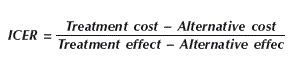Transcatheter aortic valve implantation (TAVI) is a new modality of treatment especially dedicated to patients with high surgical risk. In these patients, TAVI increased survival and improved quality of life when compared to standard treatment (drug therapy with or without percutaneous aortic balloon valvuloplasty). Our objective was to perform a costefficacy analysis of the implementation of TAVI in the Brazilian Supplemental Health System.
MethodsWe developed a predictive model to assess the cost-effectiveness of the procedure in the long-term, and a Weibull regression analysis with a time horizon of 5 and 10 years, to estimate survival data for over 24 months. In addition, a deterministic sequential Markov model was developed. Results were expressed as incremental cost-effectiveness ratio (ICER) per years of life saved and progression-free years of life.
ResultsIn a standard scenario, where the cost of TAVI was estimated as R$ 65 millions, the ICER value (cost/year of life saved) in 5 years was R$ 72,520.65. When the time horizon was adjusted for 10 years, this amount decreased to R$ 41,653.01.
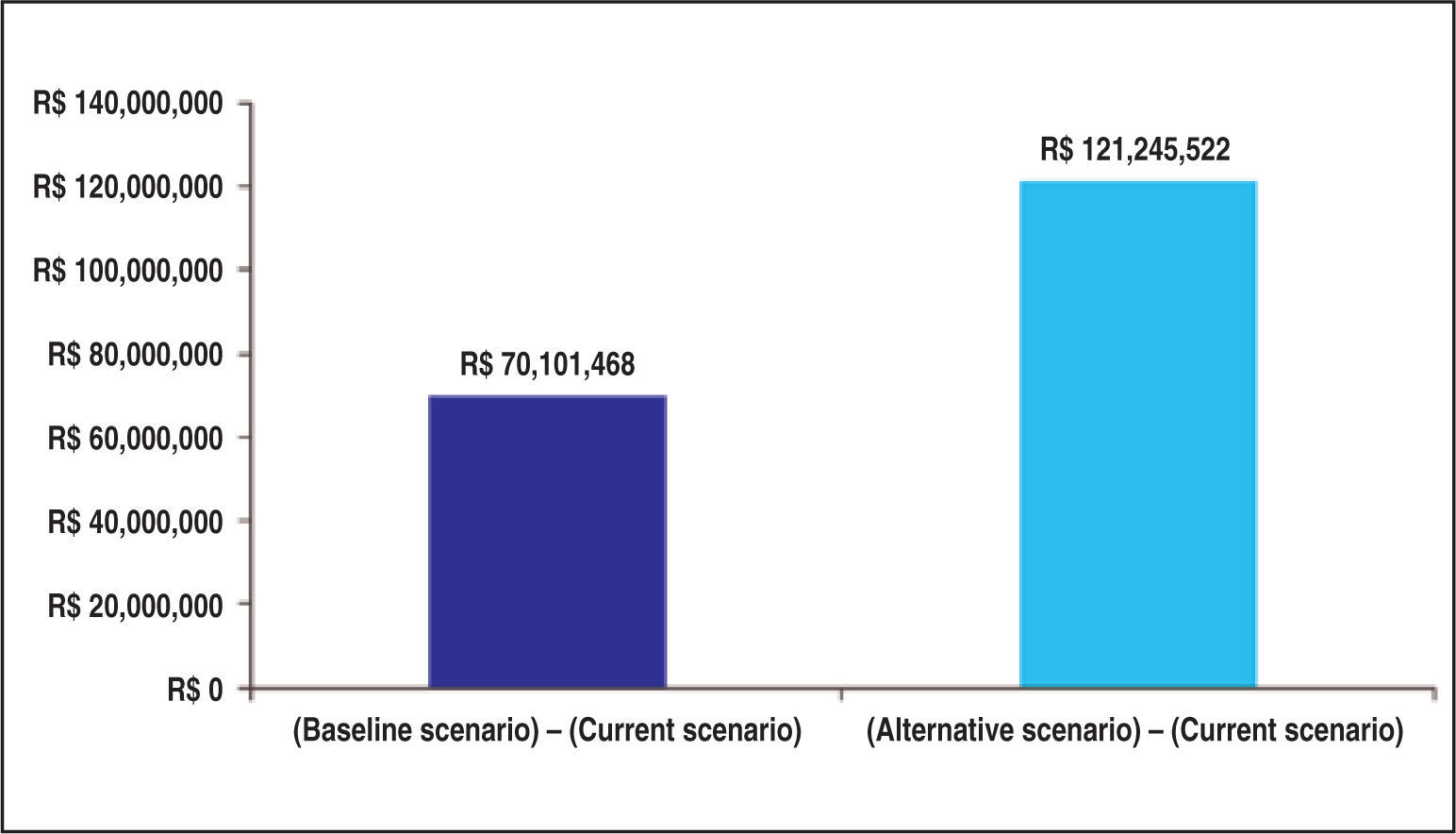
ConclusionsThe model indicated that TAVI has superior effectiveness and higher incremental cost. Furthermore, the incorporation of TAVI in the List of Health Procedures and Events of the Brazilian Supplemental Health System would have an incremental budgetary impact over the next 5 years, ranging from R$ 70 millions to R$ 121 millions, consistent with other technologies which have already been incorporated by the system.
Implante por Cateter de Bioprótese Valvular Aórtica paraTratamento de Estenose Valvar Aórtica Grave em Pacientes Inoperáveis sob Perspectiva da Saúde Suplementar – Análise de Custo-Efetividade
IntroduçãoO implante por cateter de bioprótese valvular aórtica (TAVI, do inglês transcatheter aortic valve implantation) constitui nova modalidade de tratamento destinada, sobretudo, aos pacientes com elevado risco cirúrgico. Para esses pacientes, o TAVI resultou em aumento da sobrevivência e melhora da qualidade de vida, comparativamente ao tratamento padrão (medicamentoso, com ou sem valvuloplastia aórtica percutânea). Nosso objetivo foi realizar análise de custo-efetividade da implementação do TAVI no Sistema de Saúde Suplementar brasileiro.
MétodosForam desenvolvidos um modelo preditivo, para avaliar custo-efetividade real do procedimento em longo prazo, e uma regressão de Weibull com tempo horizonte de 5 e 10 anos, para estimar dados de sobrevida por mais de 24 meses. Adicionalmente, foi desenvolvido modelo de Markov sequencial e determinístico. Resultados foram expressos como razão de custo-efetividade incremental (RCEI) por anos de vida ganhos e anos de vida livres de progressão.
ResultadosPara o cenário padrão, no qual o custo da TAVI foi estipulado em R$ 65 mil, o valor da RCEI (custo/ano de vida salvo) em 5 anos foi de R$ 72.520,65. Alterando-se o tempo horizonte para 10 anos, esse valor diminuiu para R$ 41.653,01.
ConclusõesO modelo apontou que o TAVI apresenta efetividade superior e maior custo incremental. Além disso, a incorporação do TAVI no Rol de Procedimentos e Eventos em Saúde da Agência Nacional de Saúde Suplementar acarretaria impacto orçamentário incremental nos próximos 5 anos, variando de R$ 70 milhões a R$ 121 milhões, compatível com o de outras tecnologias já incorporadas no âmbito da Saúde Suplementar.
Aortic stenosis (AS) is now the most common valvulopathy among the elderly, with an estimated prevalence of up to 5% in individuals over 75 years of age. The current relative ageing of the global population further increases the social importance of this pathology, with significant impact on public health policies.1,2
Typically, after a long period of clinical latency, symptoms of angina, syncope, and heart failure arise. Survival after the onset of symptoms without valve replacement surgery is low: 60% at one year and 32% at five years,3,4 with high risk of sudden death.5
The standard treatment for this condition is surgical valve replacement, which in observational trials proved able to increase survival and improve symptoms compared to data from historical cohorts of patients treated without surgery.5-7 The surgical procedure is considered safe, with average complication rates of approximately 4% to 5%, which may be less than 1% in patients aged below 70 years.8
However, a substantial subgroup has high surgical risk, both due to advanced age and association with comorbidities, making them ineligible for surgery. The rates of ineligibility are approximately 30% to 40% of patients.9,10 In Brazil, there are no published data evaluating these statistics. For this group of patients with no prospect of surgical treatment, the treatment consists of clinical and medical support, and of percutaneous balloon aortic valvuloplasty. Such therapeutic resources, even in association, have limited effectiveness and are not able to improve survival of patients.3,6,11 The prognosis is ominous, with mortality at one, two, and three years of 45%, 65%, and 77%, respectively.12
The implant of aortic valve bioprosthesis by catheter (transcatheter aortic valve implantation [TAVI]) is a new technique that has been successfully introduced for the treatment of patients deemed inoperable. Its main objective is to restore aortic valve function through minimally invasive techniques, thus avoiding general anesthesia and surgical procedures, such as median sternotomy, aortic clamping, and cardiopulmonary bypass.13,14 In Brazil, TAVI was introduced in 2008, and since then has been performed in different private hospitals in various regions of the country. Currently, approximately 700 such procedures have been performed in Brazil.
Several international organizations recommend and approve the use of TAVI for AS in inoperable patients, such as the National Institute for Health and Clinical Excellence (NICE) of Great Britain,15 Ontario Health Quality,12 and the National Health Committee of New Zealand.16 In Brazil, both Brazilian Society of Hemodynamics and Interventional Cardiology (Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista [SBHCI])6 and Brazilian Society of Cardiology (Sociedade Brasileira de Cardiologia [SBC])11 recommend TAVI for the group of patients considered inoperable. However, this important technique has not yet been incorporated in the list of healthcare practices in Brazil.
The present study aimed to perform an economic analysis of the impact of the incorporation of TAVI originally intended for patients with AS in the Brazilian private healthcare system, assessing its cost-effectiveness in patients not eligible for surgical treatment and determining its budgetary impact.
METHODSThe current evidence related to the effectiveness of TAVI in the treatment of patients with severe AS is restricted to a short period. A predictive model was developed in order to analyze, in the long run, the cost-effectiveness of this therapeutic modality, aiming to assess its real benefits, as well as the incremental costs of implementing this treatment. The effectiveness data were taken from the only randomized prospective clinical trial published: the PARTNER cohort B trial.17
Target populationPatients affected by severe and symptomatic AS, without surgical perspective.
Time horizonA time horizon of 60 months (five years) was considered to be more appropriate to capture patient’s cost and clinical benefit data, since data on longer periods are scarce and a model for extrapolating these data could cause excessive bias in the results.
PerspectiveThe analysis was conducted under the perspective of the Brazilian private healthcare system, and only direct costs were considered. Indirect costs, such as loss of productivity due to illness, were not included.
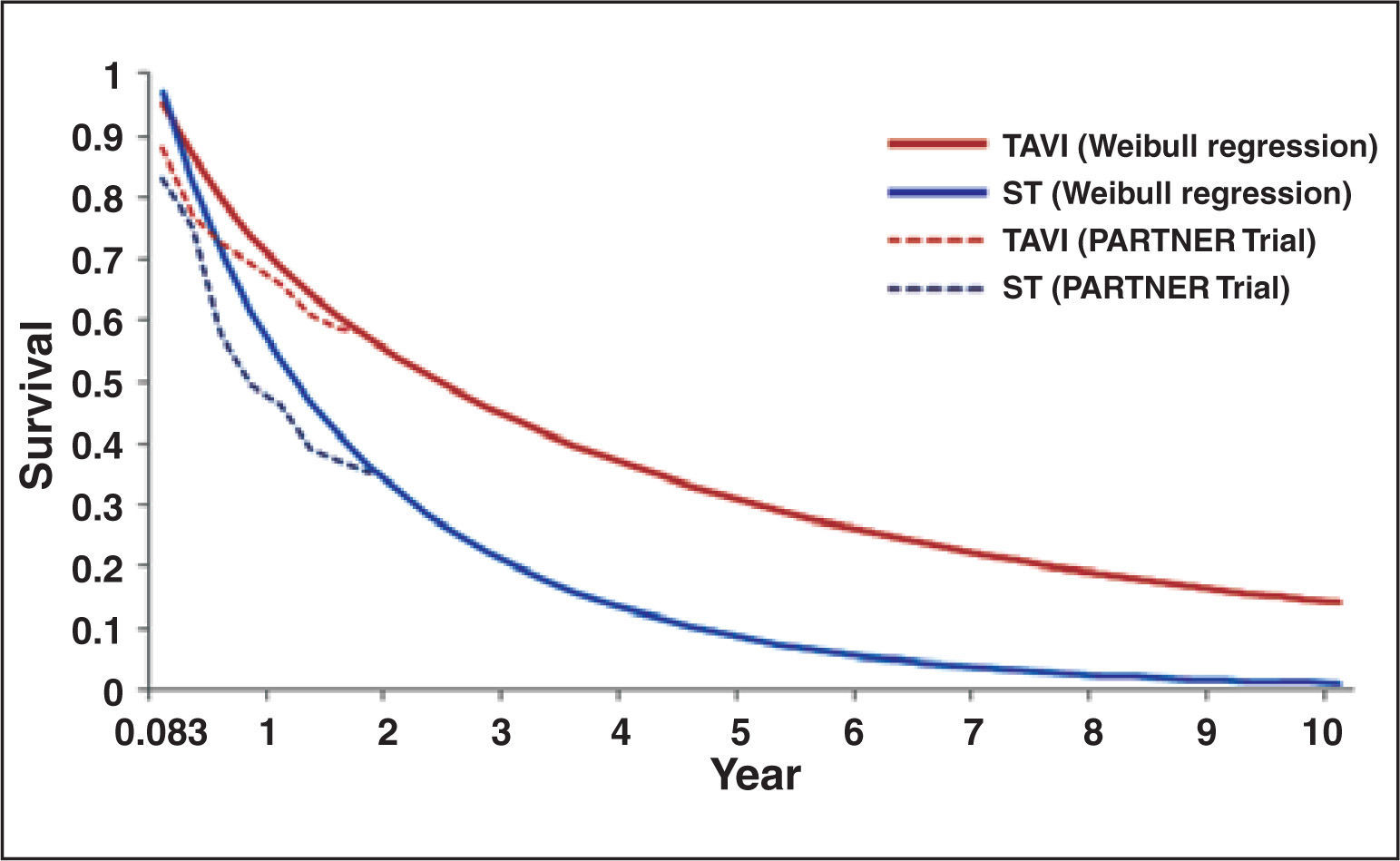
Weibull regressionIn order to estimate survival data for more than 24 months, a Weibull regression with time horizon of five and ten years was developed. From the results of the PARTNER trial,17 survival data of patients in both treatment modalities, at three months intervals, were collected. These data were used to develop the survival curve designed with the use of Weibull regression, obtaining estimates of survival data up to ten years (Figure 1).
– Weibull regression for data of survival designed for ten years in standard treatment groups (ST) and with transcatheter aortic valve implantation (TAVI), from PARTNER trial data.17
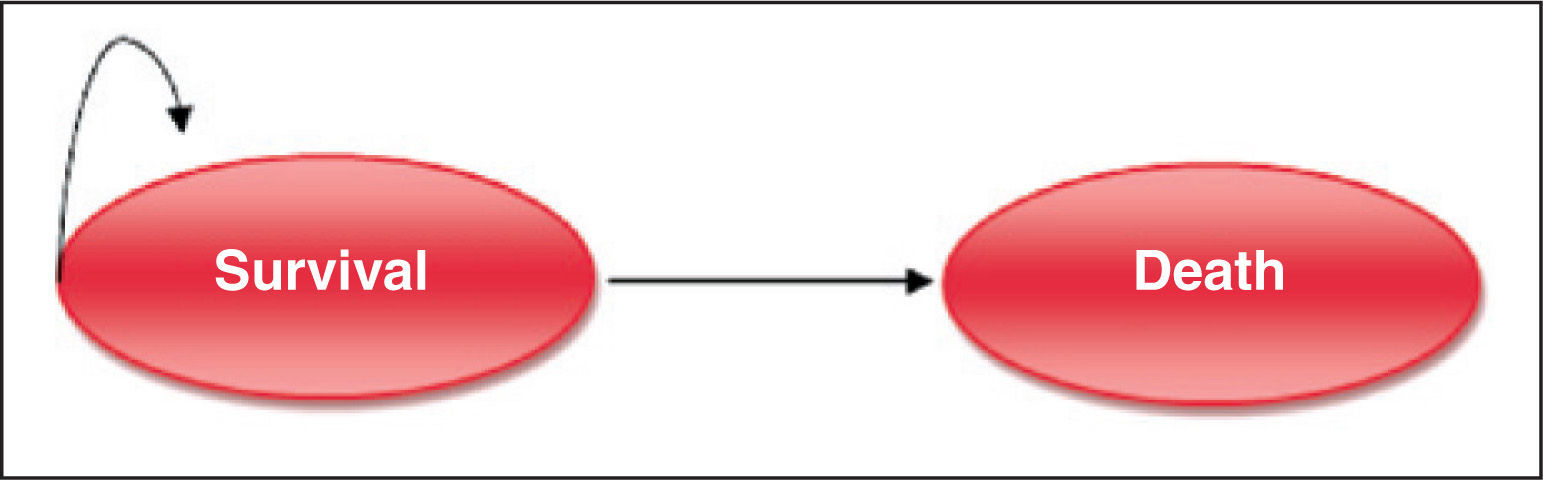
In addition, this model uses short-term data available, and relies on them in order to predict the costs by increasing the life expectancy and health benefits associated with different treatment options. Thus, a deterministic sequential Markov model was developed with an optional random element. From survival data of the patients obtained in the PARTNER trial,17 and from the corresponding estimated survival over the remaining years through the Weibull regression, the model structure considered two health states: survival and death (Figure 2).
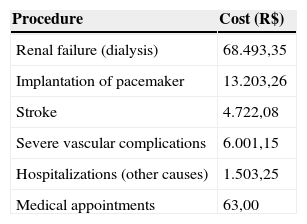
As the survival data obtained from the PARTNER study were based on observation of deaths from all causes, it was decided to construct a simple model (two states of health). To do so, all costs and likelihoods of comorbidities (stroke; serious complications, such as kidney failure and implantation of artificial heart pacemaker; and medical visits and hospitalizations) were considered. Each cycle was estimated on a time horizon of three months and, for each cycle, the chances of mortality were changed over time, in accordance with data from the PARTNER trial and the Weibull projection.
The model parameters include the mean durations in which the patient remains in each state of health, as well as the costs associated with such conditions. The model was structured in a Microsoft Excel® database. This database used quarterly cycles with probabilities for the possibility of a change in the patient health status during each period. All consequences were reported according to the expected cost and to the results on the health status of each patient.
In the model, the planning horizon used was the patient’s lifetime. Therefore, the results on the health status and the costs occurring in the future have less weight than the present results. Thereby, discounts of the global results on health status and respective costs were applied. The guidelines by the Brazilian Ministry of Health recommend a discount of 5% for costs and benefits.
CostsOnly direct medical costs, such as medications, procedures, and hospitalization, collected under the perspective of Brazilian private healthcare system for the year 2013, were considered. The considered expenditure of resources was based on data from a pre-planned economic study, conducted in parallel with the PARTNER B cohort trial,18 and the drug treatment standard was based on a registry that included high-risk patients with severe and symptomatic AS.19
Although in the PARTNER study 83.8% of control patients underwent percutaneous balloon aortic valvuloplasty, this percentage was not considered generally applicable to the therapeutic scenario observed in Brazil, where, according to the Brazilian Unified Health System database (Banco de Dados do Sistema Único de Saúde – DATASUS), only 121 percutaneous balloon aortic valvuloplasties were performed in 2011. Through a panel of experts appointed by the SBHCI,6 it was estimated that only 30% of the control group population would perform this procedure (this parameter varied in the sensitivity analysis).
Since the use of percutaneous balloon aortic valvuloplasty does not alter the survival of adult patients with unresectable symptomatic severe AS,3,10 it was considered that the effectiveness data from the PARTNER trial still prevailed, even estimating a lower use of that procedure in Brazilian population covered by this economic study.
Taking into account the perspective adopted in the analysis and aiming to establish the monthly pharmaceutical costs of the disease, the mean values of drug purchase were not considered, since, typically, healthcare plans do not reimburse patients for the drugs used in the standard treatment arm (amiodarone, digoxin, and furosemide). Thus, the patient must afford these expenses.
Based on market data, the cost of the prosthesis used for TAVI was estimated at R$ 65,000.000 and the medical procedure was estimated at R$ 23,518.42, obtained from a micro-costing data analysis, an evaluation technique that involves gathering detailed data about resources used and the value of these resources. The costs arising from events resulting from the treatment of patients with severe AS in this model were obtained from an expert panel for the collection of use of resources, consulting sources related tothe private healthcare system, such as Brazilian Hierarchical Classification of Medical Procedures (Classificação Brasileira Hierarquizada de Procedimentos Médicos – CBHPM), 5th edition; Chamber of Drug Market Regulation (Câmara de Regulação do Mercado de Medicamentos – CMED); and Simpro; among others (Table 1).
Incremental cost-effectiveness ratioThe additional cost per extra unit of benefit obtained, known as incremental (or marginal) analysis, and its results are presented as incremental cost-effectiveness ratios (ICERs). The models were used to estimate outcomes (life-years gained) and costs to guide patients on their decision regarding each treatment alternative. ICERs were calculated as follows:
Where: ICER, incremental cost-effectiveness ratio; cost, costs (in R$); effect, effectiveness (in years of life gained).
Sensitivity analysisA univariate sensitivity analysis was performed on the two main variables of the model: the time-horizon of this cost-effectiveness analysis and the cost of incorporation of the prosthesis.
Given that the distributions were adjusted to the fundamental parameters in the model, each patient among the 5,000 included in the analysis showed a different trajectory of the disease and, thus, varied results and costs. Therefore, an additional probabilistic analysis was also conducted in order to further evaluate the variability of the results, and the conclusion regarding the possibility that a given intervention would present cost-effectiveness. All distributions in the model were independently tested.
All costs of treatment (drug, procedures with a balloon catheter, and TAVI) were varied by ± 20% using gamma variation, as well as hospitalization and medical consultations costs. The rates of pacemaker implantation, stroke, serious complications, and number of hospitalizations and clinical visits were varied by ± 25%. The analyzed treatment effectiveness data were varied by ± 10%. According to the guidelines of the Ministry of Health, the discount rate was varied between 0% and 10%.
RESULTSBy employing TAVI in the treatment of inoperable patients with severe AS, the estimated total cost of treatment, over five years, was R$ 123,019.76. This value included: costs of the procedure, valve prosthesis, consultations with specialists, hospitalization expenses, and costs of adverse events. The cost of the standard treatment (pharmacological) was estimated at R$ 35,815.12, with the addition of costs of specialists and hospitalizations, among others. The values were obtained from a micro-costing study in order to assess the total cost of the procedure, considering the tables currently used by the operators of healthcare plans.
Patients treated with TAVI had longer life expectancy compared to patients who underwent only conservative therapy. In five years of analysis, the mean survival in the group treated with TAVI was 2.5 years. In the group that received only standard treatment, the mean survival was 1.53 years.
For the standard scenario, in which the cost of TAVI was set at R$ 65,000, the value of the ICER (cost/year of life saved) at five years was R$ 90,161.29. However, when changing the time horizon to the years, the value of ICER decreased to R$ 55,130.84.
Additionally, the value of the valve prosthesis was altered, between R$ 30,000 and R$ 65,000 (baseline case value). The results of the ICER of this five-year time horizon analysis, with this variance in the cost of the valve prosthesis, ranged between R$ 49,770.00 and R$ 85,957.00. The values for each change are described in Figure 3.
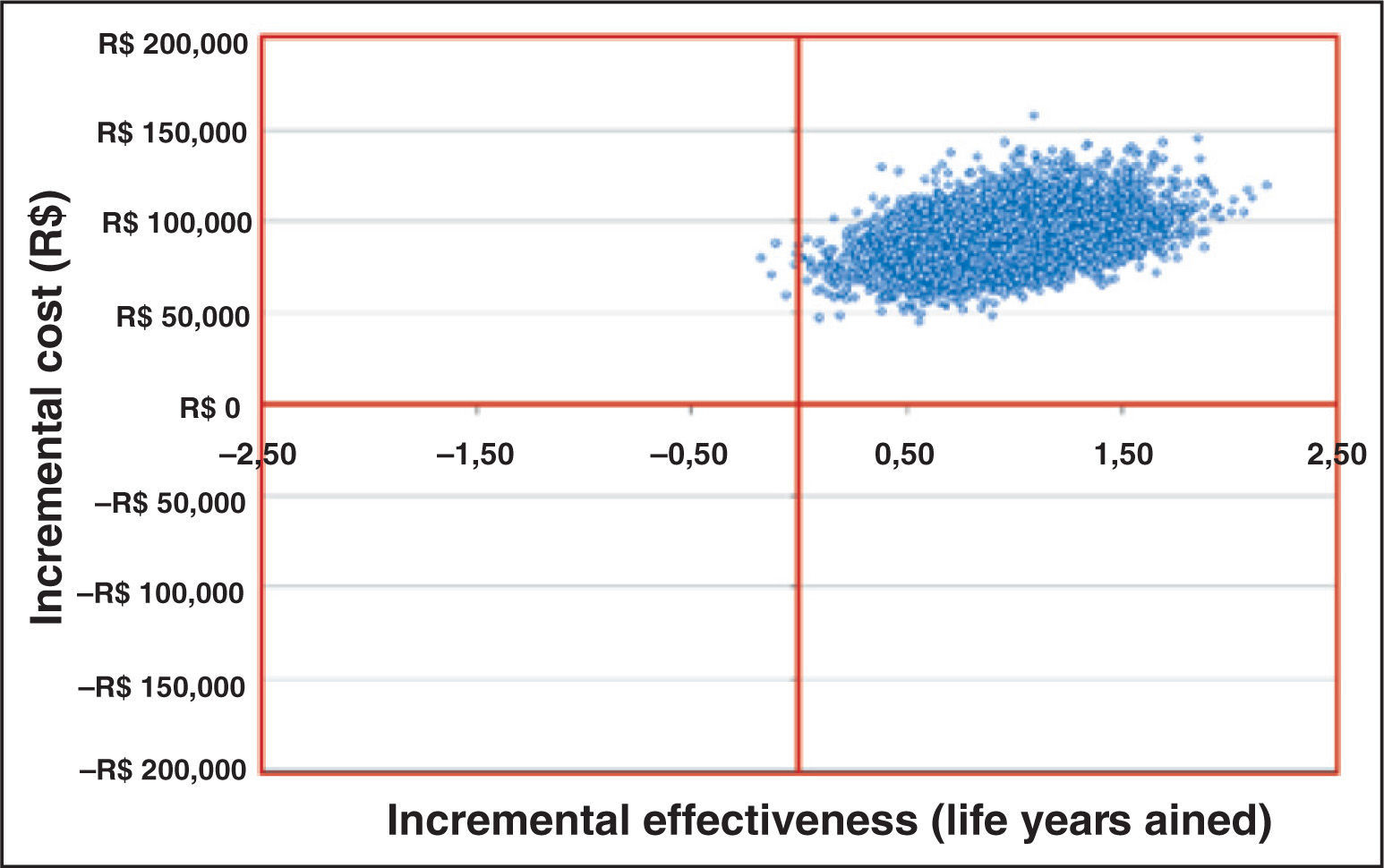
An additional probabilistic analysis was also performed, whose results were evaluated and classified as follows: quadrant 1 (incremental effectiveness > 0 and incremental cost > 0); quadrant 2 (incremental effectiveness < 0 and incremental cost > 0); quadrant 3 (incremental effectiveness < 0 and incremental cost < 0), and quadrant 4 (incremental effectiveness > 0 and incremental cost < 0).
It was observed that most of the simulations had their results in quadrant 1 (99.9%), i.e., in the simulation, almost all patients using TAVI, compared to standard treatment, were characterized by greater efficiency and also by an incremental cost, showing the robustness of the analysis model.
In addition, an analysis of budgetary impact (using Budget Impact Models [BIM]) was developed, in order to simulate the financial impact of the introduction of TAVI in the private healthcare system. This analysis was performed by comparing the cost of the first years of treatment of inoperable patients with severe AS. Thus, the budgetary impact of this new technology compared the cost of acquisition of the valve bioprosthesis (for this group of patients) with the other current treatment option.
The model was created in Microsoft Excel® database, in accordance with the guidelines of budgetary impact analyses published in 2007 by International Society for Pharmacoeconomics and Outcomes Research (ISPOR). This model estimates the budgetary impact by combining epidemiological data, hypotheses of evolution of the use of various therapies available (treatment flow), resources used, and costs of pharmacological treatment and of adverse events associated, comparing two or more different scenarios.
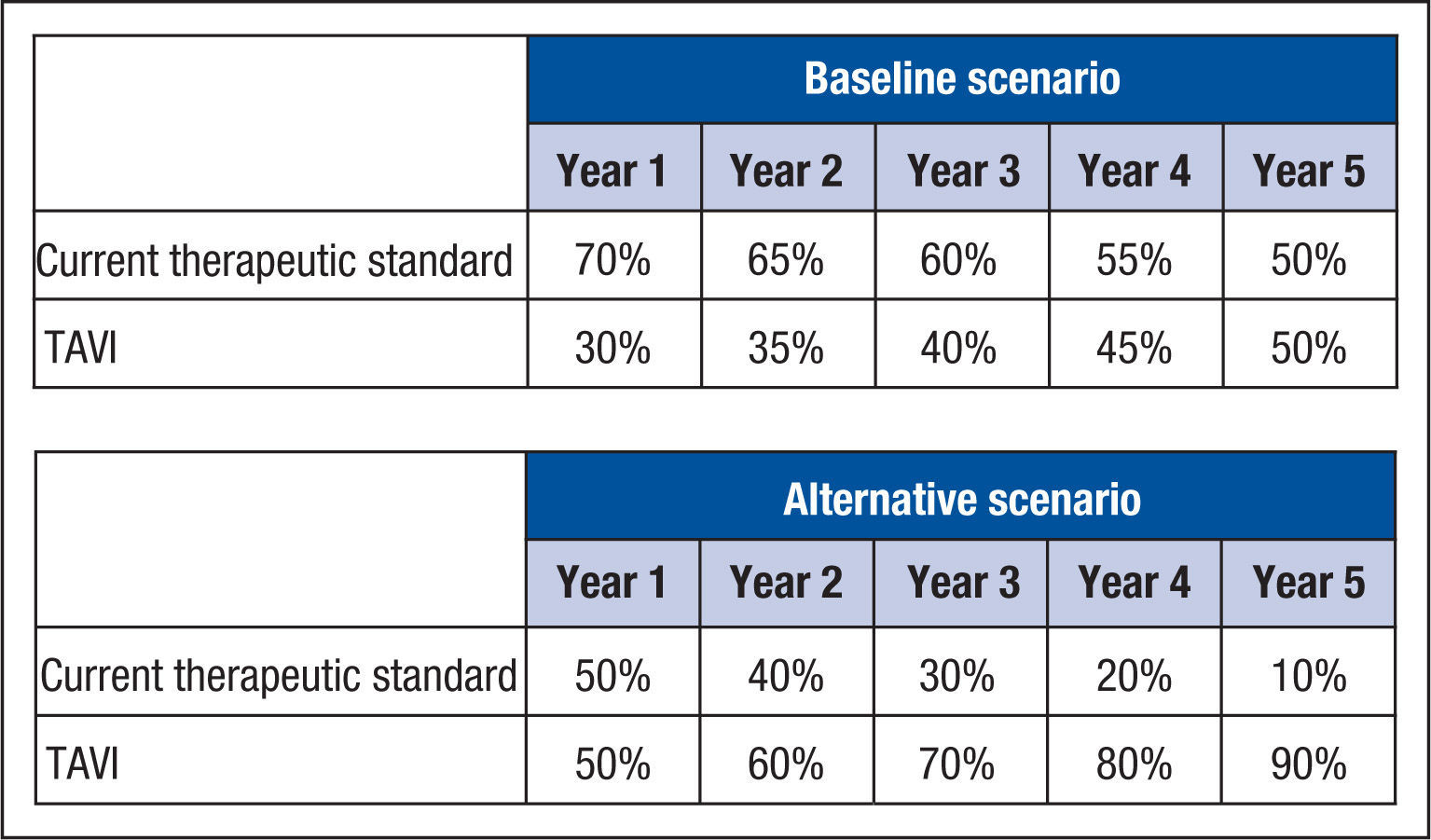
For the hypothetical scenarios envisioned (baseline and alternative scenarios), the market profile for the new technique (TAVI) over five years was estimated from the projected trend of use, since there are no real-world data for these scenarios. In the baseline scenario, the initial premise was that the transcatheter aortic valve implantation should have a usage profile derived of the opinion of the expert panel already nominated. As a sensitivity analysis, an extra scenario was considered (named “alternative scenario”), in which a higher usage profile of TAVI after the incorporation of the new technology was estimated (Figure 4).
For each year of the five-year horizon of the model, it was assumed, in the present scenario, that all eligible patients should be fully treated with the current standard therapy (drugs and balloon catheter dilation).
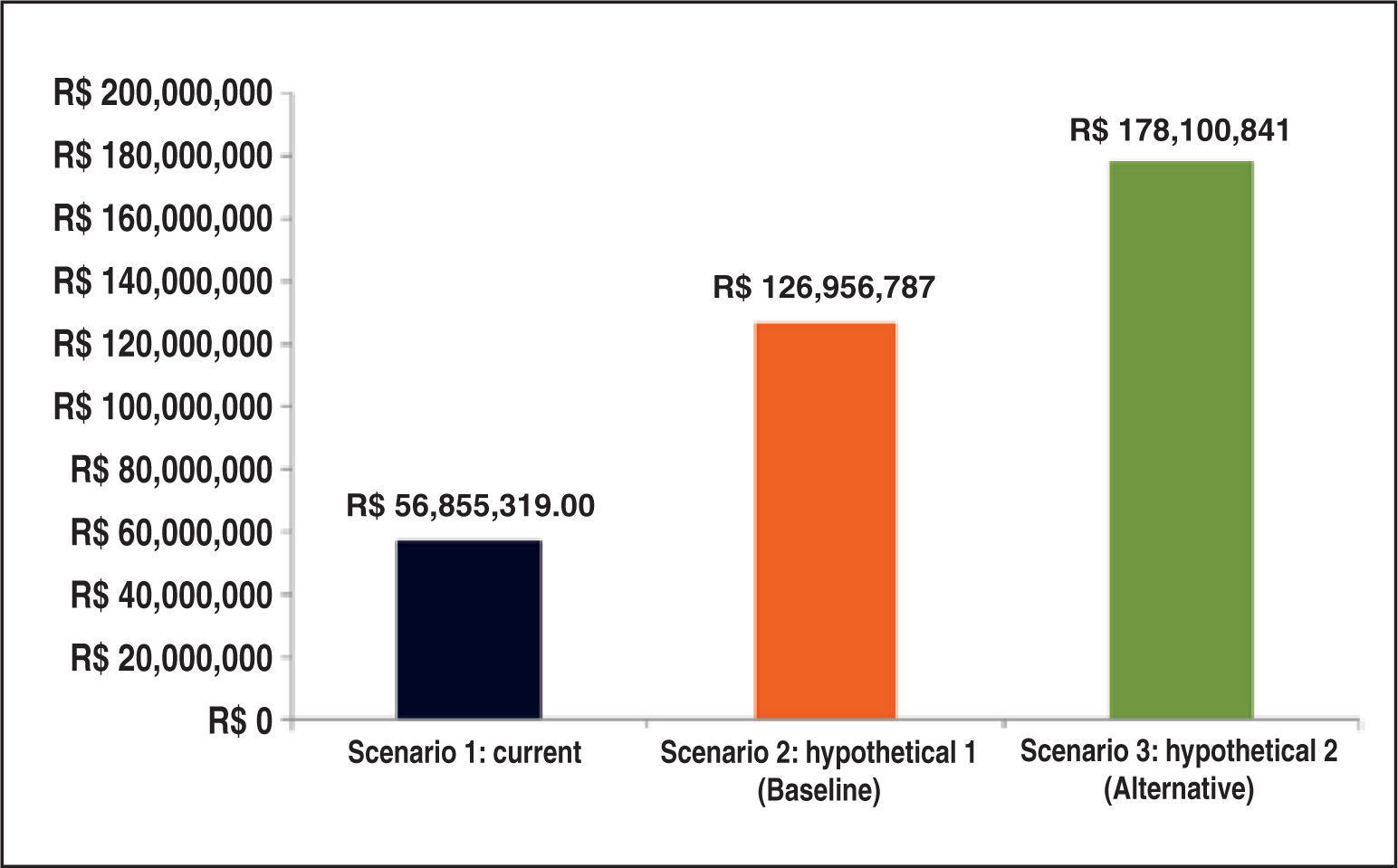
The budgetary impact analysis demonstrated that, in the current scenario, the estimate of total expenditures over the time horizon of five years would be approximately R$ 56.9 million. Both hypothetical (baseline and alternative) scenarios showed increases in budgetary impact. In the baseline scenario, in which the distribution of TAVI values was obtained from the expert panel, the budgetary impact was R$ 127 million over five years (Figure 5).
Figure 6 depicts the incremental budgetary impacts overfive years in the private healthcare system in each hypothetical scenario (TAVI incorporation) compared to the current scenario (without TAVI incorporation).
In both hypothetical scenarios, the budgetary impact is due primarily to the cost of the valve prosthesis for transcatheter implantation, accounting for approximately 43% of total costs in the baseline scenario, when compared to other costs analyzed in the model (hospitalization and medication expenses, among others). In the alternative scenario, the valve prosthesis was responsible for approximately 55% of the costs, since its market distribution would be higher in that scenario.
DISCUSSIONSince 1998, with the regulation of private healthcare in Brazil and the creation of the Brazilian National Regulatory Agency for Private Health Insurance and Plans (Agência Nacional de Saúde Suplementar – ANS), there has been sustained growth in this sector, involving operators of healthcare plans, beneficiaries, and service providers. Currently, over 48 million Brazilians use the private healthcare system. According to data from the Descriptive Document of ANS, over R$ 68 billion were spent by healthcare plan operators in 2012. Due to legal requirements, ANS assumed the task of upgrading, every two years, the List of Health Procedures and Events, a reference guide for minimum mandatory coverage. In this context, the need to assess the incorporation of new medical technologies is included; besides, an economic analysis is of major importance for the inclusion of new procedures in the list of mandatory coverage.
For Norman Daniels,20 the decisions on the allocation of financial resources in health consist of determining what kind of health services will exist in a society, who will receive them and on what basis, and who will provide these services; also, how their funding will be distrubuted, and how the power and control of these services will be distributed. In Brazil, this decision is of the responsibility of the Ministry of Health. Regarding the incorporation of new technologies, since 2012 this task has been the responsibility of the National Commission on Technology Incorporation (Comissão Nacional de Incorporação de Tecnologias, CONITEC), whose decisions are taken based on the relevance and impact of the implementation of technology in the health system, as well as the existence of scientific evidence of efficacy, accuracy, effectiveness, safety, and economic feasibility studies on the proposed technology, compared to other previously incorporated technologies.
In the sphere of private healthcare, even before the creation of CONITEC, the ANS already had, by legal determination, the power to update the list of mandatory coverage of medical procedures and, therefore, to determine the incorporation of medical technologies in its area of responsibility.
It should also be emphasized that, in the face of the population aging process, the treatment in question has the potential to benefit a significant portion of Brazilian society. According to ANS, approximately 2.5 million beneficiaries are over 70 years-old. Based on the estimated prevalence of 5% of this population with severe AS, certainly a considerable fraction may be considered as without the prospect of undergoing surgery for correction of the disease and, thus, its members will be candidates for TAVI. In Brazil, it must be emphasized that the Statute of the Elderly determines that this population should be prioritized with respect to the access to public and private services, including the right of access to healthcare.
With respect to patients with severe AS and without prospect of surgical treatment, TAVI – the scenario evaluated in this study – is the only alternative therapy that can alter the natural course of this severe cardiovascular disease, due to the significant reduction of mortality compared to the conservative strategy, as was clearly demonstrated by the PARTNER trial.17 Thus, this therapy has been evaluated and incorporated in several countries. In Brazil, although the technique has been available since 2008, with registration by the National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) of the first valve prosthesis for transcatheter implantation, there is no provision of of mandatory coverage in the national health system, which results in the exclusion of significant portion of these elderly patients from the benefit of a scientifically appropriate treatment.
As demonstrated in this study, the ICER of TAVI ranges between R$ 49,770 and R$ 85.957/year of life saved, varying mainly due to the price of the prosthesis used. This value, however, is below the limit usually accepted as a criterion for incorporation, which is US$50,000.21 The budgetary impact of the procedure is considerable, mainly due to the price of the valve prosthesis. However, since 2011, when the List of Procedures of ANS for 2010 was updated and published through the Normative Resolution 256, it can be observed that there was a significant reduction in the price of this prosthesis, of approximately 40%, compared to the reference value used in this study, and this fact should substantially reduce the expected impact. In addition, the authors emphasize that currently there are three similar prostheses with ANVISA registration and one of them is manufactured in Brazil; this fact may become even more favorable to the ICER of TAVI.
The main limitations of the economic model developed are those customary for similar studies in this country, namely the lack of local epidemiological data, the difficulty of collecting the costs, as well as the difficulty of projecting the procedure use based on a expert panel. However, these limitations are inherent to any economic study conducted in Brazil and, considering that its function is to provide a decision basis that allows the modern manager to be prepared for future funding of the procedure; this study provides important information that should be considered in the process of adoption of the technology in question.
The Brazilian guidelines published by the Ministry of Health21 with recommendations for Technology Assessment in Health, do not define a threshold ICER eligible for incorporation, unlike European countries, Canada, and Australia. However, on May 21, 2012, the Secretary of Healthcare of the Ministry of Health published in the Official Gazette a decree (SAS Decree #458) with reference to the value of US$ 50,000 as the upper limit of the ICER. Using this value as a reference, the TAVI procedure would be cost-effective for the Brazilian scenario, considering the limit of R$ 100,000, at a current exchange rate of approximately US$ 1.00/R$ 2.00. Considering the value for the ICER found in this study, is it wise to emphasise that other technologies have been incorporated by the private healthcare system with similar cost increment, with the recent example of implantable cardiac defibrillators.
Finally, it should be noted that, due to the absence of mandatory coverage for TAVI, this situation has fostered a growing judicialization in this context, aiming at the implementation of the right to receive such treatment with culminated effects. Thus, through the courts, healthcare plan operators are being forced to bear these additional costs, and in a worst-case scenario from the financial perspective. Therefore, the value of the ICER calculated in this study actually overestimates the real budgetary impact of its incorporation in the realm of private healthcare, considering that resources are already effectively being allocated for this purpose.
CONCLUSIONSSevere aortic stenosis is a disease that requires a substantial expenditure from the private healthcare system. The only treatment required in the sphere of the private healthcare system – balloon aortic valvuloplasty – does not alter the natural history of the disease. In contrast, patients treated with TAVI benefit from longer life expectancy, when compared to patients who undergo only conservative treatment. Furthermore, almost all casesusing TAVI, when compared to standard treatment, demonstrate greater effectiveness and incremental cost, evidencing its robustness in the result of economic analysis.
Additionally, the budgetary impact analysis showed that the incorporation of the transcatheter aortic valve implantation in the List of Health Procedures and Events of the National Supplemental Health Agency (Agência Nacional de Saúde Suplementar), in the form as evaluated in this trial, would involve an estimated incremental budgetary impact over the next five years between R$ 70 million and R$ 121 million, consistent with other technologies already incorporated in the private healthcare system.
CONFLICTS OF INTERESTMarcelo C. Queiroga received institutional aid and is a lecturer for Medtronic. Fábio Júnior Sandoli Brito is a speaker and proctor for Edwards Life Sciences and Medtronic. Rogério Sarmento-Leite is speaker and proctor for Medtronic. The other authors declare to have no conflicts of interest related to this manuscript.