVSD 16 | Closed |
| 31 | MFP | F | 43 | A | 6 | 31 | CPO 12-10 | Closed |
| 32 | LPCS | M | 23 | A | 1 | 23 | CPO 8-6 | Closed |
| 33 | GOR | F | 29 | D | 3 | 24 | CPO 8-6 | Closed |
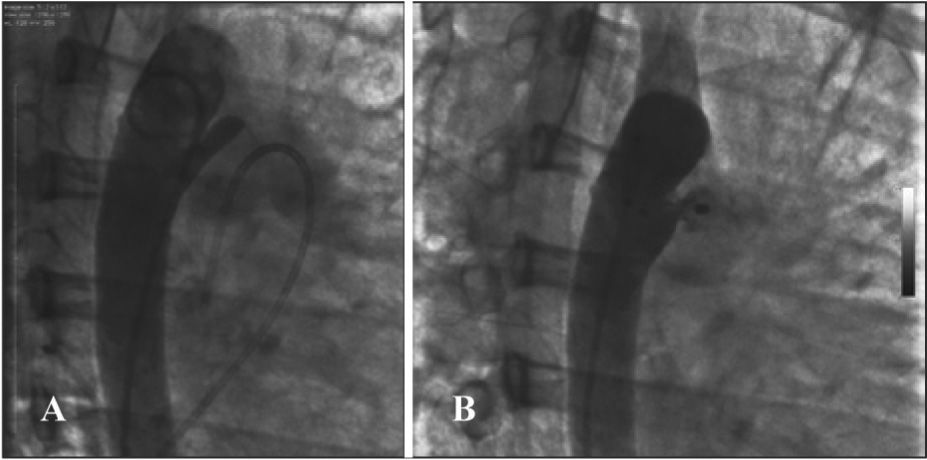
The prevalence of large calibre ductus arteriosus was a frequent finding in adults, but the smaller diameter of the ductus arteriosus showed no correlation with systolic pressure in the pulmonary artery. Pulmonary systolic pressure ranged from 17mmHg to 95mmHg (33.8±17.6mmHg) and was > 30mmHg in 51.5% of patients. One patient (30-LCMM) with severe pulmonary arterial hypertension and pulmonary vascular resistance of 4.1 Wood U had multiple VSD, also known as “Swiss cheese” ventricular septum, associated with a 10-mm type C ductus arteriosus, which was closed using a 16-mm CMVSD prosthesis (Figure 2). Two other muscular VSDs were closed in the same procedure. In a second session, three more sizeable muscular VSDs were closed, with a single restrictive VSD remaining open. After a complicated evolution, immediately after the second procedure, the patient is in NYHA functional class II, undergoing treatment protocol for pulmonary arterial hypertension with sildenafil and bosentan. Another patient (10-SCFA) with pulmonary arterial hypertension showed significant atrial fibrillation with low ventricular response. The PDA was successfully closed with a 12-mm AMVSD prosthesis, and she presented severe hemodynamic instability immediately after the procedure, requiring temporary pacemaker implantation, followed by a permanent pacemaker. The patient was discharged in good condition with a fully closed PDA, and is in good clinical condition seven years later.
Immediately after the procedure, only two patients had minimal residual shunt, with no gushing inside the devices (ADO I 12-10 prostheses in both cases), which were completely closed in the transthoracic echocardiography performed in the first postoperative month. Follow-up was achieved in 84.9% of patients, and ranged from three to 132 months (46.1±42.9months). No case had a gradient in the descending aorta or in the left branch of the pulmonary artery. The only complication was a right femoral pseudoaneurysm in a 62-year-old patient (18-JU), resolved by local compression through the ultrasound transducer; the hematoma was reasorbed by applying local heat and anti-inflammatory drug use. There were no deaths in the present series.
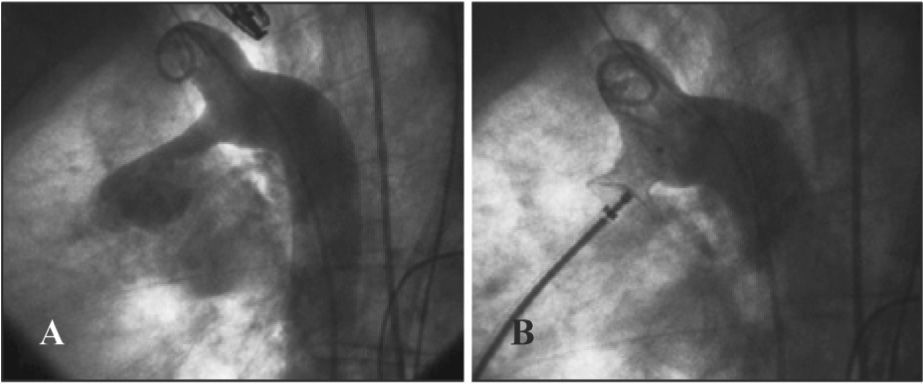
DISCUSSIONThe percutaneous closure of adult PDA has some aspects that differentiate it from procedures in children. The correct viewing of the ductus through conventional angiography is sometimes extremely difficult. The high flow through the defect, associated to the large diameter of the adult aorta, may prevent its measurement by conventional angiography. In such cases, the solution is to use sizing balloons, which, similar to the evaluation of the stretched diameter of septal defects, slightly distend the ductus, and during the passage from the descending aorta through the ductus to the pulmonary trunk, accurately outline the defect diameter, allowing the morphological analysis and accurate measurement so that the appropriate device can be chosen. In the present study, this technique was necessary in five cases, and was successful in all.
Another problem to be addressed is that the natural elasticity of the ductus arteriosus is underestimated by angiography, which draws its outline only at the moment of contrast injection, of but is not capable of assessing its distended diameter. In some cases, the ductus arteriosus appeared too small or to have a severe narrowing at its pulmonary end, but it was crossed by a diagnostic catheter, without any difficulty, allowing for the use of larger-calibre prostheses than initially estimated (Figure 3). The stringent oversizing of devices increases procedural safety, preventing embolization. It is of utmost importance that the ADO I and CPO devices are positioned so that they are compressed in their middle portion by the smallest diameter of the ductus arteriosus, leaving the pulmonary end slightly dilated, preventing slippage of the prosthesis into the descending aorta.
– In A, descending aortography in right anterior oblique view, showing ductus arteriosus type E with significant constriction in the pulmonary end. Note the gush of contrast material opacifying the pulmonary trunk and branches. Although the stenosis at the pulmonary end appeared extremely constricted, the ductus was crossed without difficulty with the multipurpose catheter (at the right of the image, positioned in the pulmonary artery), demonstrating the high elasticity of the defect, underestimated by the angiographic image. In B, the fully closed ductus with a Cera™ PDA Occluder prosthesis.
Although some patients had large-calibre ductus arteriosus, pulmonary arterial pressure was not comparatively high in most cases. This might have been due to the length of the ductus, which attenuates the transmission of systemic pressure to the pulmonary artery. In cases with pulmonary hypertension, increase in the systolic pulmonary pressure was considered mild (< 1/3 of the systemic pressure); it was > 40mm Hg in five cases, of which only two had pressure > 75% of systemic levels. In both cases in which the pulmonary arterial pressure was very high, prostheses were used to occlude the muscular VSD (12mm AMVSD and 16-mm CMVSD) for safety reasons. Sizing balloons were also used in both cases (24mm AGA and 30–40mm PTS).25-27
CONCLUSIONSThe percutaneous closure of the ductus arteriosus in adults can be performed quite safely and effectively using traditional techniques and available devices. When the analysis of the ductus arteriosus shape and size cannot be performed satisfactorily by conventional angiography, the use of a sizing balloon is a strategy that can be used to overcome this limitation.
CONFLICT OF INTERESTChamié Francisco is a consultant and proctor of Boynton (Porto Alegre, RS, Brazil). The other authors declare no conflicts of interest.