Preload dynamic tests, pulse pressure variation (PPV) and stroke volume variation (SVV) have emerged as powerful tools to predict response to fluid administration. The influence of factors other than preload in dynamic preload test is currently poorly understood in pediatrics. The aim of our study was to assess the effect of tidal volume (VT) on PPV and SVV in the context of normal and reduced lung compliance in a piglet model.
Material and methodTwenty large-white piglets (5.2±0.4kg) were anesthetized, paralyzed and monitored with pulse contour analysis. PPV and SVV were recorded during mechanical ventilation with a VT of 6 and 12mL/kg (low and high VT, respectively), both before and after tracheal instillation of polysorbate 20.
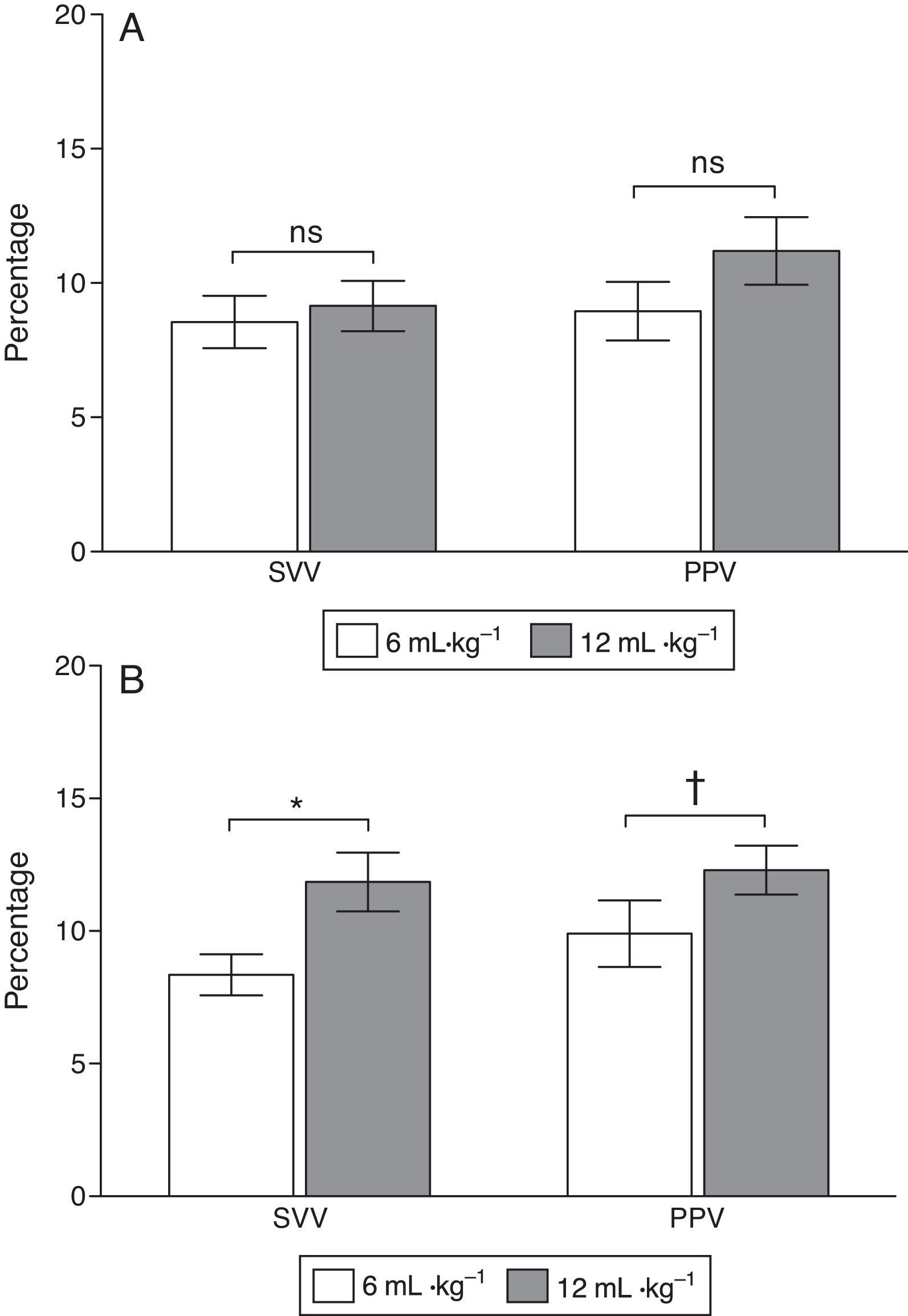
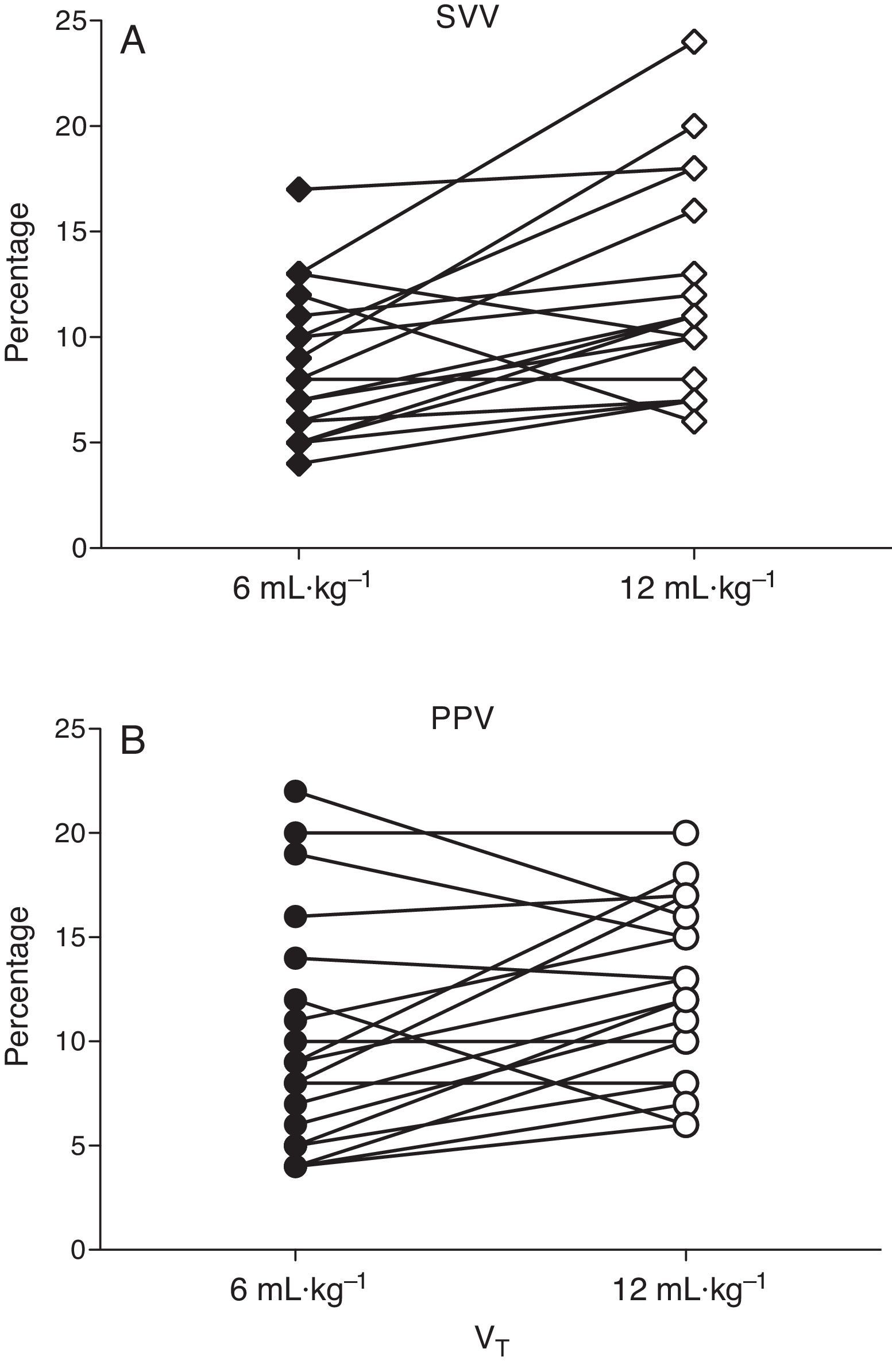
ResultsBefore acute lung injury (ALI) induction, modifications of VT did not significantly change PPV and SVV readings. After ALI, PPV and SVV were significantly greater during ventilation with a high VT compared to a low VT (PPV increased from 8.9±1.2 to 12.4±1.1%, and SVV from 8.5±1.0 to 12.7±1.2%, both P<0.01).
ConclusionsThis study found that a high VT and reduced lung compliance due to ALI increase preload dynamic tests, with a greater influence of the latter. In subjects with ALI, lung compliance should be considered when interpreting the preload dynamic tests.
Test dinámicos de precarga, variación de presión de pulso (PPV) y variación de volumen sistólico (SVV) han emergido como herramientas poderosas para predecir respuesta a la administración de fluidos. Actualmente la influencia de factores distintos a la precarga en la determinación de los test dinámicos de precarga es pobremente conocida en pediatría. Nuestro objetivo fue medir el efecto del volumen tidal (VT) sobre PPV y SVV en un contexto de compliance pulmonar normal y disminuida en un modelo porcino.
Material y métodoVeinte cerditos Large-White anestesiados y paralizados (5,2±0,4kg). PPV y SVV fueron medidos por análisis de contorno de pulso durante ventilación con VT de 6 y 12mL/kg (VT bajo y alto, respectivamente), ambos previo y posterior a lesión pulmonar aguda (ALI) químicamente inducida con instilación traqueal de polisorbato 20.
ResultadosPrevio a inducción de ALI, PPV y SVV no tuvieron cambios significativos al modificar el VT. Sin embargo, después de ALI, PPV y SVV fueron significativamente mayores durante ventilación con VT alto, respecto a VT bajo (PPV aumentó de 8,9±1,2 a 12,4±1,1%, y SVV de 8,5±1,0 a 12,7±1,2%, ambos P<0,01).
ConclusionesEste estudio encontró que un VT alto y una compliance pulmonar disminuida debido a ALI incrementan los test dinámicos de precarga, con una mayor influencia de esta última. En sujetos con ALI la compliance pulmonar debiera ser considerada al interpretar los test dinámicos de precarga.
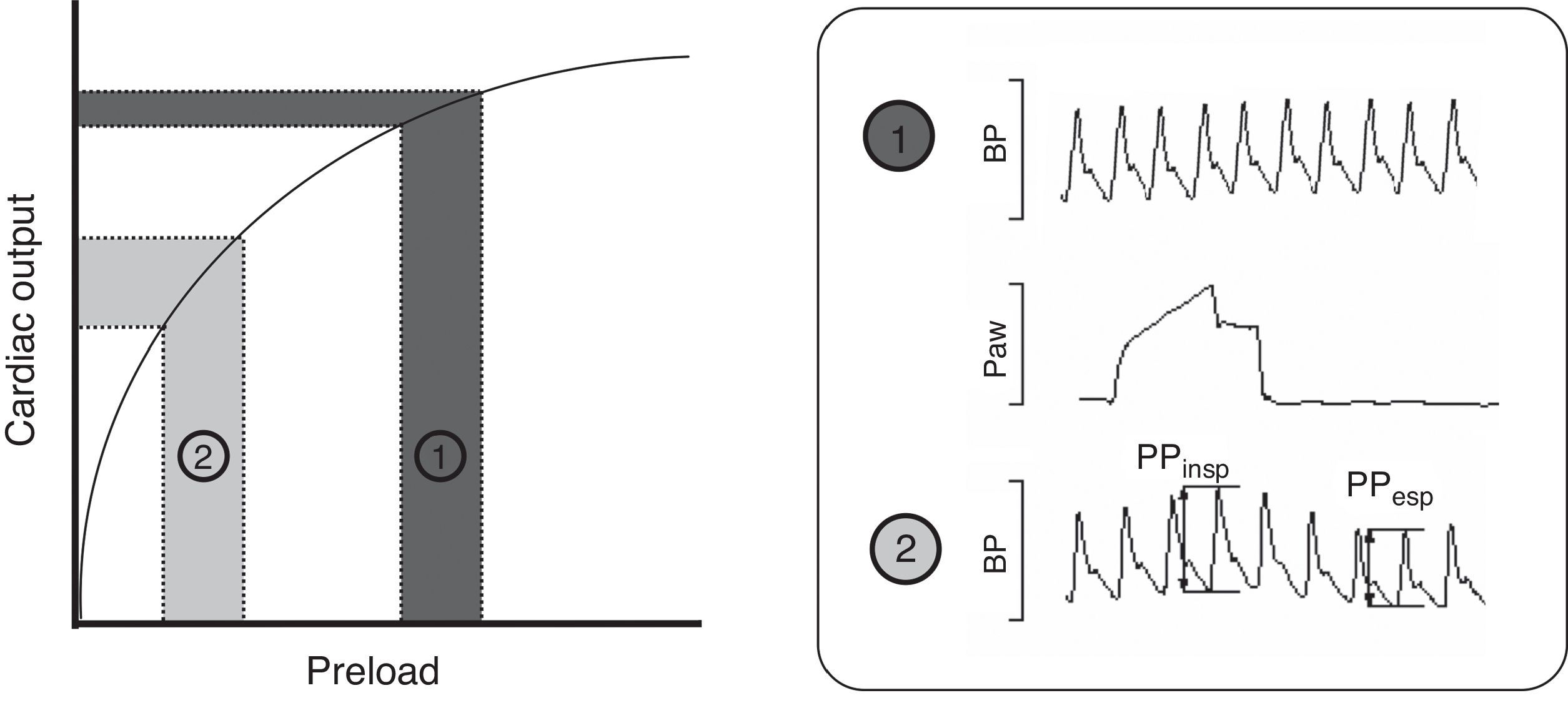
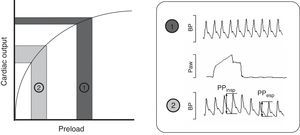
Intravenous fluid administration is a common therapy in critically ill patients, serving as the cornerstone of initial treatment in many conditions. Recent data suggest that fluid management has a major impact on the severity and outcome of critical illness.1 Knowing that excessive administration of fluids and development of fluid overload are associated with negative effects, the main question when deciding fluid administration is whether there will be beneficial effect on hemodynamics, specifically on cardiac output (CO). Preload dynamic tests, pulse pressure variation (PPV) and stroke volume variation (SVV) have emerged as powerful tools to predict response to fluid administration in different clinical settings.2–5 Many clinical and experimental studies have determined that PPV >10% and SVV >13% are associated with a significant increase in CO after fluid administration, thus predicting fluid responsiveness. These tests are based on the Frank–Starling Law, which describes the relationship between preload (end-diastolic volume or pressure) and stroke volume. As shown in Fig. 1 two zones can be identified: a steep portion of the curve where changes in preload produce significant changes in CO, and a flat portion of the curve where changes in preload do not significantly change CO (dark gray). Periodic changes in ventricular filling pressures (i.e. preload) occur due to cyclical positive pressure administered during mechanical ventilation. Cyclic changes in stroke volume are greater when the ventricles operate on the steep rather than flat portion of the Frank–Starling curve. Preload dynamic test can identify this particular situation and predict response to fluid administration.6 Of note, due to complex cardiopulmonary interactions, preload dynamic indices do not depend only on cardiac preload.7,8 Experimental and small clinical studies have demonstrated that pulmonary and other cardiovascular factors may play a role in the PPV and SVV measurements, though exact mechanisms are not well understood.9–12
Frank–Starling relationship. Once the ventricle is operating near the steep part, volume expansion induces a significant increase in cardiac output. The pulse pressure (PPV) and stroke volume (SVV) variations are marked. By contrast, once the ventricle is functioning on the flat part of the curve, fluid infusion has poor effect on the cardiac output. BP, blood pressure; Paw, airway pressure; PP, pulse pressure.
Acute lung injury (ALI) is a frequent cause of hypoxemia, loss of respiratory system compliance (CRS) and pulmonary edema. Caution has been claimed respect to the usefulness of dynamic preload tests in patients with ALI where due to low CRS the transmission of positive pressure to the vascular compartment of the lungs, great thoracic veins and the heart may be decreased.10 On the other hand, subjects with ALI have a decreased compliance resulting in higher changes in tracheal pressure for a given tidal volume (VT).11 In addition, the standard of care of ALI patients include MV with low VT. This ventilatory strategy may theoretically decrease the pressure transmitted from the airways to the pleural and pericardial spaces, diminishing the variations of SV and PP.12,13 Accordingly, the effect of a decreased VT on preload dynamic tests in ALI is unpredictable.14 Because there are marked differences in respiratory and cardiovascular physiology in children with respect to adults (e.g. chest wall compliance, airway wall compliance and resistance, heart rate, stroke volume, pulmonary and systemic vascular resistance, aortic elastance and compliance, metabolic rate), basis for many age-specific differences in the cardiovascular and metabolic responses to injury, these observations should be carefully examined.15–19
In summary, preload dynamic tests may be an inaccurate measure of filling in cases where CRS or VT are low, even more so considering the particular features pediatric physiology. The aim of this work was to determine the effect of delivered VT size on PPV and SVV in normal and reduced lung compliance conditions in a pediatric ALI model.
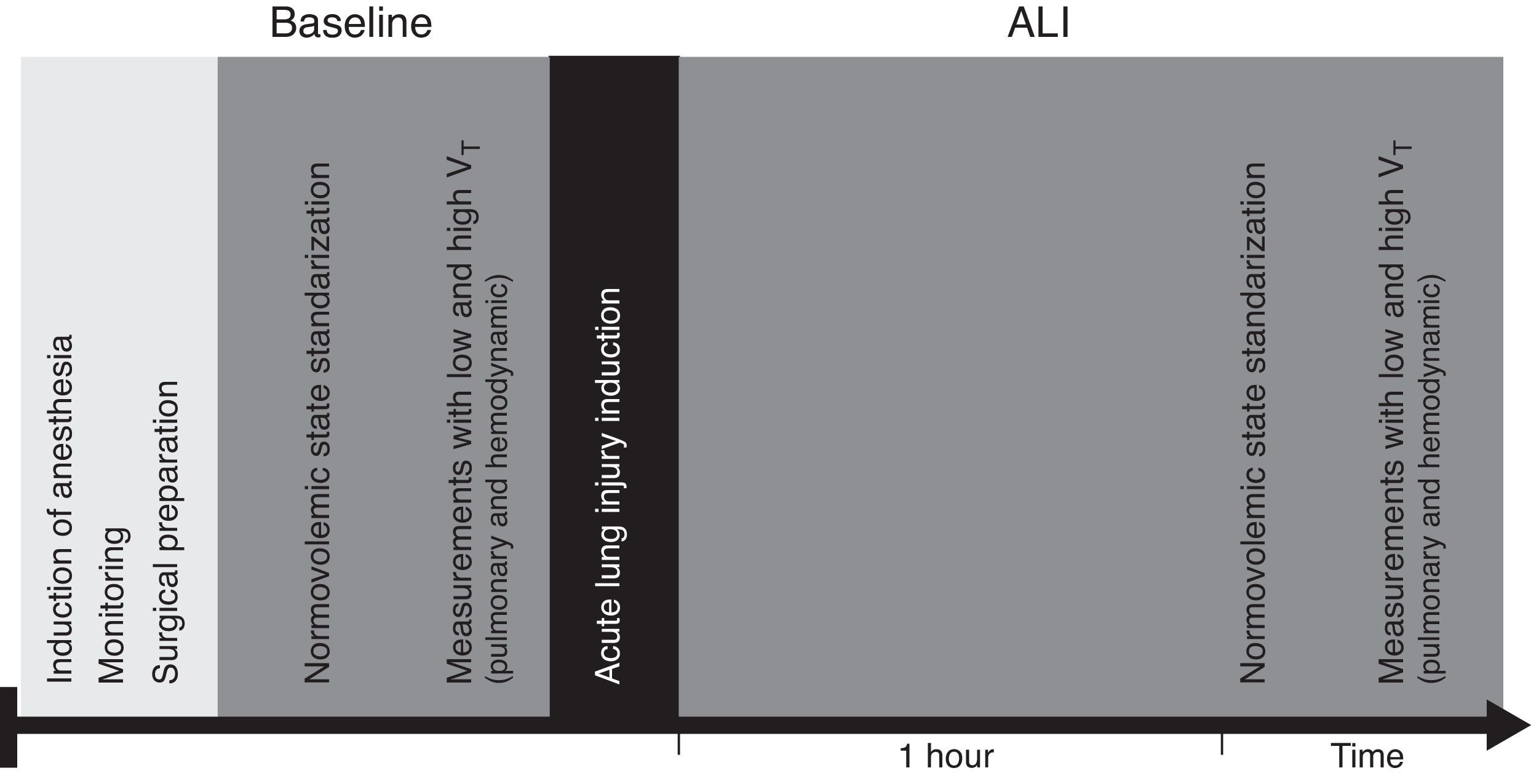
Material and methodThe experimental protocol was approved by Facultad de Medicina Clínica Alemana Universidad del Desarrollo Ethics Committee and the CONICYT (Comisión Nacional de Investigación Científica y Tecnológica) Bioethics Advisory Committee. All of the experimental procedures were consistent with the Guiding Principles in the Care and Use of Laboratory Animals adopted by the American Physiological Society.
SubjectsTwenty anesthetized and mechanically ventilated piglets (5.2±0.4kg).
Surgical preparationThe trachea was cannulated with a 3.5mm tracheostomy tube, the left jugular vein with a 4F catheter and the right axillary artery with a 4F thermistor-tipped catheter (PiCCO® PV2014L08, Pulsion Medical Systems, Munich, Germany), all via cut down. Anesthesia and neuromuscular blockade were maintained by continuous infusion of propofol (10mg/kg/h), fentanyl (4mcg/kg/h) and pancuronium (0.2mg/kg/h) during the experiment. Piglets were ventilated in a volume-controlled mode (EVITA XL®/Capnoplus, Dräger Medical, Lübeck, Germany) with a positive end-expiratory pressure of 5cmH2O, a VT of 9mL/kg, inspiratory time of 0.75s and a FiO2 of 0.5. The respiratory rate was adjusted to maintain the end-tidal carbon dioxide concentration (ET-CO2) at 45±5mmHg. Normal saline at 5mL/kg/h was administered during instrumentation. Heart rate, oxygen saturation, central temperature, mean arterial pressure (MAP) and central venous pressure (CVP) were monitored with an Infinity Delta XL® monitor (Dräger Medical, Lübeck, Germany). Zero pressure was set at the midaxillary line. Temperature was kept at 37.0±0.6°C.
Acute lung injuryAfter surgical preparation, each animal was placed in lateral decubitus and a 10% (v/v) solution of polysorbate 20 (Tween® 20 [polyoxyethylene 20 sorbitan monolaurate], Sigma-Aldrich, MO, USA) in saline (1mL/kg) was instilled in the airway of the dependent lung via a 2-mm catheter. The procedure was repeated with the animal rotated to the opposite side. Residual fluid was suctioned from the airway. Lung injury was targeted to achieve a partial pressure of oxygen (PaO2)<200Torr with FiO2=1 in the supine position at 20min after polysorbate 20 instillation. If this target was not met, polysorbate 20 instillation was repeated:
- (i)
Pulmonary measurements: Arterial blood gases were determined with an i-STAT® System and i-STAT® Cartridges EG6+ (Abbott Laboratories, Princeton, NJ) from blood samples drawn from the arterial catheter. Oxygenation was assessed by the PaO2/FiO2 ratio. Static CRS was calculated as VT/(Ppl−PEEPTOT), where Ppl is plateau pressure measured after a 4-s inspiratory hold and PEEPTOT is total PEEP measured after a 4-s expiratory hold. These variables were recorded from the ventilator display.
- (ii)
Hemodynamic measurements: cardiac output (CO) was calculated by transpulmonary thermodilution and continuous CO was obtained with PiCCO system calibrated at baseline and after ALI induction according to manufacturer's instructions. According to studies in humans at rest and under different types of environmental stress, a functional definition of ‘normovolemia’ would be by its ability to provide the heart with an optimal central blood volume, i.e. that cardiac pumping capacity is not limited by its preload. Functional normovolemia is the point in the cardiac preload/output relationship at which CO does not increase further under circumstances where venous return is unimpeded.20 Normovolemic state was standardized prior registering hemodynamic data at baseline and after ALI induction, with successive 10mL/kg intravenous 6% hydroxy-ethyl starch (HES) boluses until CO did not increase by more than 10%. HES was administered over 10min and personnel deciding fluid administration were blinded for preload dynamic indices.
- (iii)
Preload dynamic indices: PPV and SVV were obtained from the PiCCO® monitor display. According to PiCCO algorithm SVV is calculated from the mean values of four maximum (SVmax) and minimum (SVmin) stroke volumes averaged during the previous 30s (SVmean): SVV=(SVmax−SVmin)/SVmean. PPV is calculated during the same time interval: PPV=(PPmax−PPmin)/PPmean (2.7). PPV and SVV were registered at baseline and 1h after ALI induction, with low VT (6mL/kg) and high VT (12mL/kg). PiCCO® system was calibrated with transpulmonary thermodilution (TPTD) at baseline and after polysorbate 20 instillation and PPV and SVV were registered after 5min of stability (Fig. 2).
At the end of the study period, while under anesthesia, the animals were euthanized by 10% potassium chloride infusion until the detection of ventricular fibrillation or asystole.
Statistical analysisData are expressed in mean±SEM. Analysis of variance for repeated measurements was used for comparison between the preload dynamic indicators with high and low VT. Significance was set at P<0.05.
ResultsAll subjects completed the experimental protocol. After polysorbate 20 instillation, a significant decrease in PaO2/FiO2 from 345±14 to 155±8Torr and CRS from 1.58±0.13 to 0.93±0.05mL/cmH2O/kg was observed (both P<0.01). Also there was an increase in HR (133±7–162±8bpm, P<0.001) without changes in MAP (74±3–76±3mmHg), CVP (7.6±0.4–7.6±0.6mmHg) and CO (4.1±0.2–4.3±0.3L/min/m2).
Preload dynamic indicesSubjects required 14±2mL/kg at baseline and 25±3mL/kg after ALI induction for volemic status optimization. All subjects maintained sinus rhythm during experiment.
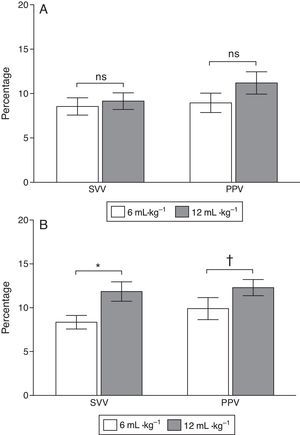
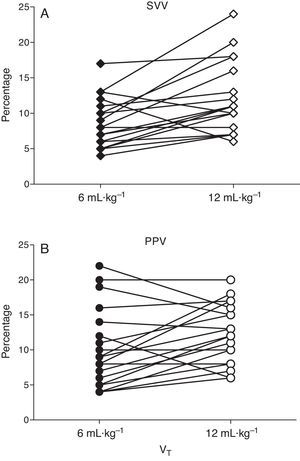
At baseline PPV was 8.9±1.2 and 10.7±1.1% with low and high VT (P=0.058) and SVV was 8.7±1.2 and 9.7±1.1% (P=0.45), respectively. After ALI induction PPV increased from 8.9±1.2 to 12.4±1.1% (P=0.037) and SVV from 8.5±1.0 to 12.7±1.2% (P=0.03) with low and high VT respectively (Figs. 3 and 4).
The main finding of this experimental study is that high VT significantly increased PPV and SVV when CRS was reduced, but not when CRS was normal in subjects with optimized intravascular volume (not hypovolemic).
Preload dynamic indices are produced by the transmission of airway pressure to the pleural and pericardial spaces, determining cyclic changes in venous return, cardiac preload and afterload. Therefore, its clinical use could be theoretically limited in conditions where the airway pressure transmitted to the intrathoracic spaces is low (i.e. low VT and low CRS). Recent studies in anesthetized large animals and critically ill adults reported that VT size influences preload dynamic indices.21,22 Our results differ with previous studies where size of delivered VT has been an indication as the major determinant of preload dynamic indices.10,23 In this model of normovolemic subjects, when VT was applied in a lung with a reduced CRS the effect was more pronounced, resulting in a leftward shift on the Frank–Starling curve and consequently a greater variation of stroke volume and pulse pressure.24 We found that the reduction of CRS was a factor that amplified the effect of VT on preload dynamic tests. In a small series of ARDS patients, Vieillard Baron13 concluded that VT and not the airway pressure itself, was the determinant for the afterload and the RV stroke work, both components of the PPV. However, an interesting prior study in a model of oleic acid-lung injury reported that the influence of lung edema in the transmission of airway pressure to the pleural space depends significantly on the peak volume during the inspiration.25 While most studies used oleic acid-lung injury, where the main pathogenic mechanism is an increase in pulmonary vascular permeability, we used a surfactant deactivation model. It has been documented that tracheal instillation of polysorbate 20 produce high-tension pulmonary edema. Despite these differences, the lung edema in subjects with injured lungs was similar to other models of lung injury.10,12,16,26 Thus, in models of pulmonary edema, transmission of airway pressure to pleural space and vascular structures may be relevant, and influence preload dynamic markers. Accordingly Huang et al. published a clinical series of patients with severe ARDS ventilated with a low VT strategy, finding that PPV maintained a good prediction for volume response. Although the VT reported was approximately 6mL/kg, the mean peak inspiratory pressure was nearly 34 cmH2O, obtaining an at least 20cmH2O driving pressure.27 These findings agree with our results, since the cyclical changes for the arterial pressure and the volume ejection would be secondary to the generated airway pressure and not to the VT absolute value.
Additionally we used a model of increased preload (normovolemia as defined, and corroborated by maintaining cardiac output and volume preload after ALI) so that the ventricle is operating on the flat portion of the Frank–Starling curve, not only is the likelihood of finding a change in PPV and SVV with increased VT reduced, but based on the literature and even in a pediatric model one would not expect the PPV and SVV to change under the condition of VT of 6mL/kg. The finding of no increase in PPV and SVV with VT 12mL/kg differs from that previously reported10,17 and could at least partially be explained by the normovolaemic model used. It would appear that a VT of 12mL/kg more closely resembles that of 10mL/kg rather than 15mL/kg if this study is compared with that by Renner et al.17
This study has some limitations. The measurement of pleural pressure would allow us to determine the chest wall compliance and specifically address its effects on preload dynamic markers, but it was not performed due to technical reasons. Additionally, tachycardia and decreased SV after ALI are also known factors that influence preload dynamic markers.
In conclusion, preload dynamic indexes are powerful tools when deciding fluid administration in critically ill subjects, but other factors, such as VT and CRS, must be considered for correct interpretation. Future clinical studies should address these variables in addition to hemodynamic parameters and preload dynamic markers for fluid management strategies in critically ill children.
FundingThis work was supported by grant Fondecyt 11075041 from CONICYT (Comisión Nacional de Investigación Científica y Tecnológica, Chile) to Pablo Cruces.
Conflicts of interestThis article meets all requirements about informed consent/acknowledgement, ethics committee, funding, animal research and lack conflict of interest, as appropriate.