To disclose our preliminary experience in inhalation sedation with sevoflorane in a standardized manner using the Anesthetic Conserving Device in intubated, critically ill patients in our ICU.
PatientsIt has been used in nine cases of adult patients, six men and three women, over 24 months.
ResultsA proper implementation of the protocol by physicians and the nursing staff has been achieved, meeting the goals established for sedation (RASS 0, −2) free of hepatic or renal adverse outcomes or side effects.
ConclusionsIn our limited experience, adjuvant inhalation sedation with sevoflorane in the ICU is safe and complementary to the use of intravenous drugs such as propofol, remifentanil and midazolam, which are currently commonly used to achieve goal-directed sedation.
Explicar nuestra experiencia preliminar en la utilización de sedación inhalatoria de manera estandarizada con sevoflorano mediante dispositivo Anesthetic Conserving Device en pacientes críticos intubados en nuestra UCI.
Pacientesse ha utilizado en 9 casos, en pacientes adultos, 6 hombres y 3 mujeres, durante un periodo de 24 meses.
Resultadosse ha conseguido una adecuada implantación del protocolo por parte de médicos y personal de enfermería, logrando los objetivos de sedación fijados en un primer momento (RASS 0, −2) y sin obtener resultados adversos ni efectos secundarios a nivel hepático y/o renal.
Conclusionesen nuestra limitada experiencia, la sedación inhalatoria con sevoflorano coadyuvante en UCI es una técnica segura y complementaria al uso de fármacos intravenosos, como propofol, remifentanilo y midazolam, utilizados habitualmente, para lograr una sedación guiada por objetivos.
Sedation is required to ensure adequate comfort and safety to ICU patients, lowering their anxiety, restlessness, and pain,1 and facilitating mechanical ventilation. Notwithstanding the availability of consensus guidelines in patients admitted to the ICU, the clinical practice is quite variable.2 Midazolam and propofol are two of the most commonly used agents for this purpose; however, these drugs exhibit pharmacological characteristics that make them less than an ideal sedative.3
Inhalation sedation using halogenated anesthetics embodies some of the characteristics of the ideal sedative: minimal accumulation, organ-independent metabolism, insignificant side effects, shorter time to awakening, and hemodynamic stability.4 Another advantage is that the drug concentration at the end of expiration may be monitored continuously to allow for adjustments in the depth of sedation. Moreover, it provides cardio and neuroprotection, in addition to bronchodilatation.5
In 2005, the AnaConDa® (AnaConDa® Sedana Medical, Uppsala, Sweden) device (Fig. 1) the acronym for Anesthetic Conserving Device (ACD), was marketed and allowed for the safe administration of inhaled agents (isoflurane and sevoflorane) with standard ICU ventilators.
To this date, there is evidence of the safe use and the existence of some advantages of inhalation sedation versus the conventional intravenous sedation, particularly for postsurgical patients.
The purpose of this paper is to disclose our results following the use of inhalation sedation for a maximum of 72h with sevoflorane, using the ACD device in intubated critically ill patients, in our ICU, with a RASS6 objective of 0–2. And further, to evaluate the effect on renal and liver function in these patients.
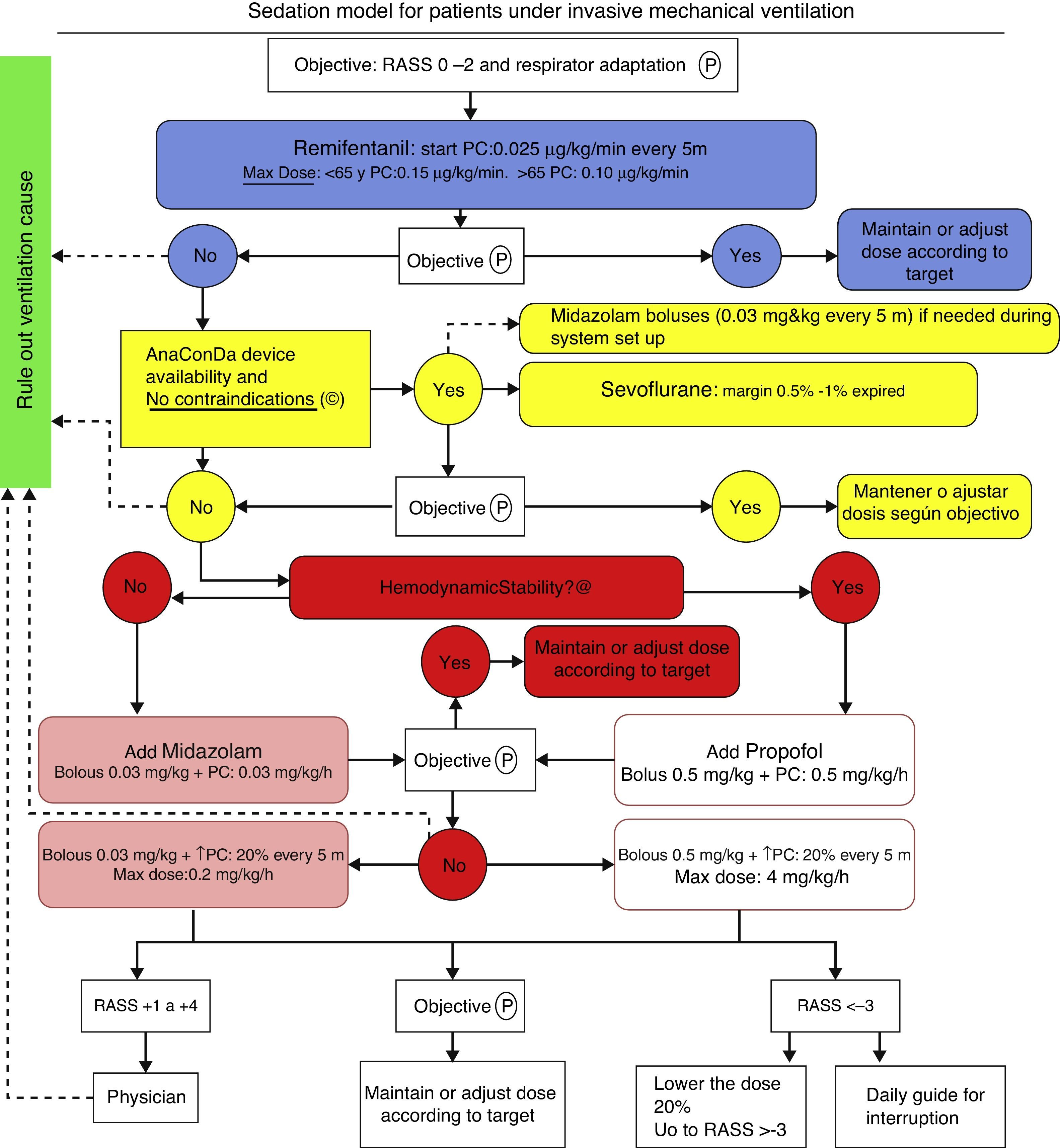
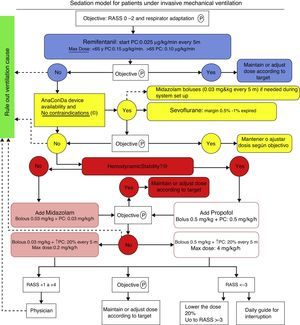
Patients and methodThe protocol illustrated in Fig. 2 has been implemented in the usual clinical practice in our ICU since early in 2012 and it is still used. The approval by an ethics committee was not required in view of the characteristics of the trial and pursuant to the local legislation.
Upon drafting the protocol and reaching a consensus among physicians, educational sessions were organized for the nursing team, resident physicians and the rest of the healthcare staff with the aim of explaining the protocol, educating on the proper use of the equipment and responding to any questions.
The protocol has been used in nine adult patients, six males and three females. Six of the nine patients had a diagnosis of acute respiratory failure and three had a diagnosis of acute over chronic respiratory failure.
Sevoflorane is used as an adjuvant when the maximum dose of remifentanil is insufficient for proper adaptation of the patient to mechanical ventilation or with a RASS score between +2 and +4. Sevoflorane is contraindicated whenever there is a history or suspicion of malignant hyperthermia and hypersensitivity to sevoflorane. We have not used Sevo in pregnant women of in patients with intracranial hypertension, since safety has not been established under these circumstances.
Our sedation objective is determined by the Richmond Agitation/Sedation Scale (RASS), with a target of 0 to −2. Every patient was evaluated according to RASS every 8h.
We began IV continuous perfusion of remifentanil sedation, titrating the dose depending on the age of the patient, starting with 0.025mcg/kg/min and increasing the perfusion every 5min until the maximum dose, if the target was not reached. When a new drug was required because of poor adaptation to the ventilator or failure to reach the RASS target, we used sevoflorane via an ACD device. Once the system was wet up, the sevoflorane end tidal % (Et sev %) was monitored, with a maximum allowance of 1%. The rate of continuous infusion of liquid sevoflorane ranges from 1 to 10ml/h, with significant patient-to-patient variation. At the time of weaning, if a new drug needs to be added, the regime illustrated in Fig. 2 is used. The device was used for a maximum of 72h, although in most patients it was only used for 48h and then was removed; plasma fluoride levels were measured and continuous ventilation was maintained. However, in patients 5 and 6 ACD was used for 72h, and then the determination was made. Additionally, serial analyses were completed for urea, plasma creatinine, GFR, total bilirubin, GOT, GPT and GGT for the assessment of renal and liver function a few hours before using the ACD device (basal), at the time of removal (after 48h) and 48h after removal (96h).
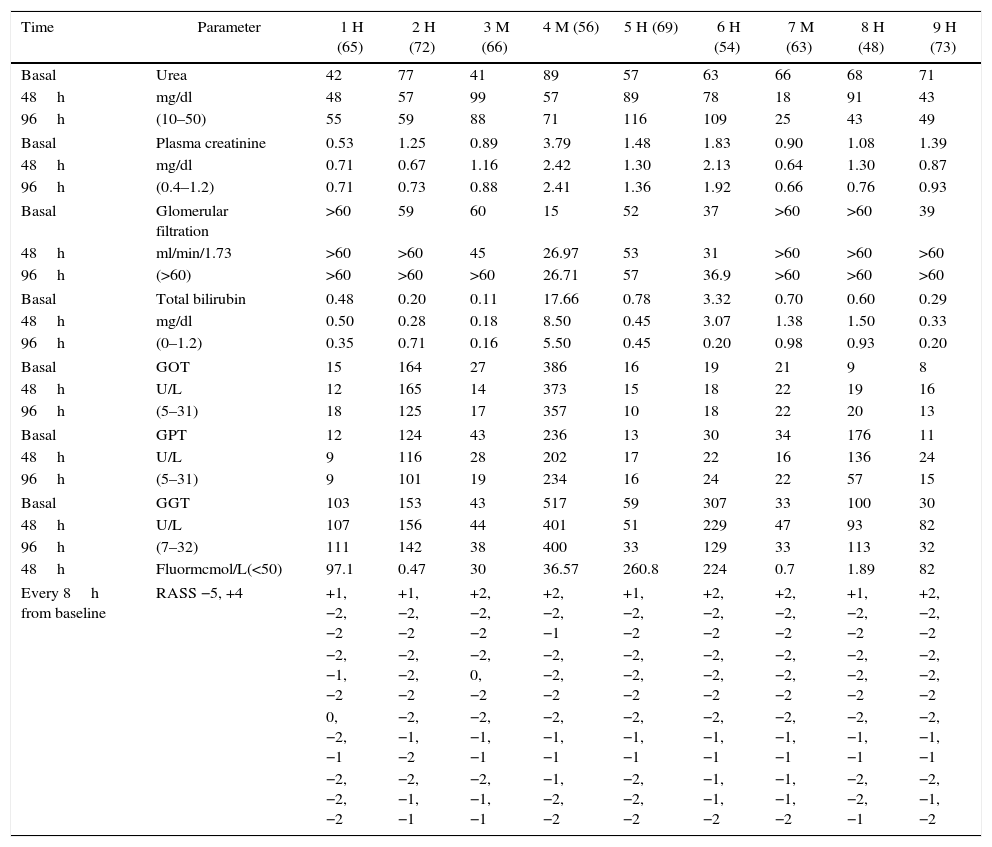
ResultsThe analytical results are presented in Table 1.
Results of 9 patients.
| Time | Parameter | 1 H (65) | 2 H (72) | 3 M (66) | 4 M (56) | 5 H (69) | 6 H (54) | 7 M (63) | 8 H (48) | 9 H (73) |
|---|---|---|---|---|---|---|---|---|---|---|
| Basal | Urea | 42 | 77 | 41 | 89 | 57 | 63 | 66 | 68 | 71 |
| 48h | mg/dl | 48 | 57 | 99 | 57 | 89 | 78 | 18 | 91 | 43 |
| 96h | (10–50) | 55 | 59 | 88 | 71 | 116 | 109 | 25 | 43 | 49 |
| Basal | Plasma creatinine | 0.53 | 1.25 | 0.89 | 3.79 | 1.48 | 1.83 | 0.90 | 1.08 | 1.39 |
| 48h | mg/dl | 0.71 | 0.67 | 1.16 | 2.42 | 1.30 | 2.13 | 0.64 | 1.30 | 0.87 |
| 96h | (0.4–1.2) | 0.71 | 0.73 | 0.88 | 2.41 | 1.36 | 1.92 | 0.66 | 0.76 | 0.93 |
| Basal | Glomerular filtration | >60 | 59 | 60 | 15 | 52 | 37 | >60 | >60 | 39 |
| 48h | ml/min/1.73 | >60 | >60 | 45 | 26.97 | 53 | 31 | >60 | >60 | >60 |
| 96h | (>60) | >60 | >60 | >60 | 26.71 | 57 | 36.9 | >60 | >60 | >60 |
| Basal | Total bilirubin | 0.48 | 0.20 | 0.11 | 17.66 | 0.78 | 3.32 | 0.70 | 0.60 | 0.29 |
| 48h | mg/dl | 0.50 | 0.28 | 0.18 | 8.50 | 0.45 | 3.07 | 1.38 | 1.50 | 0.33 |
| 96h | (0–1.2) | 0.35 | 0.71 | 0.16 | 5.50 | 0.45 | 0.20 | 0.98 | 0.93 | 0.20 |
| Basal | GOT | 15 | 164 | 27 | 386 | 16 | 19 | 21 | 9 | 8 |
| 48h | U/L | 12 | 165 | 14 | 373 | 15 | 18 | 22 | 19 | 16 |
| 96h | (5–31) | 18 | 125 | 17 | 357 | 10 | 18 | 22 | 20 | 13 |
| Basal | GPT | 12 | 124 | 43 | 236 | 13 | 30 | 34 | 176 | 11 |
| 48h | U/L | 9 | 116 | 28 | 202 | 17 | 22 | 16 | 136 | 24 |
| 96h | (5–31) | 9 | 101 | 19 | 234 | 16 | 24 | 22 | 57 | 15 |
| Basal | GGT | 103 | 153 | 43 | 517 | 59 | 307 | 33 | 100 | 30 |
| 48h | U/L | 107 | 156 | 44 | 401 | 51 | 229 | 47 | 93 | 82 |
| 96h | (7–32) | 111 | 142 | 38 | 400 | 33 | 129 | 33 | 113 | 32 |
| 48h | Fluormcmol/L(<50) | 97.1 | 0.47 | 30 | 36.57 | 260.8 | 224 | 0.7 | 1.89 | 82 |
| Every 8h from baseline | RASS −5, +4 | +1, −2, −2 | +1, −2, −2 | +2, −2, −2 | +2, −2, −1 | +1, −2, −2 | +2, −2, −2 | +2, −2, −2 | +1, −2, −2 | +2, −2, −2 |
| −2, −1, −2 | −2, −2, −2 | −2, 0, −2 | −2, −2, −2 | −2, −2, −2 | −2, −2, −2 | −2, −2, −2 | −2, −2, −2 | −2, −2, −2 | ||
| 0, −2, −1 | −2, −1, −2 | −2, −1, −1 | −2, −1, −1 | −2, −1, −1 | −2, −1, −1 | −2, −1, −1 | −2, −1, −1 | −2, −1, −1 | ||
| −2, −2, −2 | −2, −1, −1 | −2, −1, −1 | −1, −2, −2 | −2, −2, −2 | −1, −1, −2 | −1, −1, −2 | −2, −2, −1 | −2, −1, −2 | ||
Analytical results of the nine patients at the time of initiating the ACD device (basal), at 48h (after removal) and at 96h. The RASS was determined every 8h and is expressed in values ranging from −5 a +4. The plasma fluorine measurement was made 48 after the basal, except for patients 5 and 6, that were measured after 72h of administering sevoflorane treatment. The values in parenthesis are the reference normal values. GFR was measured with MDRD. Males are represented as H and females as M, with the age in parenthesis
Source: Authors.
An adequate ventilator adaptation was achieved in all patients in whom ACD inhalation sedation was administered, with a RASS score between 0 and −2. Thus the sedation using the ACD device was appropriate and met the primary goal. As illustrate in the Table, at the time of establishing inhalation sedation, most patients were restless and agitated, anxious, with poor coordination and poorly adapted to the ventilator, as expressed by RASS scores over 0. This situation improved following the administration of inhaled sevoflorane.
Moreover, none of the patients exhibited a significant decline in the kidney or liver function, despite the fact that patients 5 and 6 exhibited elevated plasma fluoride levels, as shown in Table 1. These values are considered exclusively secondary to the use of inhaled sevoflorane, though we cannot rule out other underlying causes.
DiscussionThe article discusses the preliminary results from the adjuvant use of inhalation sedation with sevoflorane in medical ICU patients, using the ACD device7 safely and explains how we have implemented a protocol in our unit. Currently there are no trials underway in our unit, although we continue to collect data to be able to undertake a study with a larger sample and stringent scientific rigor in the near future.
Until now inhalation sedation using the ACD device had mainly been used in post-surgical patients with good results.8 We present our experience using this type of sedation in an ICU where most patients are medical, with pathologies such as pneumonia, respiratory distress and septic shock.
Several papers have shown the benefit of using sevoflorane for ICU sedation using the ACD device. Röhm et al.9 showed a shorter time to extubation and hospital discharge, comparing sevoflorane versus propofol in patients undergoing heart surgery. Mesnil et al.10 showed that the quality of awakening was improved, with fewer episodes of hallucinations and agitation, when comparing sevoflorane, propofol and midazolam. These observations are consistent with our findings in terms of adaptation to the ventilator and the occurrence of restlessness episodes, measured with RASS, as shown in Table 1. Although in our case we have not recorded the time to extubation – that was not the purpose of the study – we have observed however that patients adapt better to the ventilator and are less agitated under sevoflorane sedation; so very seldom a third sedative is needed. This is the reason why we consider sevoflorane particularly helpful in situations of considerable difficulty to adapt the ventilator to the patient either because of a high need of intravenous medication or because of persistent episodes of agitation. Using this type of sedation the patients are alert and calmed, without any restlessness or anxiety; they do not exhibit violent behaviors and are not fighting the ventilator (RASS 0, −2).
Following the analyses to measure renal and hepatic function, we have observed that there were no alterations as compared to the baseline status, notwithstanding the fact that some patients exhibited very high fluoride plasma levels. Volatile anesthetic agents such as sevoflorane, produce inorganic fluoride metabolites associated with renal dysfunction. A value of 50mcmol/L has been postulated as a threat to develop renal failure11 and it is believed to potentially cause liver failure as well.12 However, although some of our patients exhibit significantly high levels, none of them has developed kidney or liver failure associated with this type of sedation, as established in further follow-up.
Notwithstanding the limitations of the studies so far completed, most of them show the superiority of volatile anesthetics against the traditional intravenous drugs for ICU sedation. Volatile agents reduce the time to extubation and discharge, and consequently reduce the risk of infection and complications, at a cost similar to that of intravenous agents. The excellent pharmacokinetics13,14 of volatile anesthetics makes them a good choice for ICU sedation based on the rapid patient evaluation and faster awakening, extubation, and discharge.
For all these reasons, we suggest the use of this sedation modality in conventional ICUs, stressing the importance of proper training of all the staff members involved in the care of these patients. Adjuvant sevoflorane is a safe sedation alternative to the current use of the regular intravenous agents and is a valid and effective option for the everyday ICU practice.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingNone.
Please cite this article as: López-Ramos JM, Gómez-Sainz JJ, Manzano-Canalechevarria A, Aguilera-Celorrio L. Sevoflorano como coadyuvante en la sedación durante ventilación mecánica en pacientes médicos de UCI: resultados preliminares en una serie de casos. Rev Colomb Anestesiol. 2016;44:52–57.