The wide morphological variety of coarctation of the aorta (CoA) and some complications resulting from the implantation of conventional stents has made the utilization of covered stents (CS) desirable. We describe our experience with the use of CS to treat CoA in children and adults.
MethodsThe records of patients that received CS were retrospectively reviewed. The procedures were performed according to the established technique. Use of CS as primary treatment were assessed, as well as those deployed due to complications resulting from the initial procedure.
ResultsBetween 2007 and 2014, CS were used in 14 patients, 9 (64.3%) of whom were males. The mean age was 19.5 ± 10.5 years, and the mean weight 61.7 ± 25.5kg. Bicuspid aortic valve was present in 74% of cases, and two patients had patent ductus arteriosus. Subatretic aortic coarctations were found in five patients. Eleven patients had systemic arterial hypertension, and 73% had normalized blood pressure levels after stent dilation. Implantation was possible in all cases. Primary implants were performed in ten (71.4%) patients with native coarctation and in four patients as a second device to correct problems originating from previous procedures. The mean time of follow-up was 51.7 ± 29.8 months. Three minor complications were related to procedures, and there were no deaths.
ConclusionsThe use of CS was safe and effective in this small case series. Further studies focusing on the long-term evolution and the possibility of CS redilation are needed to support its use in children.
A ampla variedade morfolo¿gica das coarctações da aorta (CoA) e algumas complicac¿o¿es derivadas do implante de stents convencionais tornam desejáveis a utilização de stents recobertos (SR). Descrevemos a experie¿ncia com o uso de SR para tratar CoA em crianc¿as e adultos.
MétodosForam revisados, retrospectivamente, os registros dos pacientes nos quais foram utilizados os SR. Os procedimentos foram realizados segundo a técnica consagrada. Foram estudados casos em que os SR foram utilizados como primeiro tratamento e também aqueles realizados em complicações derivadas do procedimento inicial.
ResultadosEntre 2007 e 2014, foram utilizados SR em 14 pacientes, sendo 9 (64,3%) do sexo masculino. A média de idades foi 19,5 ± 10,5 anos, e a média dos pesos, 61,7 ± 25,5kg. Valva aórtica bicúspide estava presente em 74% dos casos, e dois apresentaram persistência do canal arterial. Coarctações subatréticas foram encontradas em cinco pacientes. Onze pacientes apresentaram hipertensão arterial sistêmica, e 73% normalizaram as cifras tensionais após a dilatação com stents. O implante foi possível em todos os casos. Foram realizados implantes primários em dez (71,4%) pacientes portadores de coarctações nativas e em quatro como segundo dispositivo, para corrigir problemas derivados de procedimentos anteriores. O tempo médio de seguimento foi de 51,7 ± 29,8 meses. Três complicações menores estiveram relacionadas aos procedimentos, e não houve registro de óbito.
ConclusõesO uso de SR foi seguro e eficaz na nossa pequena série de casos. Mais estudos, enfocando a evolução de longo prazo e a possibilidade de redilatação dos SR, são necessários para corroborar seu uso em crianças.
The treatment of coarctation of the aorta (CoA) has been evolving over time, and balloon angioplasty has become a less invasive alternative to surgery.1
Recently, the use of balloon-expandable stents has become the treatment of choice for CoA in adolescents and adults.2,3 The wide morphological variety of CoA and some complications resulting from the utilization of conventional stents has made the use of covered stents (CS) desirable.4,5
In this study, the authors describe their experience with the CS to treat CoA in children and adults. They also discuss their indications for use in native lesions and their applications in addressing some complications of this procedure.
MethodsThe records of all patients referred for percutaneous treatment of CoA in whom CS were used were retrospectively analyzed. All procedures were conducted under general anesthesia and orotracheal intubation, after a minimum fasting period of 8hours.
Heparin was administrated at doses of 100 IU/kg in children and 5,000 to 10,000 IU in adults, after obtaining the arterial access. Antibiotic prophylaxis with cefazolin (50mg/kg in children or 2g in adults) was routinely administered. Patients underwent left heart catheterization by femoral artery access.
Lesions were retrogradely crossed using a pre-shaped catheter for the right coronary (JR), into which a straight-tip hydrophilic guidewire was advanced. Once the ascending aorta was reached, pressures were recorded above and below the lesion, and the gradient was obtained.
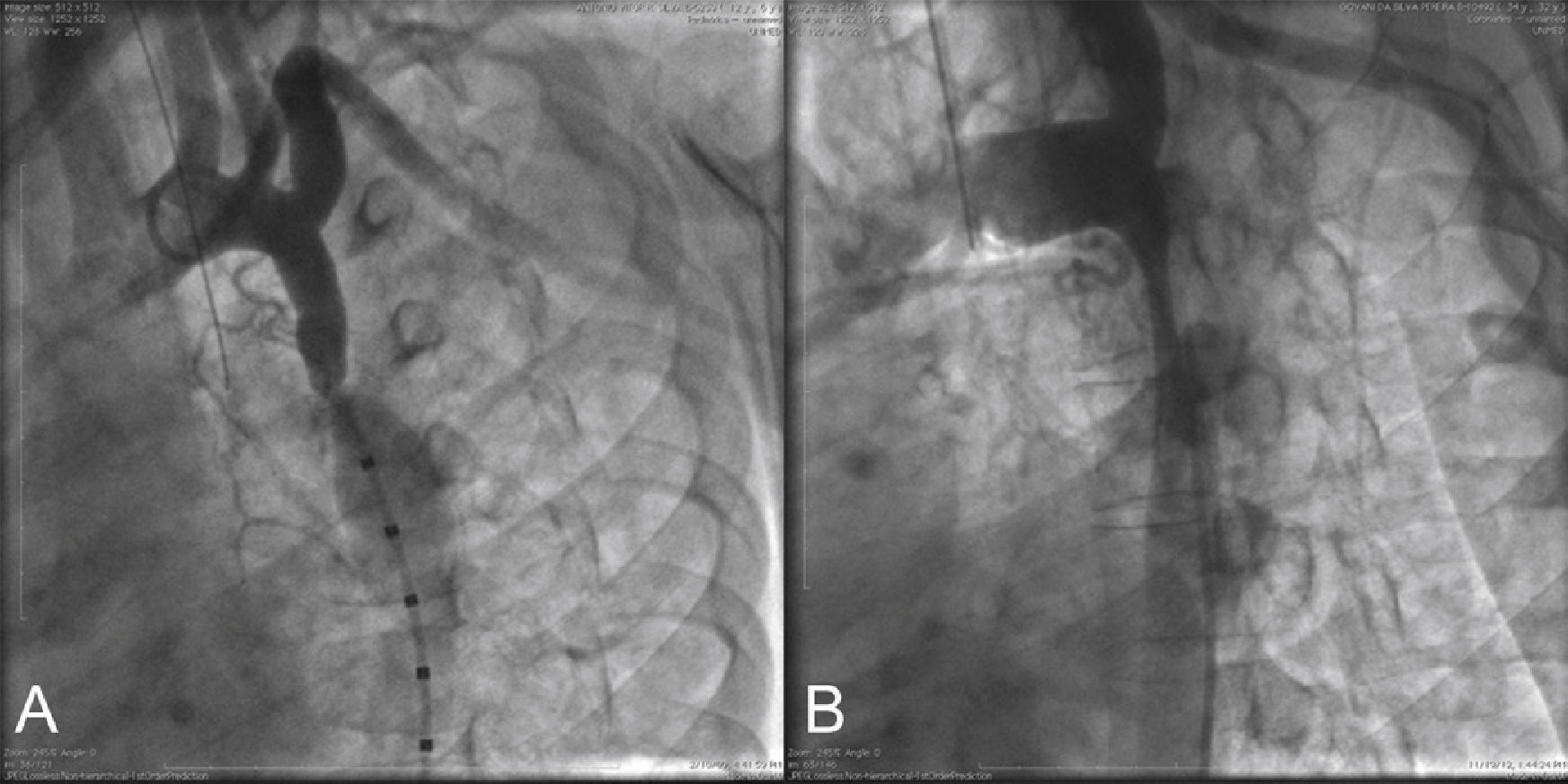
Using a calibrated pigtail catheter, a panoramic aortography was performed in the left anterior oblique projection at 30°, and a descending aortography was performed in the following projections: left anterior oblique at 60°, right anterior oblique, and left lateral. In some cases, a small caudal angulation was added for better visualization of the obstruction site (Fig. 1).
Descending aortography in the left projection. In (A), there is a gradual reduction in the aorta diameter, from the origin of the left subclavian artery, ending in the focal coarcted area at the end of the aortic isthmus (tubular hypoplasia). Note the post-stenotic dilation of the descending aorta. In (B), severe segmental coarctation is observed, encompassing the entire aortic isthmus. The reduced caliber of the descending aorta can be observed, which does not present the usual post-stenotic dilation.
After the angiography, the transverse aorta diameters between the carotid arteries, the isthmus region at the origin of the left subclavian artery, the obstruction site, and the descending aorta below the lesion were measured. The length was measured from the base of the left subclavian artery to the descending aorta region, in order to completely encompass the coarcted area.
After lesion documentation, an exchange guidewire, rigid or super-rigid, was positioned in the left subclavian artery or ascending aorta above the valve. Over it, a long sheath with a gauge of at least 3 F greater than the nominal balloon catheter caliber was introduced and advanced beyond the obstructed area.
Through the sheath, the chosen CS was advanced, manually mounted on a balloon with a diameter of 1 to 2mm larger than that of the isthmic region or the transverse aorta, between the carotid arteries, measuring 1 to 2cm longer than the stent length. The authors chose stents whose length were close to that obtained by the angiographic measurement of the distance from the lower border of the transverse aorta until the descending aorta was reached, in order to place the affected region in its central portion.
The balloon/stent set was advanced over the guidewire and positioned with the aid of contrast injections through the lateral access of the long sheath. Then, the balloon was inflated by manual inflators using pressures lower than the rated burst pressure. Correct apposition of the CS to the aortic wall was checked and the balloon was completely deflated and removed through the interior of the sheath, carefully so as not to displace the exchange guidewire. The pigtail catheter was reintroduced over the guide and the record of the pressures above and below the CS was performed. Control angiograms were performed using the same previous projections.
New dilations, with balloons of greater diameter than the original one, were performed when stent expansion was considered insufficient or adequate apposition of the upper end of the device to the aortic walls was not achieved. At the end of the procedure, hemostasis was performed by manual compression or with the use of auxiliary hemostatic systems, when available.
Patients were referred to the intensive care unit, where they remained under observation until the following day, undergoing electrocardiographic and blood pressure monitoring. They were discharged after undergoing transthoracic echocardiograms.
Follow-up with transthoracic echocardiography was conducted after 1, 3, and 6 months. After 6 months, thoracic aorta CT angiographies were performed to verify the adequate CS apposition to the aortic wall, as well as to rule out the presence of aneurysms or dissections. A stress test was also requested to assess the blood pressure curve during exercise.
Types of stents usedThe Advanta V12 CS (Atrium Medical – Hudson, USA) are made of a laser-cut stainless steel structure with a double cover of expanded polytetrafluoroethylene (e-PTFE), placed outside and inside of the stent metal structure. The stents have an open-cell geometry and are mounted on the balloon by the manufacturer. They are marketed at lengths of 29, 41, and 61mm, mounted on balloons with 12, 14, and 16mm of diameter. They can be dilated to a maximum diameter of 22mm and their shortening rate is approximately 25%.6
The Cheatham Platinum CS (Numed – Hopkinton, USA) are made of a platinum-iridium alloy wire, shaped and welded into a cylindrical form. They are covered only on their external surface by an e-PTFE membrane. The devices have a circumference consisting of eight diamond-shaped cells, called “8 zigs”. The unexpanded cell measures 11mm. Other cell cylinders can be longitudinally welded to the initial one, increasing the stent length. The minimum recommended diameter for dilation is 8mm and the maximum, 24mm, with a nominal shortening rate of 20%. From December 2002 onwards, the welds were reinforced with gold.7
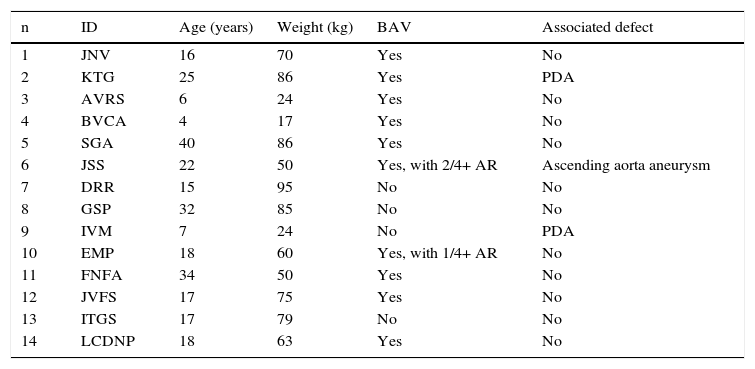
ResultsFrom September 2007 to December 2014, CS were used in 14 patients, of whom nine were males (64.3%), with a mean age of 19.5 ± 10.5 years (range 4 to 40 years), and mean weight of 61.7 ± 25.5kg (ranging from 17 to 95kg).
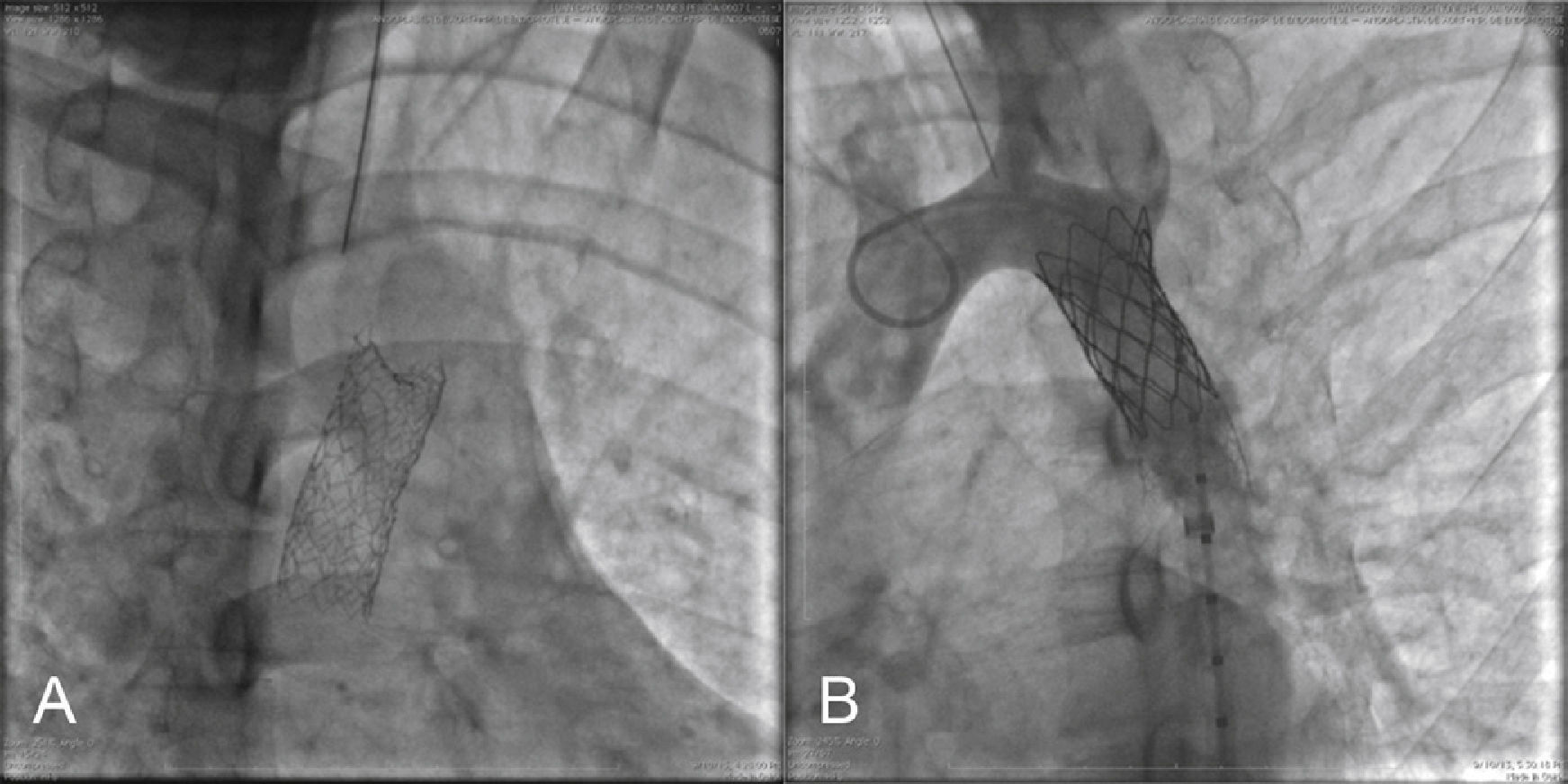
Ten patients (71.4%) had associated bicuspid aortic valves. Only two had aortic regurgitation − mild (1/4 +) in one case and moderate (2/4 +) in another one, which also had an ascending aortic aneurysm (Case 6). Two patients had patent ductus arteriosus (PDA; Cases 2 and 9; Fig. 2).
In all patients, the CoA was located in the usual region, at the end of the aortic isthmus. Only one patient had segmental hypoplasia after the left subclavian artery origin. The lesion was focal in the other cases (Table 1).
Demographic data.
| n | ID | Age (years) | Weight (kg) | BAV | Associated defect |
|---|---|---|---|---|---|
| 1 | JNV | 16 | 70 | Yes | No |
| 2 | KTG | 25 | 86 | Yes | PDA |
| 3 | AVRS | 6 | 24 | Yes | No |
| 4 | BVCA | 4 | 17 | Yes | No |
| 5 | SGA | 40 | 86 | Yes | No |
| 6 | JSS | 22 | 50 | Yes, with 2/4+ AR | Ascending aorta aneurysm |
| 7 | DRR | 15 | 95 | No | No |
| 8 | GSP | 32 | 85 | No | No |
| 9 | IVM | 7 | 24 | No | PDA |
| 10 | EMP | 18 | 60 | Yes, with 1/4+ AR | No |
| 11 | FNFA | 34 | 50 | Yes | No |
| 12 | JVFS | 17 | 75 | Yes | No |
| 13 | ITGS | 17 | 79 | No | No |
| 14 | LCDNP | 18 | 63 | Yes | No |
ID: patient identification; BAV: bicuspid aortic valve; PDA: patent ductus arteriosus; AR: aortic regurgitation.
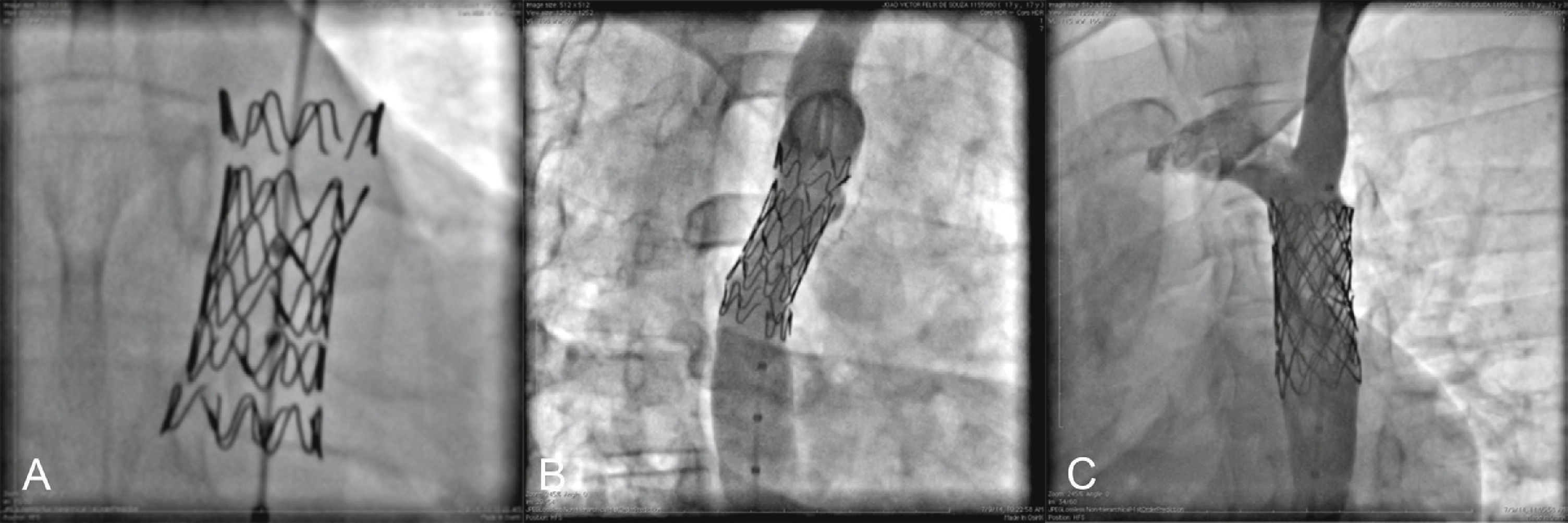
The mean diameter in the CoA region was 6.2 ± 4.7mm (range 1.7 to 15mm). The lesion was considered subatretic (less than 3mm in diameter) in five cases (Fig. 3).
Before the procedure, 11 patients (78.5%) had hypertension and were receiving different treatments prescribed by their attending physicians. After the stent implantation, eight patients normalized their blood pressure levels at rest and on exertion, and anti-hypertensive medication cessation was recommended.
Stent deployment was possible in all cases. Primary implantations were carried out in ten (71.4%) patients with native coarctation. No lesion pre-dilation was necessary in any case. In four cases (Cases 11-14), a second device was implanted to correct problems arising from previous procedures.
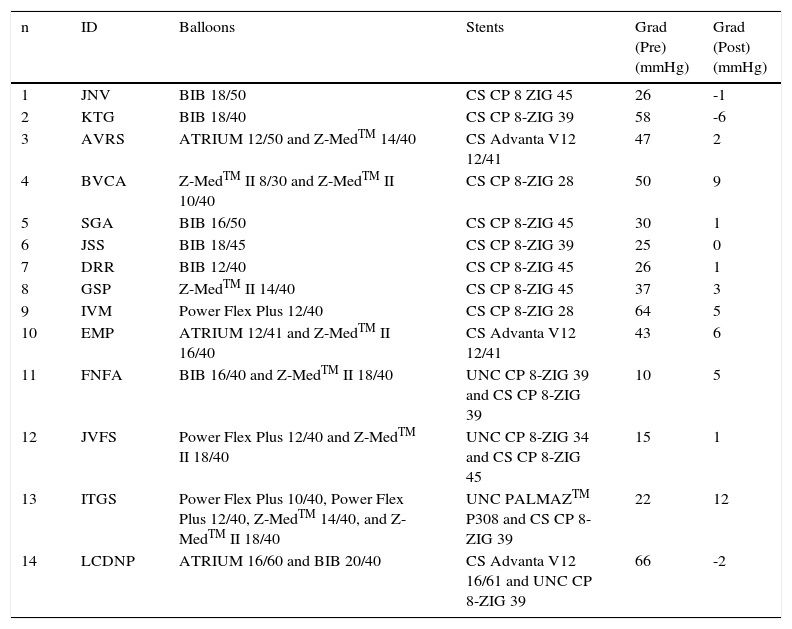
Eighteen stents were deployed in 14 patients. The devices used are detailed in Table 2.
Balloons and stents used.
| n | ID | Balloons | Stents | Grad (Pre) (mmHg) | Grad (Post) (mmHg) |
|---|---|---|---|---|---|
| 1 | JNV | BIB 18/50 | CS CP 8 ZIG 45 | 26 | -1 |
| 2 | KTG | BIB 18/40 | CS CP 8-ZIG 39 | 58 | -6 |
| 3 | AVRS | ATRIUM 12/50 and Z-MedTM 14/40 | CS Advanta V12 12/41 | 47 | 2 |
| 4 | BVCA | Z-MedTM II 8/30 and Z-MedTM II 10/40 | CS CP 8-ZIG 28 | 50 | 9 |
| 5 | SGA | BIB 16/50 | CS CP 8-ZIG 45 | 30 | 1 |
| 6 | JSS | BIB 18/45 | CS CP 8-ZIG 39 | 25 | 0 |
| 7 | DRR | BIB 12/40 | CS CP 8-ZIG 45 | 26 | 1 |
| 8 | GSP | Z-MedTM II 14/40 | CS CP 8-ZIG 45 | 37 | 3 |
| 9 | IVM | Power Flex Plus 12/40 | CS CP 8-ZIG 28 | 64 | 5 |
| 10 | EMP | ATRIUM 12/41 and Z-MedTM II 16/40 | CS Advanta V12 12/41 | 43 | 6 |
| 11 | FNFA | BIB 16/40 and Z-MedTM II 18/40 | UNC CP 8-ZIG 39 and CS CP 8-ZIG 39 | 10 | 5 |
| 12 | JVFS | Power Flex Plus 12/40 and Z-MedTM II 18/40 | UNC CP 8-ZIG 34 and CS CP 8-ZIG 45 | 15 | 1 |
| 13 | ITGS | Power Flex Plus 10/40, Power Flex Plus 12/40, Z-MedTM 14/40, and Z-MedTM II 18/40 | UNC PALMAZTM P308 and CS CP 8-ZIG 39 | 22 | 12 |
| 14 | LCDNP | ATRIUM 16/60 and BIB 20/40 | CS Advanta V12 16/61 and UNC CP 8-ZIG 39 | 66 | -2 |
ID: patient identification; Grad: gradient across the aortic coarctation region; BIB: balloon-in-balloon; CS: covered stent; UNC: uncovered stent.
One patient (Case 4) required hyperexpansion of the stent with a second balloon with a diameter 2mm greater than the previous one, to attain the correct apposition of the device to the aortic wall in the same procedure.
Four patients had clinical and echocardiographic signs of recoarctation. In the first three cases (Cases 3, 10, and 14) Advanta V12 CS were implanted. Angiographies performed in subsequent cardiac catheterizations showed that in two patients (Cases 3 and 10), both devices showed good apposition to the aortic wall, but disclosed intimal proliferation inside and were redilated with balloon, obtaining satisfactory results (Fig. 4).
In the third case (Case 14), the restenosis was caused by collapse of the upper border of an Advanta V12 16/61 CS, approximately 1 year after the implantation performed in another hospital. Dilation was performed with the successful implantation of a second device, a Cheatham Platinum 8 ZIG-39 uncovered stent (Fig. 5).
The other case (Case 11) had recoarctation due to fracture of a Cheatham Platinum uncovered stent, discovered 7 years after implantation. It was treated with dilation and implantation of a new Cheatham Platinum 8-ZIG 39 CS inside the first one.
Late aneurysm formation was observed in two patients. In one (Case 12), there was a circumferential fracture in the first and final weld lines of a Cheatham Platinum 8-ZIG 34 uncovered stent. A posterior-superior strut fracture resulted in the formation of a small aneurysm. In the second patient (Case 13), the aneurysm was formed during the PALMAZTM P308 uncovered stent implantation, whose borders were sharp. Treatment was successful in both cases, with implantation of the Cheatham Platinum 8 ZIG-45 CS and Cheatham Platinum 8-ZIG 39 CS, respectively (Figs. 6 and 7).
In (A), detail of a Cheatham Platinum uncovered stent implanted 13 years ago. Note the circumferential fracture of the first and last row of “zigs”. In (B), descending aortography in the right anterior artery, showing a dissection line with a small aneurysm in the region of the first row of fractured weld. In (C), control aortography after implantation of a Cheatham Platinum covered stent with aneurysm exclusion.
The mean diameter of the balloons used in the procedure was 15.2 ± 3.1mm (ranging from 12 to 20mm). The mean systolic gradient through the obstructed area was reduced from 34.2 ± 16.0mmHg (ranging from 10 to 64mmHg) to 2.5 ± 4.6mmHg (ranging from -6 to 12mmHg) after stent implantation. The mean diameter of the coarcted segment increased from 6.2 ± 4.7mm to 15.6 ± 2.8mm.
The mean time of follow-up was 51.7 ± 29.8 months (ranging from 2 to 88 months).
There were three complications related to procedures: an arterial stenosis at the site of a long sheath insertion, which required surgical repair (Case 4), one pseudoaneurysm treated by local compression (Case 6), and a case of right upper limb paresthesia due to hyperextension of the brachial plexus during the procedure (Case 14). There were no deaths in this series.
DiscussionBalloon-expandable stents, covered by a layer of e-PTFE, have been used for the treatment of CoA.8–12 The most commonly used are the Cheatham Platinum CS and the Advanta V12 LD. They are usually indicated in the following situations:13 patients older than 30 years; in cases of critical obstruction (diameter < 3mm in the region of the CoA); when there is atresia of the aortic lumen; when the CoA is associated with PDA; when there are significant degenerative changes in the aortic wall; when it is necessary to perform aneurysm exclusion or treat aorta dissections; in case of circumferential fracture with misalignment between the proximal and distal portions of a previously implanted stent; and in cases of partial or circumferential stent fracture, with strut protrusion against the aortic wall.
In the Platinum Cheatham CS, the e-PTFE membrane is sutured to the external area of the struts. This device must be mounted manually on the delivery balloon. Great care must be taken in order not to damage the outer membrane, which cannot be wetted and should be handled with dry and clean gloves. To avoid dislodging the cover, it is necessary to prevent excessive friction with the introducer sheath, which must have a caliber at least 3 F higher than the sheath recommended for the balloon use. This can be a limiting factor for the use of the Cheatham Platinum CS in children or small patients, with smaller-caliber arteries. When crossing the hemostatic valve of the long sheath, one of the supplied protection cylinders must be used together with the stent. If not available, they can be manufactured from a segment cut from a sheath of the same diameter used for the stent deployment. It would be desirable for the Cheatham Platinum CS to come pre-assembled by the manufacturer, which would reduce the time and increase the safety of the procedure, allowing its use in a larger number of small patients and even in children.
As they are non-permeable cylinders, due to the e-PTFE cover, the CS must show complete apposition into the aortic wall in the cephalic region of the isthmus and must not be positioned above the lower border of the transverse aorta. The presence of free space between the stent borders and the aortic wall would create a sac where the high speed flow of the descending aorta could displace the stent or even cause its collapse, resulting in obstruction, as reported in Case 14.14,15 The expansion of the lower border to the diameter of the dilated descending aorta does not seem necessary.
Redilation of the CS, although possible, is avoided by some authors; therefore, its use should be carefully evaluated in children.16–18
Aortic dissection and rupture are important reasons for the emergency use of CS, which should be available for the rescue of the more severe cases.19
There is a small risk of spinal artery occlusion with the use of CS, although it originates well below the coarctation level. Additionally, it has not been a significant problem when using endoprosthesis for the treatment of major thoracic aortic aneurysms, occurring in only 1 to 4% of cases.20
It is important to be careful to avoid unintentional exclusion of important vessels, such as the subclavian and carotid arteries, when using CS.
ConclusionsThe use of covered stents was safe and effective in this small case series. A reduction in the diameter of the long sheaths would be desirable. Further studies focusing on the long-term evolution and the possibility of redilation of covered stents, are needed to support their use elective, in children.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.