Renal artery aneurysms are rare and constitute a challenge to endovascular treatment. Our objective was to describe and analyze the techniques and strategies for the endovascular treatment of renal artery aneurysms verifying short and medium-term results in a consecutive series of cases.
MethodsRetrospective study of procedures performed from January 2010 to December 2013, analyzing technical and therapeutic success, morbidity and mortality, the rate of endoleaks and reinterventions.
ResultsIn a total of six patients treated, mean age was 41±5 years and all patients were female. The majority of the patients had type 2 saccular aneurysms (83.3%). Remodeling techniques using stent and coils were used in four cases; embolization of renal polar branch was used in one case and treatment with a Multilayer® endoprosthesis in another case. Technical and therapeutic success rates were 100% and 83.3%, respectively. In one patient there was upper renal pole ischemia, which progressed to uncontrollable hematuria and pain, requiring nephrectomy. There were no deaths or occlusion of the native renal artery and its branches during the 1-year follow-up.
ConclusionsEndovascular treatment of renal artery aneurysm proved to be a feasible alternative to conventional surgery with low morbidity. A detailed study of renal vasculature and aneurysm location determines the choice of the endovascular technique to be used. Type II renal artery aneurysm was the most frequent morphology observed and may be successfully treated by remodeling techniques using stents and coils.
Técnicas e Táticas no Tratamento Endovasculardo Aneurisma da Artéria Renal
IntroduçãoOs aneurismas da artéria renal são raros e constituem um desafio ao tratamento endovascular. Nosso objetivo foi descrever e analisar as técnicas e táticas no tratamento endovascular do aneurisma da artéria renal, verificando os resultados a curto e médio prazos de uma série consecutiva de casos.
MétodosEstudo retrospectivo, de procedimentos realizados no período de janeiro de 2010 a dezembro de 2013, em que foram analisados: o sucesso técnico e terapêutico, a morbimortalidade, e a taxa de vazamentos e de reintervenções.
ResultadosEm um total de seis pacientes tratados, a idade média foi de 41±5 anos e todos eram do sexo feminino. A maioria apresentou aneurismas saculares tipo II (83,3%). Foram utilizadas técnicas de remodelamento com uso de stent e molas em quatro casos, embolização segmentar renal em um caso e tratamento com endoprótese Multilayer® em outro. O sucesso técnico e terapêutico foi de 100 e 83,3%, respectivamente. Em um paciente, houve isquemia de polo superior renal, que evoluiu para hematúria e dor incontrolável, necessitando de nefrectomia. Não ocorreram óbitos e nem oclusão das artérias renais nativas e de seus ramos durante o acompanhamento de 1 ano.
ConclusõesO tratamento endovascular do aneurisma de artéria renal demonstrou ser uma alternativa viável à cirurgia convencional com baixa morbidade. O estudo detalhado da vascularização renal e da localização do aneurisma determina a escolha da técnica endovascular a ser utilizada. O aneurisma da artéria renal do tipo II foi a morfologia mais frequentemente encontrada e pode ser tratado com sucesso por técnicas de remodelamento com o uso de stent e mola.
The renal artery aneurysm (RAA) is a rare event whose prevalence is 0.01%.1,2 In the last decade, with the use of non-invasive diagnostic methods, such as Doppler ultrasonography (DUS) and computed tomography (CT), the number of diagnosed cases has increased.3
Although the natural history of the RAA is scarcely known and symptoms are nonspecific or nonexistent, potential complications, such as embolization and rupture, are reported in 5% to 10% of cases, leading to a high mortality rate, especially in pregnant women or in patients with polyarteritis nodosa (PAN).4,5 Therefore, the indications for treatment include symptomatic patients, pregnant women or women of childbearing age, and asymptomatic patients with aneurysms>2cm or with PAN.3,6
Both the conventional surgical treatment and kidney autotransplantation have mortality rate ranging from 0% to 4%, with a complication rate of 10% to 30%.7,8 In recent years, with the progress of endovascular treatment and development of new devices, RAAs have been approached with less morbidity and with preservation of the native vascular tree.9
The aim of this study was to describe and analyze the techniques and tactics of endovascular treatment of RAA, assessing technical and therapeutic success, morbidity and mortality, and the rate of endoleaks and reinterventions in a consecutive series of patients followed for one year.
METHODSStudy characteristicsThis is a retrospective, longitudinal, observational study carried out in a referral center for cardiovascular pathologies. Inclusion criteria were the following: patients of both sexes with symptomatic RAA, RAA diameter>2cm, RAA in pregnant women or women of childbearing age, and RAA associated with PAN. Patients with serum creatinine>2.0mg/dL or creatinine clearance<30mL/min were excluded.
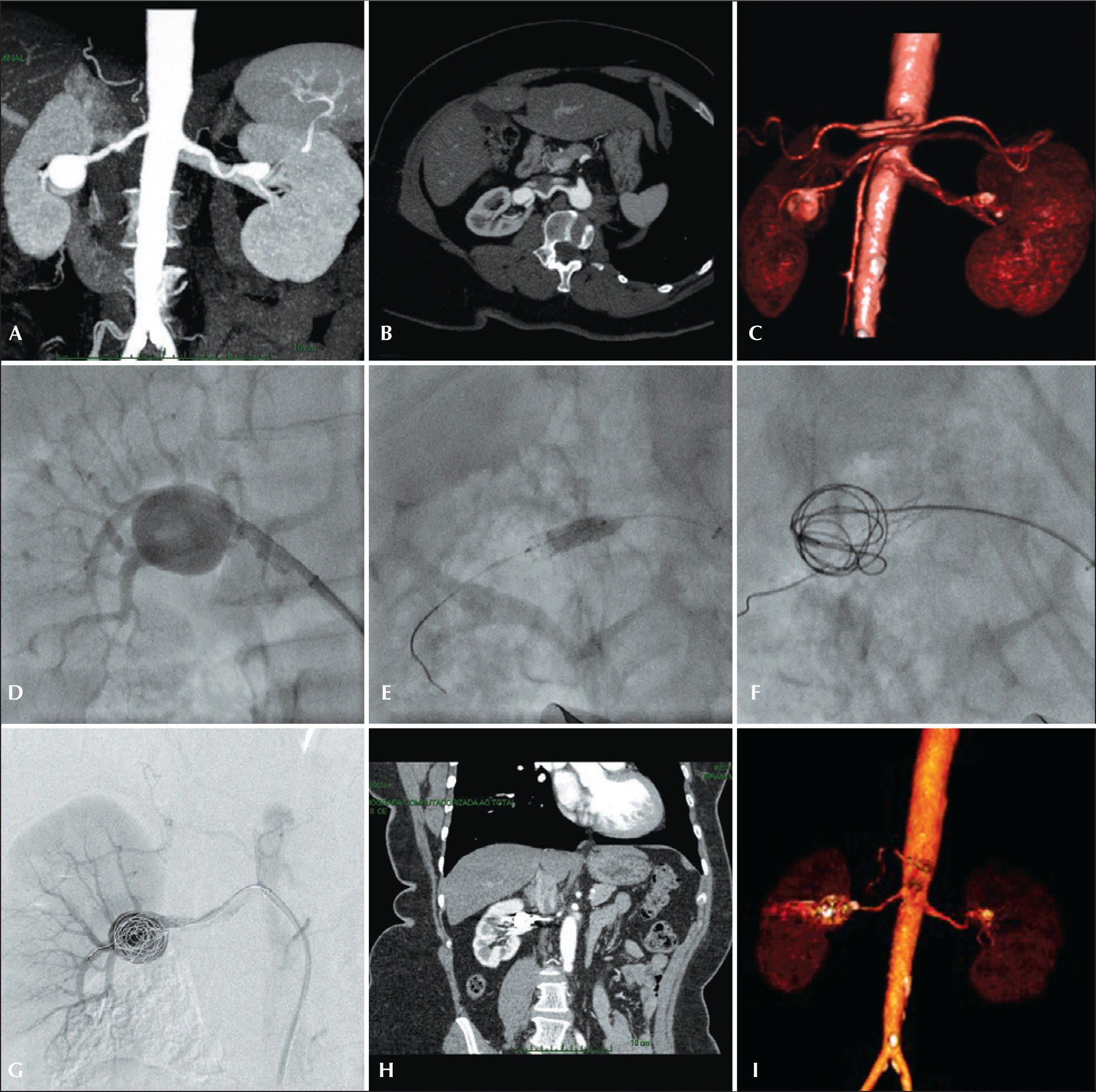
The therapeutic schedule was performed using CT in all cases; preoperative arteriogram was an optional diagnostic method. All tomographies were reconstructed in the OsiriX® MD software in three-dimensional mode and multiplanar reconstruction mode (Figure 1).
– Preoperative planning, endovascular treatment and postoperative follow-up of type II renal aneurysm. (A and B) Angiotomography coronal and axial views. (C) Volumetric reconstruction of renal artery aneurysm. (D) Renal arteriography. (E) Balloon-expandable-stent implantation in the renal aneurysm neck. (F) Release of coils in the aneurysmal sac after aneurysm catheterization within the stent mesh. (G) Final arteriography showing no leakage or occlusion of renal branches. (H) Postoperative computed tomography (CT) angiography in coronal view. (I) Volumetric reconstruction of postoperative CT angiography.
Previous angiotomography and/or arteriography disclosed the following: aneurysm morphology (saccular or fusiform), presence of draining branches, aneurysm neck extension, involvement of bifurcation areas, and the distance from the renal artery origin to the aneurysm and from the aneurysm to the renal artery bifurcation.
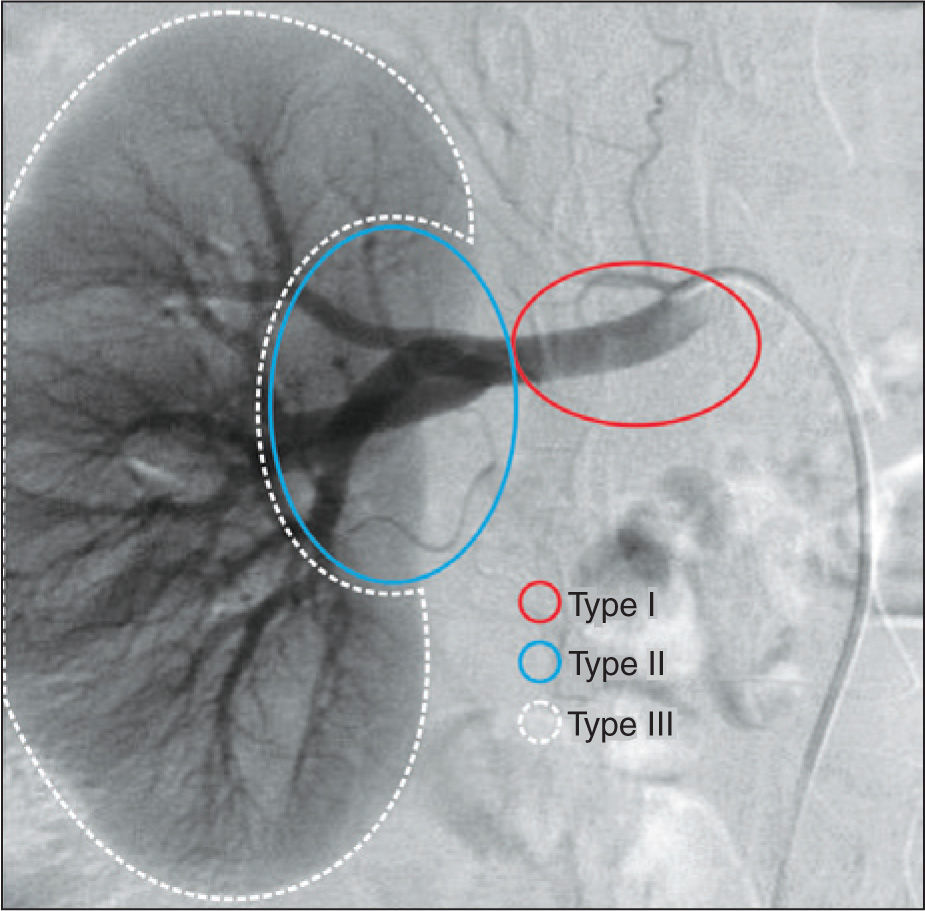
The planning of endovascular treatment of RAAs was performed according to their topographic10 classification into type I (aneurysms located in the main renal artery), II (aneurysms located in the hilum), and III (intrarenal aneurysms) (Figure 2).
– Classification of types of renal artery aneurysm according to their location. Type I, aneurysms located in the main renal artery; type II, aneurysms located in the renal hilus region, after arterial bifurcation of the main renal artery; and type III, aneurysms of the intraparenchymal renal artery.
a) Endoprosthesis GORE® VIABAHN® (W. L. Gore & Associates Inc. – Flagstaff, United States)
This is a treatment option for type I RAA located up to 15mm from the renal artery ostium or hilum without nurturing branches coming from the aneurysmal sac. When these limits are not taken into account, there is a risk of endoleaks and perpetuation of flow within the aneurysmal sac, with consequent therapeutic failure.
This endoprosthesis must not be used in type II RAA due to risk of covering important renal branches, or in type III RAA, due to its high profile and little flexibility to navigate thin distal vessels, as well as risk of thrombosis or thromboembolism when used in these scenarios.
b) Selective embolization with coils
It can be used in three types of aneurysms, but is better indicated for saccular aneurysms with neck<4mm in diameter or aneurysm/neck ratio>2:1.9 In such cases, techniques that use microcatheters for superselective aneurysm catheterization are needed, as well microcoils with 0,018 profile. The authors prefer AZUR® coils (Terumo Interventional Systems, Somerset, United States) and the 2.4F Progeat® microcatheter (Terumo Interventional Systems, Somerset, United States). An important point is that the size of the first coil must be equal to the aneurysmal sac, to allow a circular configuration within the aneurysm.
This technique should not be used in complex aneurysms, those with wide necks or when arterial branches leave the aneurysmal sac. In such cases, remodeling techniques are recommended.
Techniques and tactics for type II renal aneurysmsa) Multilayer® endoprosthesis (Cardiatis, Isnes, Belgium)
The Multilayer® endoprosthesis is used primarily for the treatment of peripheral aneurysms; however, its use in renal aneurysms has been reported in recent studies. The stent structure, consisting of several intertwined and connected layers, creates a configuration in multiple planes, which acts by slowing the turbulent flow within the aneurysmal sac, contributing to its thrombosis, while improving the laminar flow in the main artery and its branches.11,12
Its little flexibility and high profile limit its use in type III aneurysms.
b) Remodeling techniques: stents and coils
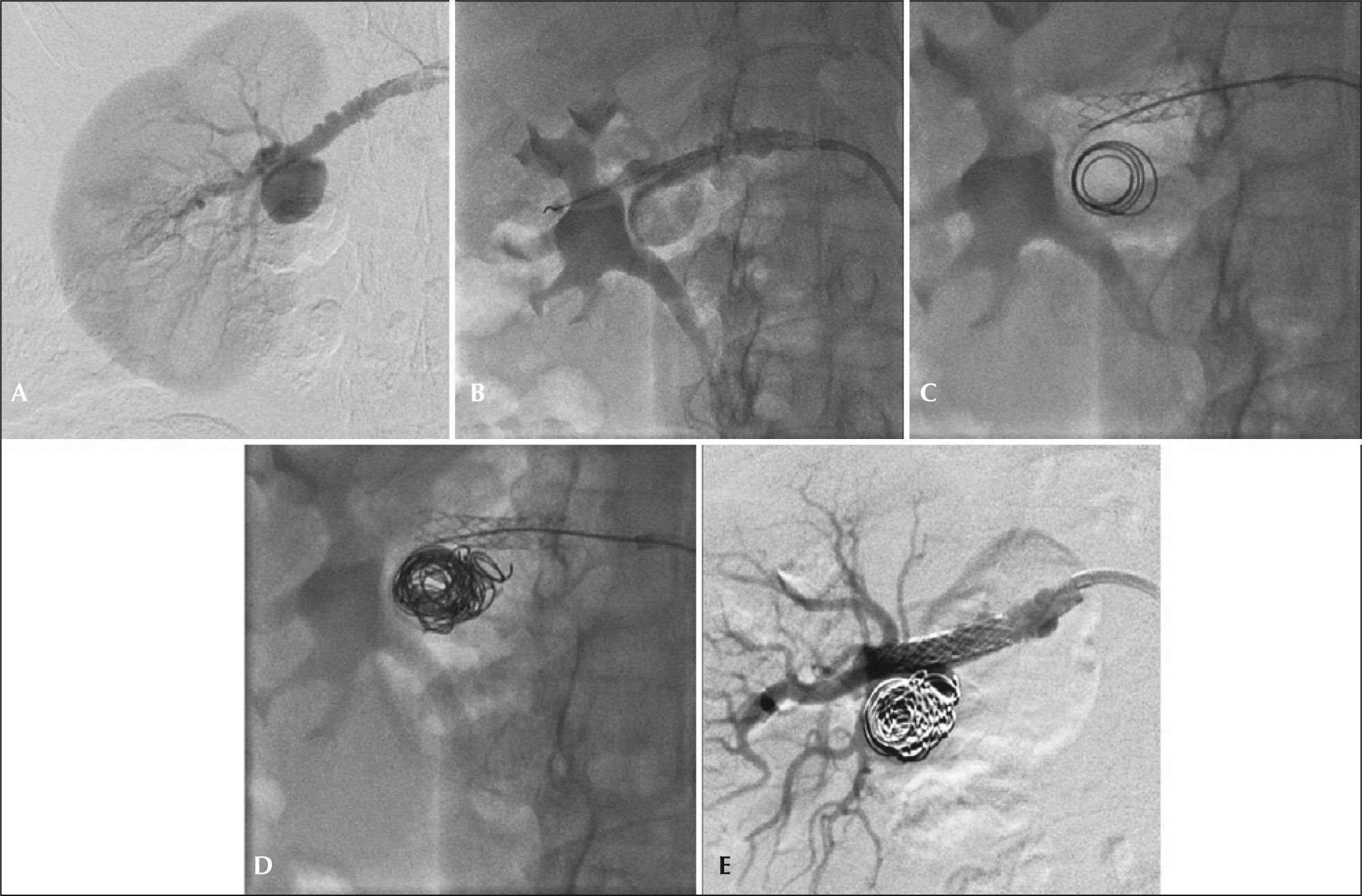
This technique is an important tool in the treatment of types I and II aneurysms with wide necks. The use of balloon expandable stent works as support for the release of microcoils within the aneurysmal sac after selective catheterization with 4F diagnostic catheters or microcatheters within the stent mesh (Figure 3). With that, there is a change in local hemodynamic parameters, with redirection of flow through the native artery and subsequent endothelization of the stent mesh occurs. The authors prefer the Palmaz® Blue® balloon expandable stent (Cordis Corporation, Warren, United States) associated with the use of 0.018 profile AZUR® coils (Terumo Interventional Systems, Somerset, United States).
– Step-by-step remodeling technique for treatment of type II renal artery aneurysms with a microcatheter. (A) Arteriography showing type II aneurysm and associated fibrodysplasia disease. (B) Positioning and release of the balloon-expandable-stent in renal aneurysm neck. (C) Implant of 0.018 microcoils after micronavigation with 2.4F microcatheter within the stent mesh. (D) Final aspect of coil compactness in the aneurysmal sac. (E) Final arteriogram showing no leaks or occlusions of native vessels.
a) Superselective embolization of renal segmental branch using N-butyl cyanoacrylate Histoacryl® (B Braun, Tuttlingen, Germany)
Histoacryl® is a tissue adhesive which, when in contact with ionic fluids such as blood, solidifies, resulting in vessel occlusion. The glue is used in a mixture with Lipiodol® (Guerbet, Anlney, Sous-Bois, France), so that it becomes visible to the X-ray and slows polymerization. The authors used a concentration of around 30% to 50% of the glue in the solution.
This technique is used for type III aneurysms, in which the occlusion of the native segmental vessel causes a small renal infarction area. The use of microcatheters for careful micronavigation of renal segmental vessels is required to perform this technique.
ProcedureAll procedures were performed in the Hemodinamic Laboratorycath lab at the Endovascular Intervention Center, or in hybrid room, at Instituto Dante Pazzanese de Cardiologia.
Patients were treated under local anesthesia. Antibiotic prophylaxis was performed with 1.5g of cefuroxime at the time of anesthesia induction. The approach was preferentially performed through the common femoral artery, through unilateral puncture. When that approach was not possible, or when there was significant tortuosity of the renal artery to be catheterized, the left brachial artery approach was chosen.
Radiographic control was performed in a Siemens® Artis Flat Panel device, or in the hybrid room, in a Siemens® Artis Zeego Hybrid device.
Intraoperative angiography was performed in all patients. The immediate postoperative period was carried out in the ward in all cases.
Postoperative follow-upPatients were followed through outpatient evaluation at 15, 30, 180, and 360 days after the procedure and annually thereafter. CT control was performed at 30 days of follow-up. The USG-D was performed at 30 and 180 days, and annually thereafter, in order to assess the presence of flow in the aneurysmal sac.
Outcomes and definitionsTechnical success was defined when the technique chosen for the treatment of RAA was performed as previously planned. Therapeutic success was defined when the technique chosen for the treatment of RAA was performed as previously planned without the presence of leaks or other complications that would influence appropriate aneurysm occlusion. The renal artery patency was defined as achieving patency of the treated renal artery without signs of ischemia of the corresponding renal parenchyma.
After one year, morbidity and mortality, as well as rates of leakage and reoperation were evaluated.
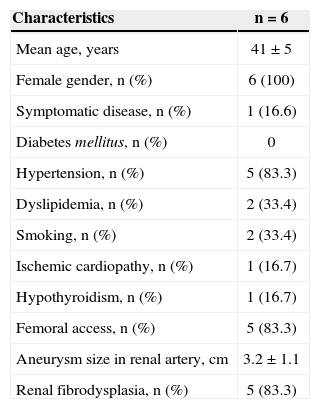
RESULTSFrom January 2010 to December 2013, six consecutive endovascular repairs of RAAs were performed. Demographic characteristics, comorbidities, and indications for treatment are shown in Table 1. Mean age was 41±5 years. All patients were females and, 83.3% were asymptomatic, having been incidentally diagnosed on imaging assessments. Hypertension was the most prevalent comorbidity. There was a high incidence of fibrodysplasia (83.3%), and there were no cases of PAN. Aneurysm>2cm in women of childbearing age was the main indication for endovascular treatment of RAA. All patients were treated electively. The femoral artery approach was used in five cases (83.3%), whereas the brachial approach was used in the remaining case.
Clinical and angiographic characteristics
| Characteristics | n=6 |
|---|---|
| Mean age, years | 41±5 |
| Female gender, n (%) | 6 (100) |
| Symptomatic disease, n (%) | 1 (16.6) |
| Diabetes mellitus, n (%) | 0 |
| Hypertension, n (%) | 5 (83.3) |
| Dyslipidemia, n (%) | 2 (33.4) |
| Smoking, n (%) | 2 (33.4) |
| Ischemic cardiopathy, n (%) | 1 (16.7) |
| Hypothyroidism, n (%) | 1 (16.7) |
| Femoral access, n (%) | 5 (83.3) |
| Aneurysm size in renal artery, cm | 3.2±1.1 |
| Renal fibrodysplasia, n (%) | 5 (83.3) |
Technical success was 100% and therapeutic success was 83.3% (n=5). The mean time of endovascular procedure was 40 minutes (range 34-61 minutes), and the mean lenght of in-hospital stay was five days (range 2-15 days).
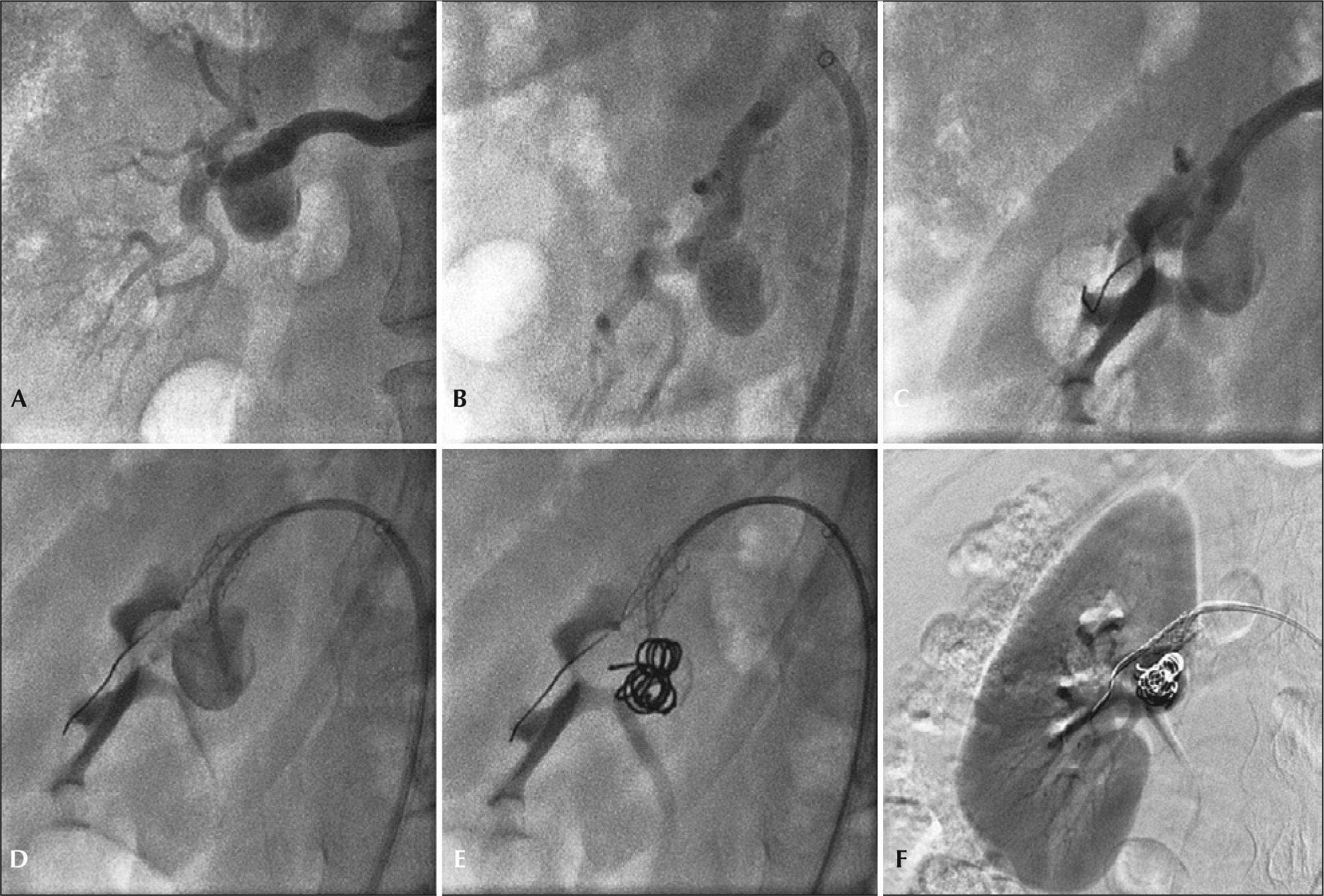
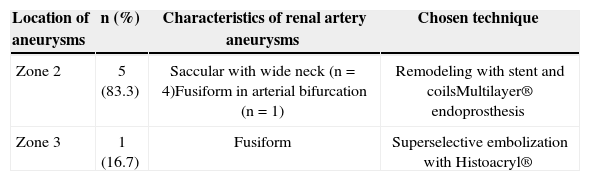
The techniques used for the endovascular repair of RAA are described in Table 2. Four endovascular treatments were performed using the remodeling technique, employing 5 or 6mm×15 or 18mm Palmaz® Blue® balloon expandable stent, associated with 0.018’ AZUR® microcoils. The release of the coils was performed after catheterization of the aneurysmal sac with 4F diagnostic catheters or 2.4F Progreat® microcatheter, navigated by a 0.014×180cm guidewire (Figure 4). There were no renal artery dissections, perforations of the renal parenchyma, or arteriovenous fistulas in the periprocedural period.
Technical details of endovascular renal artery aneurysm corrections
| Location of aneurysms | n (%) | Characteristics of renal artery aneurysms | Chosen technique |
|---|---|---|---|
| Zone 2 | 5 (83.3) | Saccular with wide neck (n=4)Fusiform in arterial bifurcation (n=1) | Remodeling with stent and coilsMultilayer® endoprosthesis |
| Zone 3 | 1 (16.7) | Fusiform | Superselective embolization with Histoacryl® |
– Step-by-step remodeling technique for treatment of the type II renal artery aneurysm with 4F catheter. (A) Arteriography of the right renal artery. (B) Incidence used to visualize the distal main renal artery. (C) Balloon-expandable-stent implant in the aneurysm neck. (D) Catheterization of the aneurysmal sac with 4F JR catheter within the stent mesh. (E) Release of 0.035 coils in the aneurysmal sac. (F) Final renal arteriography.
One patient had a type II fusiform aneurysm in the outlet of a large renal artery branch and the chosen treatment was the Multilayer® endoprosthesis, which was successfully implanted. On the second day after the surgery, the patient developed pain in the right lumbar region associated with macroscopic hematuria. Renal CT showed ischemia of the entire upper renal pole. Despite treatment with analgesics and opioids, the patient developed uncontrollable pain, and a radical nephrectomy was performed. Currently, the patient is undergoing outpatient treatment, with no pain or renal function deterioration (Figure 5).
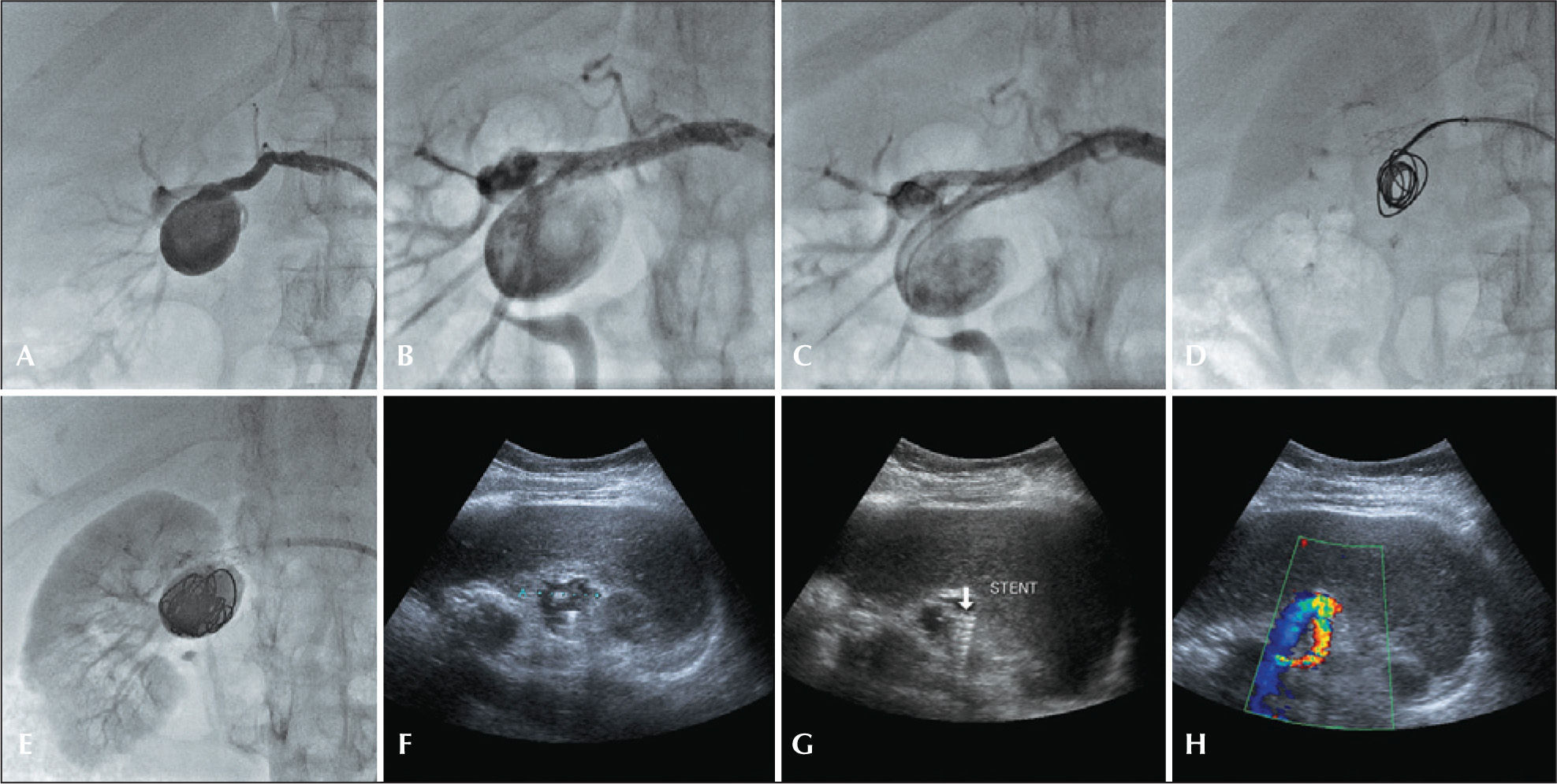
– Treatment of bulky type II renal artery aneurysm by the remodeling technique and postoperative follow-up with Doppler ultrasonography. (A) Right renal arteriogram demonstrating bulky type II renal aneurysm. (B) Arteriographic incidence. (C) Balloon-expandable-stent implant and catheterization with a JR 4F catheter within the stent mesh. (D) Release of 0.035 coils in the aneurysmal sac. (E) Final arteriogram. (F) Postoperative follow-up with B-mode Doppler ultrasonography demonstrating the complete filling of the aneurysmal sac with coils. (G) B-mode Doppler ultrasonography demonstrating adequate stent location in the renal aneurysm neck. (H) Color Doppler ultrasonography demonstrating stent patency and absence of flow in the aneurysmal sac.
Another patient had a type III fusiform aneurysm in the distal segmental renal artery, in which the micronavigation technique was used with superselective embolization of the feeder branch with Histoacryl®. The patient had no complaints of back pain, hematuria or worsening renal function postoperatively. The control USG showed elimination of renal aneurysm.
During the one-year follow-up (5-21 months), no endoleaks or back flow into the aneurysmal sac was observed in the RAAs (Figure 6). Renal artery patency at one year was 83.3%. There were no deaths, reoperations or nephrectomies. Of the five patients who had hypertension, blood pressure control was better, with decrease in the number of antihypertensive medications postoperatively.
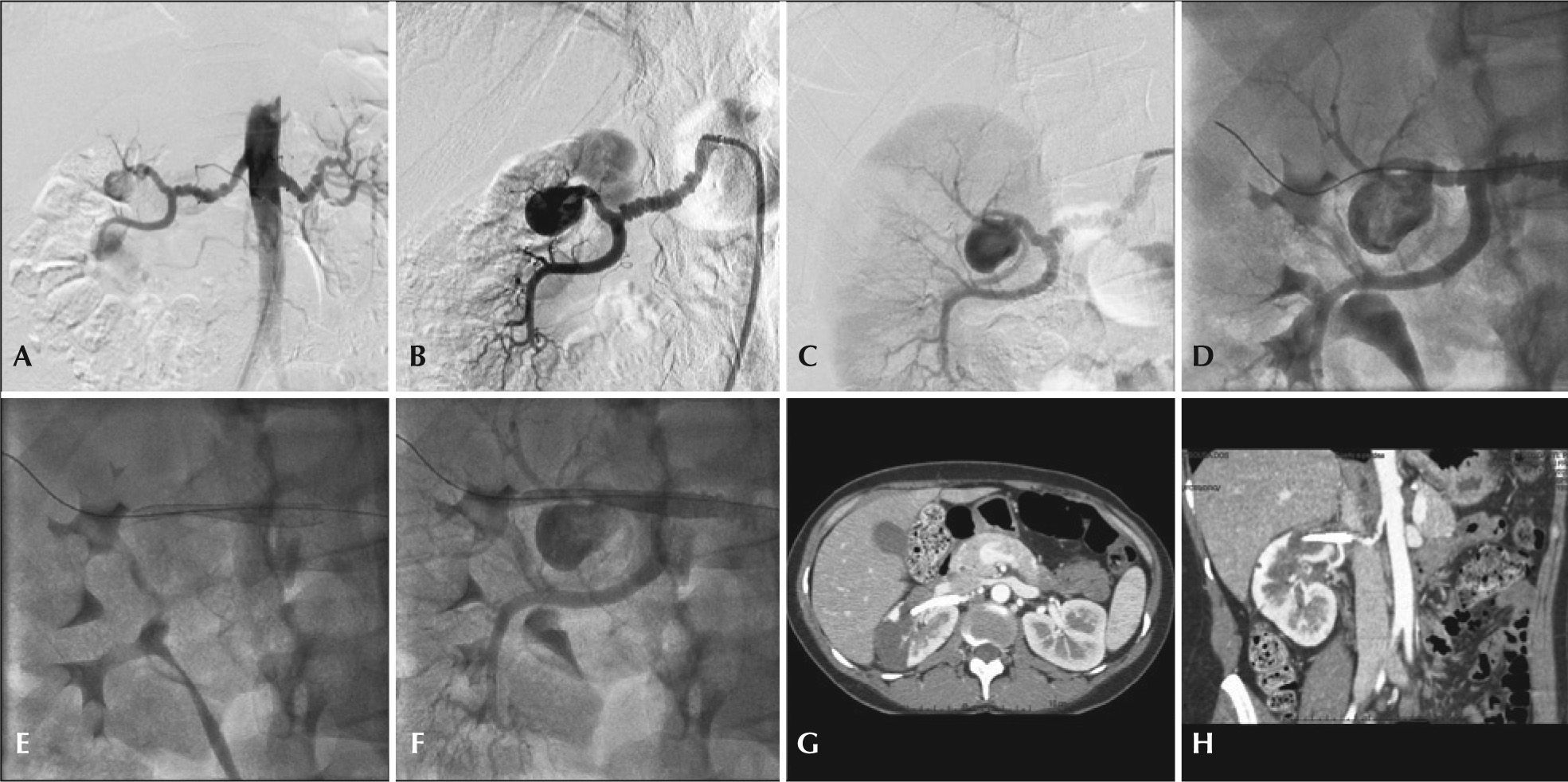
– Treatment of type II renal artery aneurysm using Multilayer® endoprosthesis. (A) Aortography showing bilateral fibrodysplasia disease and type II renal aneurysm to the right. (B) Selective right renal arteriography. (C) Incidence with best visualization of renal bifurcation and its association with the aneurysm neck. (D) Passage of 0.018 guidewire through the distal main renal artery. (E) Multilayer® endoprosthesis positioning and release under the main renal artery and at the aneurysm neck. (F) Final renal arteriography. (G) Postoperative angiotomography in axial view demonstrating upper renal pole ischemia. (H) Computed tomography angiography in coronal view demonstrating patency of renal trunk branch.
RAAs are rare. The prevalence in autopsy studies is 0.01%; however, in patients selected for renal arteriography, their prevalence can reach 0.3 to 1%. They are predominant in females due to their strong association with renal muscular fibrodysplasia.8,13 Among patients from the United States Registry for Fibromuscular Dysplasia, 91% were women, and 5.6% had RAA.14 In the present study, all patients were females, and five of them (83.3%) had renal artery fibrodysplasia.
Its natural history is poorly known. It is known that the RAAs can occur due to trauma, infection and arteritis, such as PAN, Kawasaki disease, atherosclerosis, or vascular dysplasias (renal artery fibrodysplasia or Ehlers-Danlos syndrome). RAA rupture is a rare event, with a reported incidence of 5% to 10%, but it is associated with mortality rates of up to 80%. Among the three types of RAAs, type III has greater risk of rupture.2,8,9,15,16
There is a general consensus in the literature that RAAs should be treated when the following criteria are met: (1) RAA>2cm or documented aneurysmal growth; (2) symptomatic patients; (3) RAA with documented distal embolization; (4) RAA in women of reproductive age or pregnancy; (5) RAA associated with significant stenosis, and poor renal perfusion.1,6,9,16 In the present study, most patients had indication due to aneurysm>2cm, and most were also asymptomatic and still of childbearing age.
With the currently available imaging methods, detailed preoperative planning is a key part in determining the technique and choice of materials for effective endovascular repair and adequate postoperative followup. CT allows for the evaluation of the renal vascular anatomy, as well as the characteristics of aneurysms to be treated. When this diagnostic method cannot demonstrate all the necessary information for correct endovascular management, preoperative arteriography should be performed. In cases of complex aneurysms, in which the best incidence for visualization of the aneurysm neck should be obtained, the rotational angiography with three-dimensional reconstruction is essential for a better understanding of the renal vascular angioarchitecture.9 However, in the postoperative follow-up, the CT has limits for adequate visualization of coils and leaks, because of artifacts generated by the presence of metal,17 and thus, the USG-D and magnetic resonance angiography are appropriate methods for patient follow-up.3,9
The experience acquired with endovascular management of renal artery stenosis has contributed to the learning of the endovascular approach of RAAs.18 Studies on the endovascular treatment of these aneurysms are restricted to small samples or case reports.19-23 Antoniou and Antoniou24 published a review of 22 aneurysms treated with stenting, with 91% of technical success and 27% of minor complications such as occlusion of small polar arteries. During the eight-month follow-up, only one case of renal artery restenosis was reported. Elaassar et al.19 reported the treatment of 13 patients using three different techniques: coil embolization (n=8), embolization with liquid agents (n=2), and remodeling technique with coils and stenting (n=3).
They obtained 100% technical success, and the rate of occlusion of small renal branches was 23%. Brazilian authors have reported the treatment of 11 complex renal artery aneurysms treated with remodeling techniques in eight patients and embolization in three. They obtained 100% of technical success with only one case of branch occlusion in a type II RAA treated with coil embolization. They observed no leaks or recanalization in aneurysms treated during a followup of 32 months.9
In the present study, technical success was achieved in 100% of cases, and three techniques were used for the treatment of RAAs, depending on the location and anatomy. Due to the predominance of type II RAAs (83.3%), the remodeling technique with stenting and coils was used in four cases, with no significant renal branch occlusions or leaks with this technique. In one case of type III RAA, the embolization technique with liquid agent was used, with total occlusion of the aneurysm and the feeder vessel, with no significant renal ischemia, pain or macroscopic hematuria postoperatively. In one case of type II RAA, the Multilayer® endoprosthesis was used. Even though it was implanted in the desired location, the patient developed ischemia of the entire upper renal pole and uncontrollable pain, and a nephrectomy was performed. This led to a therapeutic success rate of 83.3%.
Rundback et al.10 classified the renal aneurysms according to their angiographic location, helping to jestablish treatment strategies. In type I lesions, in which the RAA originates from the main renal artery, the treatment with covered stent is sufficient for proper occlusion of the aneurysm, while respecting the limit of 15mm between the aneurysm and the origin of the renal artery, and from the latter to its bifurcation. In the treatment of such aneurysms, preference is given to the use of stents with greater flexibility and more accurate release, as small stent migration caused by renal artery tortuosity or the chosen materials lack of precision can occlude renal artery branches, with consequent renal ischemia. Selective embolization with coils or adhesive may be an option in these types of saccular aneurysms with small necks.2,9,15 There were no type I RAAs in the present series.
Type II RAAs were previously treated only by conventional surgery or nephrectomy. These lesions represent a therapeutic challenge for the endovascular technique due to the anatomical difficulty of these lesions. These aneurysms comprised most of the aneurysms treated in the present study. In such cases, the use of remodeling techniques with stenting, associated with the use of coils or coils and glue, is fundamental for the preservation of native vessels and proper aneurysmal occlusion.2,4,9,15,20
As for the type III aneurysms, which emerge from small segmental arteries and supply a small portion of the renal parenchyma, they may be embolized with the occlusion of the feeder vessel. Liquid agents, such as glue and Onyx® (Ev3 Inc., Plymouth, United States) may be used for this purpose. The present study comprised one case of type III RAA, which was adequately embolized using Hystoacril®.2,9,19,24
Study limitationsDue to the small number of cases, heterogeneous group of patients, and types of procedures performed, as well as the short-term follow-up, the comparison between the techniques and their association with outcomes limit the results of the present study. Finally, result accuracy may be affected by the retrospective data analysis.
CONCLUSIONSEndovascular treatment of renal artery aneurysms was shown to be technically feasible and associated with low morbidity and mortality rates in the short and medium term. Detailed studies of the renal vasculature and location of the aneurysm determine the choice of endovascular technique to be used. Type II renal artery aneurysm was the most frequent morphology observed and may be successfully treated by remodeling techniques using stents and coils.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
FUNDING SOURCENone.