To evaluate the release of sodium hypochlorite from three different commercial brands of heat-polymerized acrylic resin immersed in water and submitted to mechanical or chemical polishing after disinfection with hypochlorite at different concentrations.
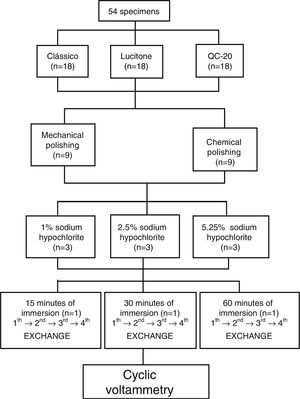
Material and methodsFifty-four disk-shaped specimens (n=18) were made for each resin (Lucitone 550, QC-20, and Classico) and assigned to two groups according to the type of polishing. Specimens were divided in three sub-groups in relation to sodium hypochlorite concentration (1%, 2.5%, and 5.25%), and the groups were immersed for 10-min periods in each sodium hypochlorite concentration. The electrochemical method used for detecting the release of sodium hypochlorite in each specimen was the cyclic voltammetry.
ResultsIn the specimens of Clássico resin polished mechanically and immersed in 5.25% sodium hypochlorite, as well as Lucitone and QC-20 resins immersed in 2.5%, the amounts of disinfectant solution released in the four 15-min water exchanges were higher than the four 60-min exchanges.
ConclusionThere were differences in hypochlorite release from the three commercial brands of denture-base acrylic resins subjected to mechanical polishing. However, no hypochlorite release from the same resins was observed when they were subjected to chemical polishing.
Evaluar la liberación de hipoclorito de sodio de 3 marcas comerciales diferentes de resinas termo-polimerizadas sumergidas en agua, y sometidas a pulido mecánico o químico después de la desinfección con hipoclorito a diferentes concentraciones.
Material y métodosCincuenta y cuatro especímenes en forma de disco (n=18) fueron confeccionados para cada resina (Lucitone 550, QC-20 y Clássico) y asignados a 2 grupos de acuerdo con el tipo de pulido. Las muestras se dividieron en 3 subgrupos en relación con la concentración de hipoclorito de sodio (1, 2,5 y 5,25%), y los grupos se sumergieron durante períodos de 10min en cada concentración de hipoclorito de sodio. El método electroquímico usado para la detección de la liberación de hipoclorito de sodio en cada espécimen fue a través de voltametría cíclica.
ResultadosEn las muestras pulidas mecánicamente de resina Clássico inmerso en hipoclorito de sodio al 5,25%, así como en las resinas Lucitone y QC-20 inmersas a 2,5%, la cantidad de solución desinfectante liberada en los 4 intercambios de agua de 15min fue superior a los 4 de 60min.
ConclusiónHubo diferencias en la liberación de hipoclorito de las 3 marcas comerciales de resinas acrílicas sometidas a pulido mecánico. Sin embargo, no se observó liberación de hipoclorito en las mismas resinas cuando se sometieron a pulido químico.
New dentures and especially those that need repair and adjustments may be contaminated with viruses, bacteria and fungi.1 To prevent the transmission of disease and cross-infection, infection control procedures such as the disinfection of dental prostheses by immersion in a chemical solution must be performed before and after clinical procedures.2 The most appropriate chemicals for disinfection by immersion of dentures are chlorine compounds, aldehydes and peracetic acid. It has been observed that 10min of immersion in 1% sodium hypochlorite, or 2% glutaraldehyde has proved to be effective in reducing the number of microorganisms on the dentures.3 Soaking dentures in sodium hypochlorite is a widely used method of chemical disinfection.3
Hypochlorite has many advantages of the ideal disinfectant, as broad spectrum antimicrobial, rapid bactericidal action, solubility in water, among others. However is irritating to mucous membranes and present deleterious effects on metals1 and moderate toxicity.4
The hypochlorite is considered to have a high antimicrobial activity due to oxidation of proteins in cells by hypochlorous acid; however, this mechanism has not yet been demonstrated experimentally.5 Besides the antimicrobial activity, its tissue-dissolving and detergent actions, and its capacity to neutralize toxic products is recognized.6 When sodium hypochlorite is used as a disinfectant must be carried out a pre-cleaning surfaces process, because the excess of organic material available reacts with chlorine and reduces the effectiveness of the disinfectant, so the prosthesis to be disinfected should be carefully washed before its use.7
Symptomatic inflammatory reactions in oral mucosa in contact with the prostheses are often observed in patients who wear dentures continuously. Several compounds including residual monomer, methyl methacrylate and additives such as hydroquinone, benzoyl peroxide, N,N-dimethyl-p-toluidine and formaldehyde are released from acrylic polymers, diffusing into saliva and when are in contact with the mucosa, causes redness and burning sensation in the adjacent areas.8 Besides these intrinsic components that causes some symptoms, exist the chemical disinfectants that may produce the same reaction. During the prostheses disinfection process, the resin may adsorb water in combination with the disinfectant solutions, which may be released later in the saliva.9 It should be noted that all chlorine products have some level of toxicity, which makes these effective microbicides.10 The potential of sodium hypochlorite in the skin and mucosa irritation may be due to hypochlorite ions (OCl−), sodium hydroxyde (NaOH) or hypochlorous acid (HOCl), seeming to be this last the principal agent involved in causing the irritants effects.11 Sodium hypochlorite may produce hypochlorous acid and chloramines, which are proved that may cause injury to some cells and tissue damage at low concentrations (10–20μM), inducing cell lysis and damaging proteins of cell membranes.12
In the literature it is no proven the hypochlorite action over integral oral mucosa, but there is evidence about ocular, nasal and intestinal mucosa irritation.11 Considering the toxicity and allergic reactions by contact in some patients with hypersensitivity to sodium hypochlorite, is important to consider the disinfection of items during procedures with prosthetic implants, such as direct fabrication on the interim implant restoration.13 In implant therapy, especially in cases of immediate load implants, prosthesis need to be disinfected, but disinfecting substances cannot be released into the wound.
The three main factors that affect in vivo toxicity of disinfectants are: a) bioavailability of residual disinfectant after absorption and washing of instruments and other medical items, b) way by which residual disinfectant enters to the body and c) relative toxicity of disinfectant.4
In the literature, some studies are found about the liberation of denture resin components in saliva,14–17 but have no information about disinfectant solutions liberation to the same. According to Costella et al.,18 water sorption and diffusion coefficient depend of the structure of copolymer, nature of monomer, filler composition, degree of polymerization, crosslinking, environmental temperature, concentration of catalyst and initiator systems and cycle of water sorption, among others.
The aim of this study was to evaluate the release of sodium hypochlorite from three different commercial brands of heat-polymerized acrylic resin immersed in water submitted to mechanical or chemical polishing, after disinfection with hypochlorite at different concentrations. The work hypothesis would be that does not exist differences in sodium hypochlorite release of acrylic resins with mechanical and chemical polishing after chemical disinfection.
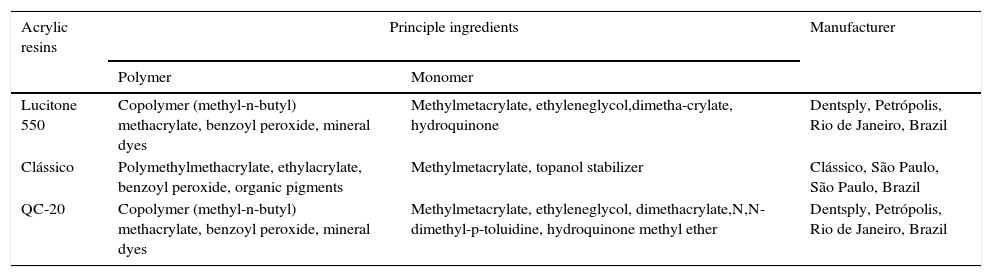
Material and methodsA rectangular metal matrix, with a central opening of 15mm diameter×4mm height, was used to make wax patterns (Wilson; Polidental Manufacturing and Trade Ltd., São Paulo, SP, Brazil). These wax patterns were invested in flasks with dental stone (Gesso Rio, Rio Claro, SP, Brazil), which was coated with a thin layer of sodium alginate (Cel-Lac; SS White Dental Products, Rio de Janeiro, RJ, Brazil). The monomer and polymer of the heat-cured acrylic resins Clássico, Lucitone 550 and QC-20 (Table 1) were proportioned, mixed, packed, and pressed into the flask following the respective manufacturers’ instructions. Lucitone 550 and Clássico are conventional heat-cured resins and QC-20 is a dual-polymerizing acrylic resin that uses thermal and chemical activators and is classified as a quick-polymerizing resin.
Heat polymerized acrylic resins.
| Acrylic resins | Principle ingredients | Manufacturer | |
|---|---|---|---|
| Polymer | Monomer | ||
| Lucitone 550 | Copolymer (methyl-n-butyl) methacrylate, benzoyl peroxide, mineral dyes | Methylmetacrylate, ethyleneglycol,dimetha-crylate, hydroquinone | Dentsply, Petrópolis, Rio de Janeiro, Brazil |
| Clássico | Polymethylmethacrylate, ethylacrylate, benzoyl peroxide, organic pigments | Methylmetacrylate, topanol stabilizer | Clássico, São Paulo, São Paulo, Brazil |
| QC-20 | Copolymer (methyl-n-butyl) methacrylate, benzoyl peroxide, mineral dyes | Methylmetacrylate, ethyleneglycol, dimethacrylate,N,N-dimethyl-p-toluidine, hydroquinone methyl ether | Dentsply, Petrópolis, Rio de Janeiro, Brazil |
After resin polymerization, the flasks were cooled at room temperature before opening. The disk-shaped resin specimens were then deflasked. Fifty-four specimens were fabricated for each resin (n=18), divided into two groups depending on the type of polishing (mechanical and chemical). Specimens were divided in three subgroups (n=3) in relation with sodium hypochlorite concentration (1, 2.5 and 5.25%). Sample grouping and specimen distribution are illustrated in Fig. 1. Excess of acrylic resin was removed with tungsten steel bur #1508 (Edenta AG, Hauptstrasse, AU/SG, Switzerland) at low speed with additional hand smoothing with #320-grit silicone carbide paper (Norton, Guarulhos, São Paulo, Brazil) using water as a coolant.
In order to simulate a complete denture base, one side of the resin specimen was polished and the other surface was not. The non-polished side did not receive any further surface treatment in addition to the #320-grit silicone carbide paper hand smoothing; the polished side received additional polishing with #400- and 600-grift silicone carbide papers. Mechanical polishing was done with a horizontal machine (Struers DPU-10; Panambra, São Paulo, São Paulo, Brazil) using a rag wheel with polishing pastes (pumice/water followed by zinc oxide/water).
Specimens subjected to chemical polishing were processed in a chemical polishing machine (PQ 9000; Termotron Ltd., Piracicaba, São Paulo, Brazil) by soaking them in a methyl methacrylate-based solution (Clássico) at approximately 80°C for 10s, and then placing them on a glass plate to dry.
All specimens were stored in water at 50°C for 1h, with the purpose of releasing intrinsic substances such as methyl methacrylate and formaldehyde.16 After this, specimens were stored in distilled water at room temperature for 7 days to ensure the elimination of any residual intrinsic substance of resins.
The specimen groups were immersed for 10min periods in each sodium hypochlorite concentration. After disinfection, each specimen was washed and immersed in distilled water at different periods (15, 30, and 60min) with four subsequent water exchanges in each period (Fig. 1).
Electrochemical assays were carried out with a Microquimica MQPG 01 potentiostat (Microquimica, Palhoça, SC, Brazil) by employing conventional electrochemical cells with three electrodes; the data were recorded through an interface computer. A fiber carbon electrode was used as working electrode. A platinum wire was used as the counter electrode and all potentials are referenced to a sodium saturated silver/silver chloride electrode [Ag/AgCl/KCl(sat)] without regard for the liquid junction potential. The cyclic voltammetric studies were carried out at 100mV/s sweep rate in 5mL of phosphate solution pH 8.5. Since the cyclic voltammograms were recorded at a window potential range, between −0.4V up to 0.6V, oxygen-free solution was not necessary. Each solution analyzed were stirred for 30s and allowed to rest for 30s for equilibration before recording.
The supporting electrolyte used in this study was a phosphate solution at pH 8.5 based on studies to determine basic solution of sodium hypochlorite19 and hypochlorous acid concentrations with an electrode in a phosphate buffer solution (pH 8.5).20 The pH solution helps to adjust the sodium hypochlorite dissociation and to release the resin to the immersion medium.
The specimens of each type of resin and polishing were immersed in disinfectant solutions of sodium hypochlorite at 1% (Cloro Rio Rioquímica, São José do Rio Preto, São Paulo, Brazil) for a 10-min period. After this, each sample was rinsed with 80mL of distilled water and dried with absorbent tissue. Later, the specimen was placed in a glass container with 5mL of phosphate buffer solution and kept immersed for 15min (first water exchange, first sample analyzed); then this body sample was dried with absorbent paper and placed in the second glass container that had 5mL of basic phosphate, and maintained for the same immersion time (15min) until the fourth exchange of water (fourth water exchange). The same procedure was performed on the specimens after disinfection in sodium hypochlorite solutions at 2.5 and 5.25% concentration and immersed for a period of 30 to 60min in phosphate buffer solution (Fig. 1).
The main purpose of consecutive changes of immersing solution was to allow the release of all the adsorbed disinfectant, thus avoiding the saturation of the solution. Water exchanges with longer immersion periods, such as 120 and 240min, were not feasible in laboratory and clinical practice. In addition, water exchanges in shorter immersion periods help the release of sodium hypochlorite quickly.
Statistics analyses were carried out using SPSS software v.15.0. The analysis of the frequency distribution of the sample data and the homogeneity test of variances (Cochran test) indicated that the sample was not normal and non homogeneous, indicating the use of nonparametric tests. The values used in the statistical analysis were obtained through the difference between the current produced by the test samples and the standard phosphate solution (0.1M/pH 8.5; white solution), without sodium hypochlorite waste at scan rate of 20mVs−1. Wilcoxon test was used to compare the immersion time and water exchange on each hypochlorite concentration and commercial brand of resin.
ResultsThe specimens with chemical polishing of all brands of resin, after disinfection, did not release sodium hypochlorite at any immersion time, while those with mechanical polishing released.
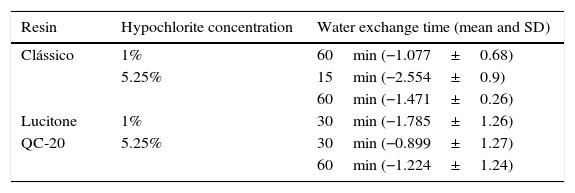
Wilcoxon test showed significantly differences (p≤0.05) between the different water exchanges periods (Table 2) and comparing the immersion time (Table 3).
Values with significantly differences (p=0.05) for Wilcoxon test between water exchanges in different commercial brand resins.
| Resin | Hypochlorite concentration | Water exchange time (mean and SD) |
|---|---|---|
| Clássico | 1% | 60min (−1.077±0.68) |
| 5.25% | 15min (−2.554±0.9) | |
| 60min (−1.471±0.26) | ||
| Lucitone | 1% | 30min (−1.785±1.26) |
| QC-20 | 5.25% | 30min (−0.899±1.27) |
| 60min (−1.224±1.24) |
Values with significantly differences (p=0.05) for Wilcoxon test of comparison between water immersion periods.
| Resin | Hypochlorite concentration | Water exchange time compared (mean and SD) |
|---|---|---|
| Clássico | 5.25% | 15min (−2.554±0.94)×60min (−1.471±0.26) |
| Lucitone | 2.5% | 15min (−2.091±0.7)×60min (−1.509±0.57) |
| QC-20 | 1% | 30min (−1.148±0.82)×60min (−1.778±0.16) |
| 2.5% | 15min (−2.081±0.7)×60min (−1.869±0.54) |
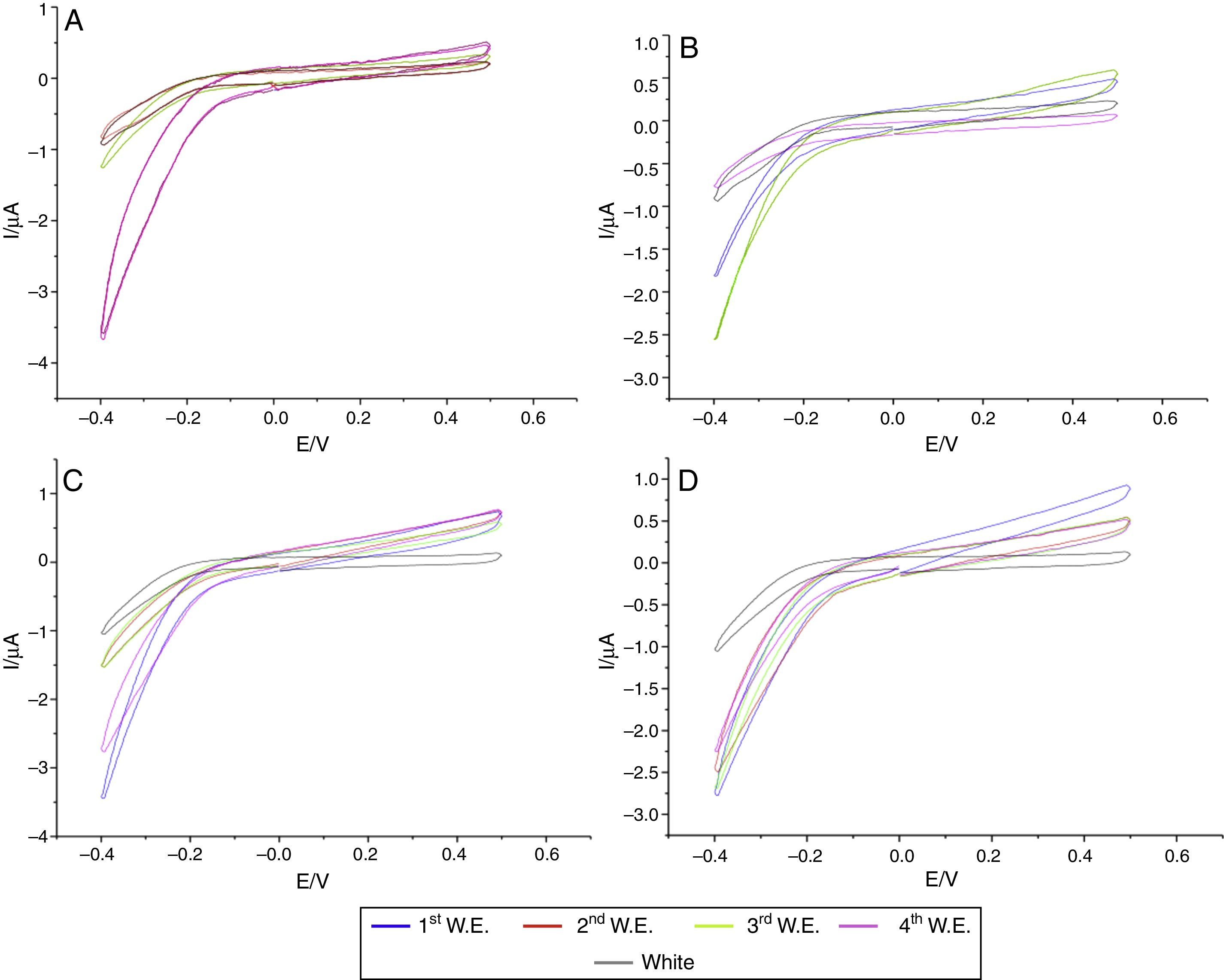
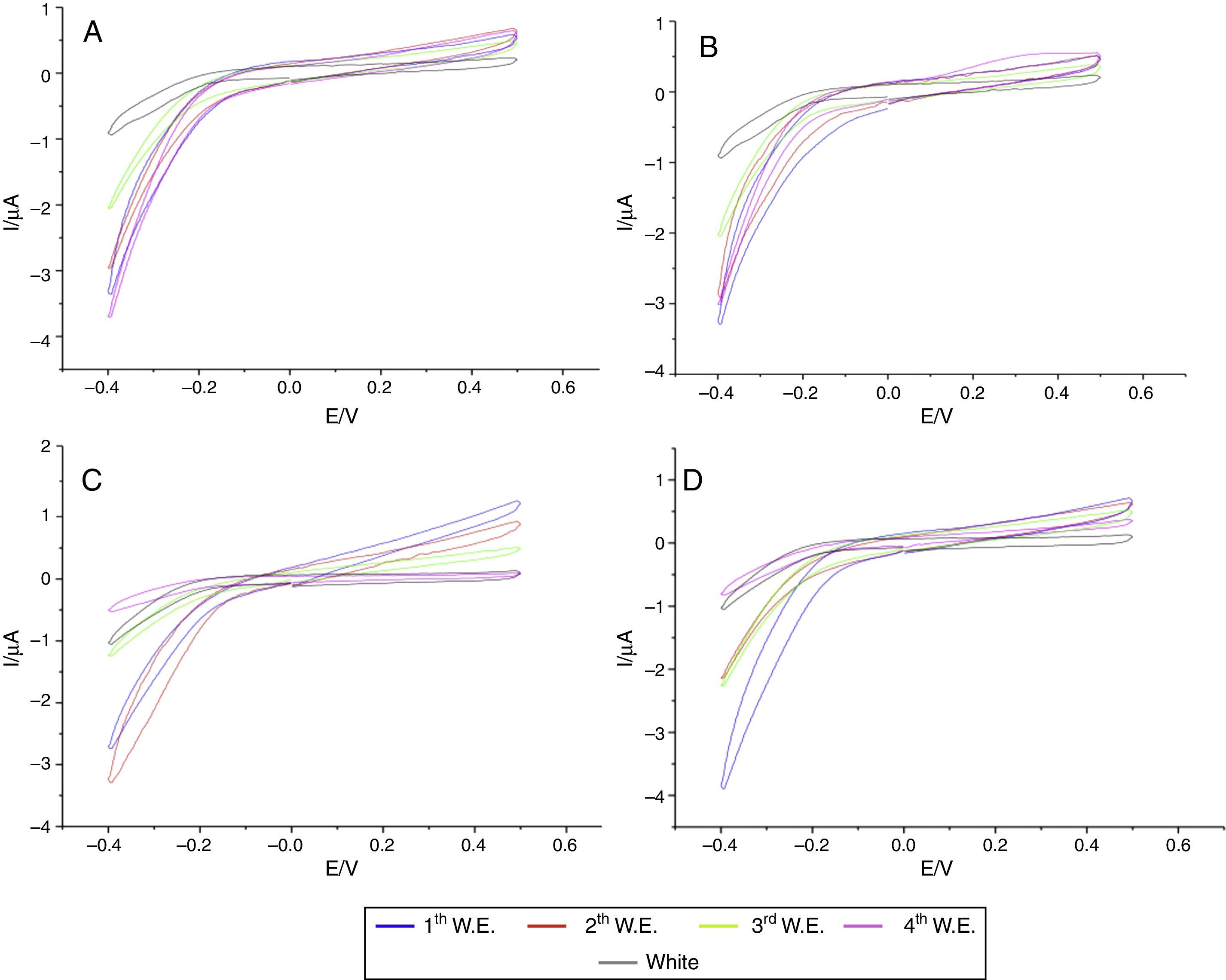
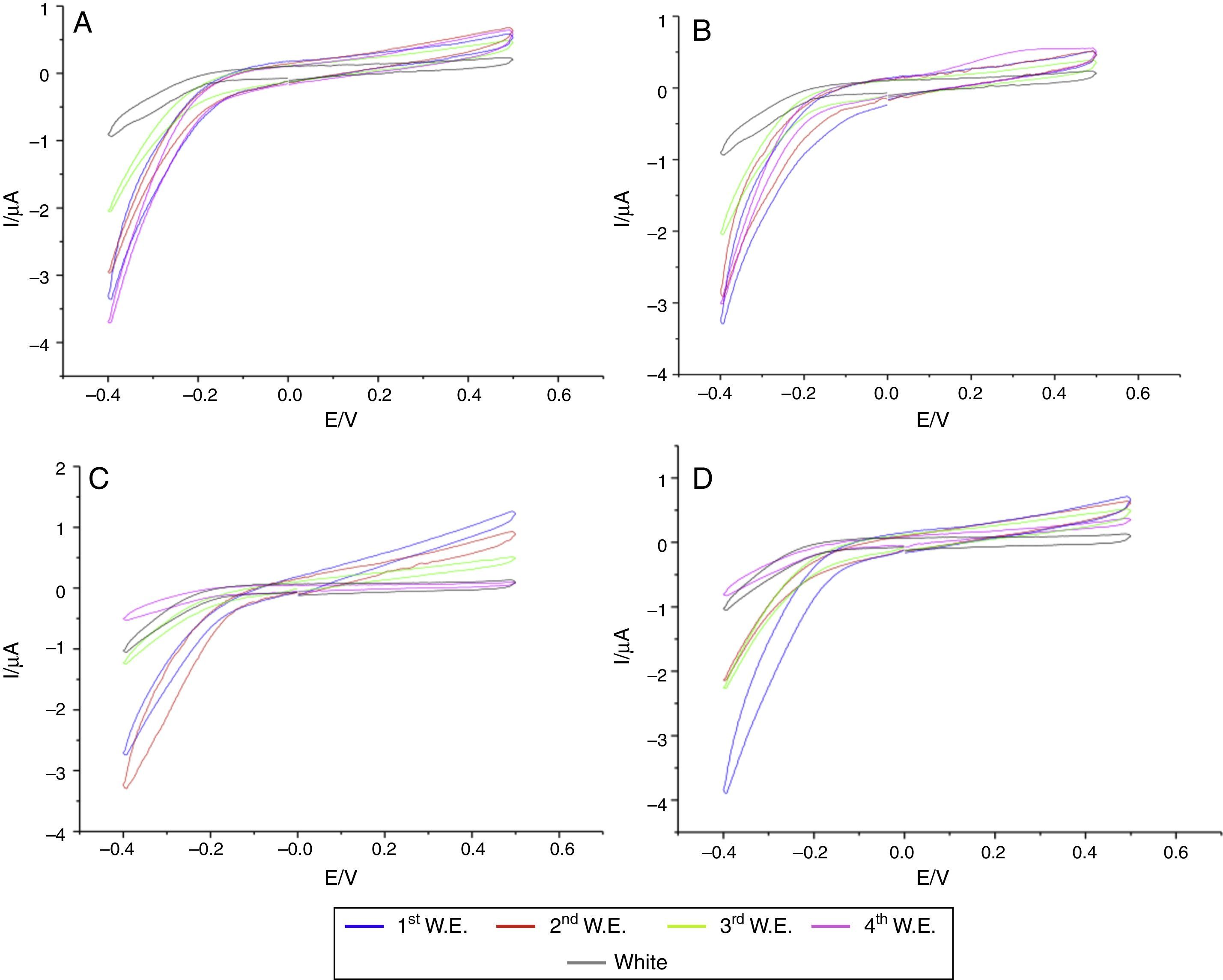
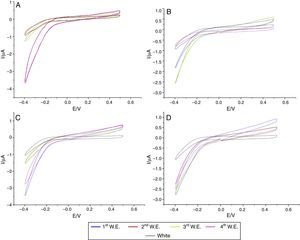
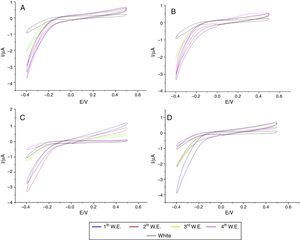
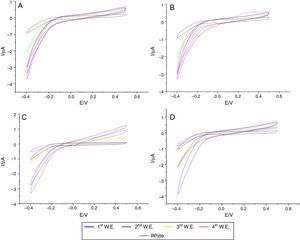
In the specimens of Clássico resin immersed in sodium hypochlorite at 5.25% (Fig. 2) as well as Lucitone (Fig. 3) and QC-20 resins (Fig. 4) immersed at 2.5%, the amount of disinfectant solution released in the four 15-min water exchanges were higher than the four of 60-min.
Considering the water exchange factor, Clássico resin disinfected with 1% sodium hypochlorite showed differences between the 60-min water exchanges, observing in the first three water replaces intermediate quantities of disinfectant were released whereas at fourth water exchange, no hypochlorite waste was found. For the samples of Clássico resin disinfected with sodium hypochlorite at 5.25%, in the 30-min water replaces a high disinfectant release was identified in the first three water exchanges while in the period of 60min, there was hypochlorite waste only in the first. For Lucitone resin, significantly voltammetry values were observed for the samples disinfected at 1% during the 15-min water exchanges, showing the lowest disinfectant waste at fourth water replace. Besides, QC-20 resin showed significantly values for samples disinfected at 5.25% in 30-min and 60-min water exchange, observing no hypochlorite release from the third water exchange of 30-min period. However, in 60-min period, no disinfectant waste was identified only in the fourth water replace (Figs. 2–4).
DiscussionThe greatest reduction in microbial contamination of the internal aspects of acrylic resin has been reported by immersion in 1% sodium hypochlorite and 2% glutaraldhehyde.3 Moreover, the porosity of resins allows them to adsorb water4 and certain components of disinfection solutions could penetrate in them, being later released to the saliva as some components of the resins are released, which are then swallowed.16,17
The work hypothesis was rejected, as long as mechanically polished acrylic resins release residual hypochlorite, regardless of the tested concentration. By their turn chemical polishing did not result in significant release.
All resin specimens subjected to chemical polishing did not release sodium hypochlorite after disinfection at any time. This is in accordance with findings9 who did not observe release of glutaraldehyde after chemical polishing of the same brands of denture resins. These results may be due to the fact that surface finishing was achieved with methyl methacrylate-based substances, leading to the formation of a film that covered the resin surface, rendering waterproof and preventing penetration of liquids. Thus, rinsing in running water can be enough to remove all disinfectant residues, as it remains only on the surface.
On the other hand, all resins of the three commercial brands released sodium hypochlorite after mechanical polishing, which concurs with the results of a study on release of glutaraldehyde9 who identified the release of glutaraldehyde in the same resin brands after the same polishing method. It could be related because the three commercial brands of resins are different in their composition, polymerization reaction, and probably in their porosity, this being the last most important property that could be related with the adsorption of liquids.
After disinfection with sodium hypochlorite at concentrations of 1% and mechanically polished, the QC-20 and Lucitone resins showed qualitatively similar behavior in the four water exchanges of three periods. In 30-min periods, the small amount of disinfectant solution waste was detected, proving that the periods are not enough to allow the release of sodium hypochlorite from inside of the resin. Also, after immersion in sodium hypochlorite solution at 1 and 2.5%, the Clássico resin samples subjected to mechanical polishing released hypochlorite in all of four water exchanges of 15 and 30min. In another study,9 Clássico resin had the highest release of glutaraldehyde in the 30-min and 60-min periods.
The QC-20 resin samples immersed in sodium hypochlorite at 2.5% showed a large amount of disinfectant waste in the three periods as well as the four water exchanges.
All samples of the three commercial heat-cured brands, after disinfection with sodium hypochlorite at 5.25%, released the disinfectant during all water exchanges and at any period time. Thus, 60-min periods of water immersion were not enough for the sodium hypochlorite release. The above could be explained by considering that at high concentration of sodium hypochlorite, a film is formed, which probably holds large amount of disinfectant solution that is continually released in the water exchanges.
The principal objective of the water exchanges was to prevent wrong results due to water saturation with sodium hypochlorite. The immersion periods of 15, 30, and 60min were selected because they are clinically viable and potentiate the quick release of the disinfectant.
There is concern that denture immersion in chemical solutions used for cleaning and disinfection could lead to the absorption of these solutions with subsequent release in the saliva. It is necessary to establish an appropriate protocol to eliminate the residues of disinfectant after disinfection.
The potential of sodium hypochlorite solutions to cause allergic reactions is frequently reported in the medical literature, but is really scarce in dental literature.21 Also, an in vitro study of sodium hypochlorite in cell culture concluded that a 5% concentration is highly toxic for clinical use during root canal irrigation whereas the same disinfectant at 0.5% showed cytopathological effects in some cells.22
Kaufman and Keila21 verified in their study the ability of sodium hypochlorite solutions to cause allergic reactions after contact with intact skin and some patients that may be hypersensitive to these solutions. Thus, wastes of this disinfectant in dentures can cause allergic reactions in patients with hypersensitivity to sodium hypochlorite.
The greater the toxicity of a disinfectant, washing longer periods should be used for devices and/or medical and dental items, to reduce the level of disinfectant below the dose that cells are damaged. Washing and rinsing cycles can provide adequate reduction of waste below toxic levels for a specific disinfectant, but these procedures may not be effective for others substances.4
As a limitation of this study could be the few specimens used, however, it may be considered as a first step to further trials to determine the action of other disinfectants on physical and mechanical properties of acrylic resins.
It is essential to introduce in oral rehabilitation some disinfection procedures effectively proven and practical, but without damage the patient's health neither the physical and mechanical properties of dentures.
ConclusionIn the present in vitro study, there were differences in hypochlorite release from the three commercial brands of denture-base acrylic resins subjected to mechanical polishing. Disinfection with sodium hypochlorite at 5.25% of samples mechanically polished promoted the highest amount of released substance. Also, in cases of dentures subjected to mechanical polishing and after disinfection, these should be immersed in water during 60min or more to achieve the highest amount of hypochlorite elimination. However, the chemical polishing seems to be the most indicated procedure because there is no hypochlorite release.
Ethical disclosuresProtection of people and animalsThe authors state that for this investigation have not been performed experiments on humans or animals.
Confidentiality of dataThe authors declare that this article does not appear patient data.
Right to privacy and informed consentThe authors declare that this article does not appear patient data.
Conflict of interest statementThe authors declare that there is no conflict of interest.
We acknowledge the financial support for this study provided by scientific research grant #00/11515-1 from the São Paulo State Research Foundation (FAPESP).