Central neuropathic pain after spinal cord injury continues to represent a therapeutic challenge, perhaps due to the lack of understanding and consensus as to the neuropathophysiological symptomatic etiology. Spinal cord stimulation is a sophisticated minimally invasive alternative for the treatment of peripheral neuropathic pain; however, in this case, we present a patient in whom thoracic spinal cord stimulation was the only successful approach to the treatment of lower extremity neuropathic pain after an incomplete cervical spinal cord injury.
El dolor neuropático que sigue a la lesión medular espinal puede representar un reto clínico y terapéutico. Después de que múltiples modalidades terapéuticas, incluyendo alternativas farmacológicas y no farmacológicas, han fallado, la mejoría sintomática es improbable. La estimulación medular puede resultar beneficiosa en pacientes con dolor intratable.
La estimulación medular es vista como una alternativa terapéutica viable para el manejo del dolor neuropático periférico, pero su uso en el dolor central es controvertido, con solo un limitado número de casos exitosos reportados. El siguiente caso es de una paciente en quien la resección de un ependimoma cervical con siringomielia resultante condujo a dolor neuropático intratable en las extremidades inferiores. El control sintomático exitoso se logró obtener tras la utilización de un estimulador medular torácico.
Neuropathic pain following spinal cord injury may pose a clinical and therapeutic challenge. After multiple therapeutic modalities have failed, including pharmacological and non-pharmacological options, improvement of symptoms is improbable. Spinal cord stimulation (SCS) may be beneficial for patients with intractable pain.
SCS is considered as a viable therapeutic option for the management of peripheral neuropathic pain, but its use in central pain is controversial with only a limited number of successful cases reported. The following is the case of a female patient in whom the resection of a cervical ependymoma with associated syringomyelia led to intractable pain of the lower limbs. Successful symptom control was achieved after thoracic SCS.
Case reportA forty-nine year-old female patient was assessed by the neurosurgery service for pain in the left hemithorax radiating to the ipsilateral upper limb and the inter-scapular region. She reported a sense of tightness in the chest accompanied by diaphoresis and intermittent loss of sphincter control.
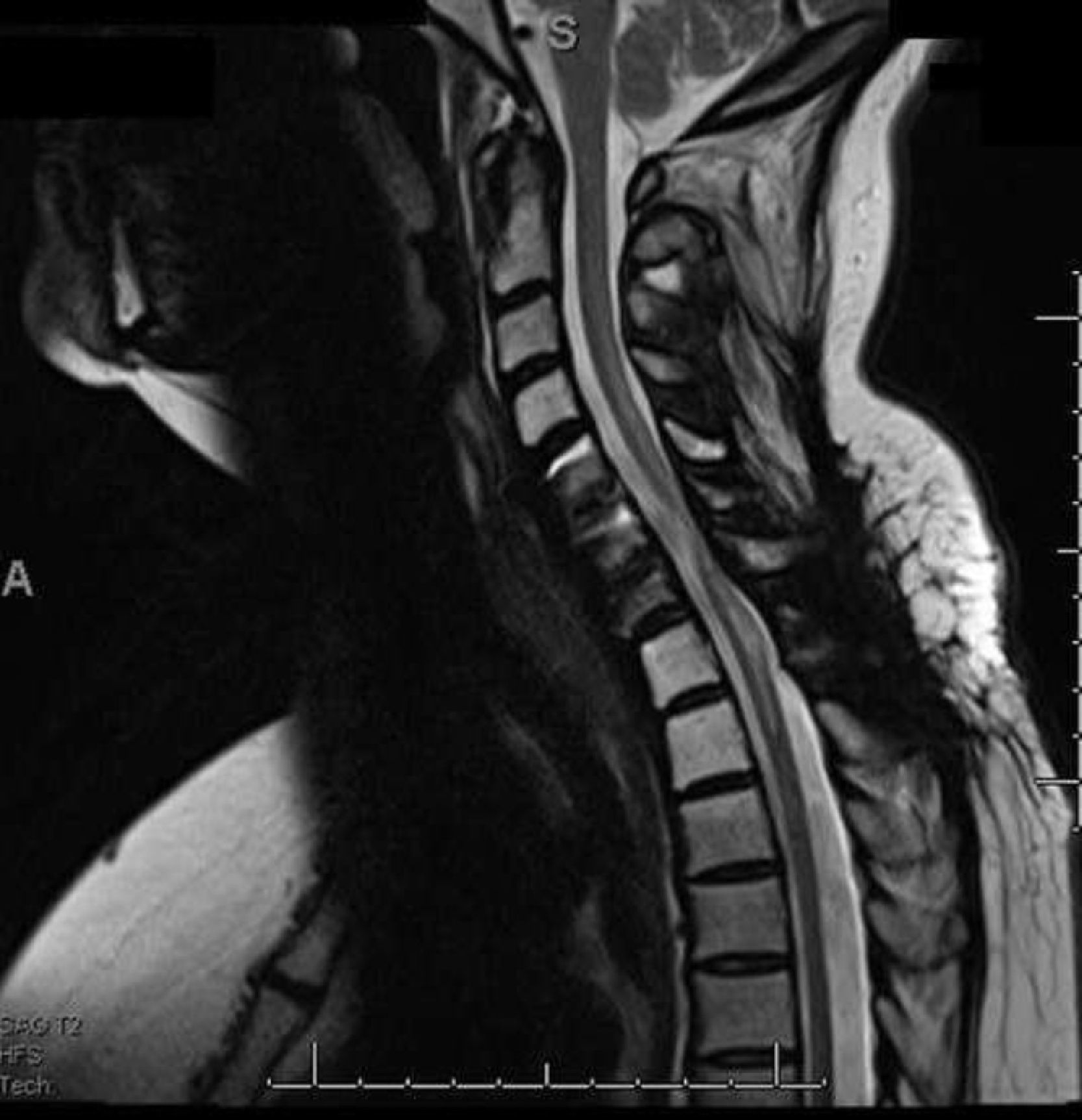
A magnetic resonance imaging (MRI) study revealed an intramedullary tumor at C6 (ependymoma) with associated syringomyelia above and below the lesion (C5-T2) (Fig. 1). The lesion was excised through a cervical laminectomy. Persistent pain after surgery required a new MRI that showed focal enhancement in C6-C7 with spinal canal stenosis secondary to disc protrusions. The patient was taken to surgery for additional tumor resection with discectomy and arthrodesis of C5-C7.
During the immediate postoperative period, the patient reported total resolution of pain in the upper limbs, but complained of new pain in the lower limbs accompanied by numbness and shooting sensation from the feet to the knees. The postoperative MRI revealed absence of new tumor growth, but presence of mild myelomalacia in C4-T1 (Fig. 2).
The patient received cervical spinal radiation over the course of the next four months. Post-operative electromyography/nerve conduction studies (EMG/NCS) revealed basically normal findings except for absent response from the lateral femoral cutaneous nerves. She was referred to the pain clinic, where she was treated with multiple oral and topical agents (gabapentin, pregabalin, topiramate, amitriptyline, nortriptyline, sertraline, naproxene, tramadol, oxycodone, hydromorphone, methadone and capsaicin), and experienced severe adverse effects including sedation, cognitive dysfunction and imbalance. Despite all the efforts, pain continued to be intractable. Although spinal cord stimulation is a controversial option for the treatment of central neuropathic pain,1 this therapeutic alternative was considered, offered and accepted on the basis of a few successful case reports.2,3
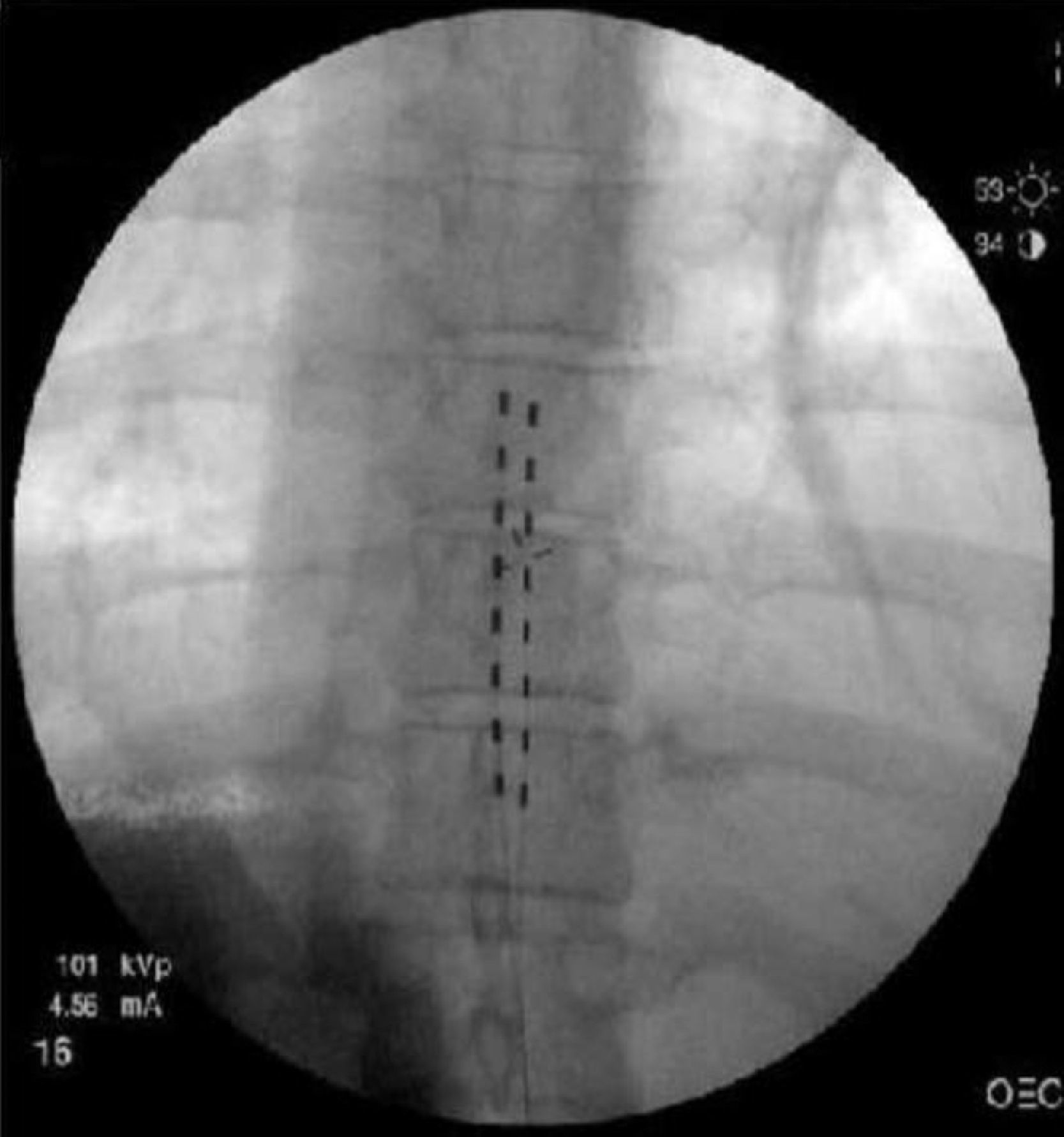
Two Octrode (Medtronic, Inc., Minneapolis, MN) test leads were advanced percutaneously into the posterior peridural space up to the vertebral body of T8 (Fig. 3). Electric stimulation was instituted with complete topographic paresthetic overlap. The patient was discharged and returned to the clinic four days later, having used the system 96% of the time. The amplitude was set at 1.9A in electrode A, and 3.1A in electrode B, and the pulse width used was between 440–450μs. On follow-up, she reported complete pain resolution (100%) with the use of the stimulator.
Subsequently, the neurosurgery service implanted a Medtronic Specify 5-6-5 (Medtronic, Inc., Minneapolis, MN) surgical electrode at the level of T9. On the follow-up visit three months after the implantation, having required one reprogramming during that period, she reported improvement of her symptoms. Multiple drugs were discontinued and cognition improved. The patient finally decided to continue her management with physicians nearer to her place of residence and we lost contact. Later we learnt of her demise due to tumor relapse two years later.
DiscussionCentral pain origin and mechanisms are not well understood. Close to 30–40% of patients with spinal cord injuries develop pain.4 A recent study in rats showed that perilesional myelomalacia is associated with inflammation, astrogliosis, and apoptotic cell death. This, in turn, results in late neurological and behavioral deficits, and neuropathic pain, reflecting a key feature of post-traumatic clinical presentation of syringomyelia in humans.5 The role of spinothalamic tracts in the development and persistence of central pain syndromes is uncertain and, consequently, a current topic of debate.6 The continuous activity of intact residual spinothalamic tracts (“spinal pain generators”) appears to trigger abnormal thalamic activity.7 Spinal neuronal hyperexcitability may be due to an increased release of glutamate, an excess population of sodium channels, the activation of glial cells secondary to inflammation, and/or the loss of descending modulation pathways.8
Spinal cord stimulation was inspired on the gate theory proposed by Melzac and Wall, whereby the activation of large low-threshold fibers inhibits (closes the gate) the transmission of nociceptive information along the small high-threshold fibers.9 However, this single explanation of the mechanism of action of spinal cord stimulation is insufficient, considering that spinal cord stimulation is ineffective for nociceptive pain. Several other mechanisms of action have been proposed, including the following: (a) antidromic activation of the dorsal columns causing inhibition of segmental transmission and suppression of the hyperexcitability of the wide dynamic range neurons10,11; (b) inhibition of the neuroexcitatory transmission (glutamate and aspartate)12; (c) promotion of GABAergic transmission13; and (d) supraspinal activation of the modulation loops affecting rostral transmission.14 Consequently, spinal cord stimulation may be an appropriate therapeutic option for managing intractable neuropathic pain in patients with intact dorsal horns.
Many clinicians argue that spinal cord stimulation is invasive and costly, and that its use is not justified considering its technical complexity, less-than-ideal effectiveness, and its rates of adverse effects and complications. Although there is a growing trend toward evidence-based medicine, lack of evidence should not be equated with absence of effect. Although conventional treatments such as psychotherapy and physical therapy have huge scientific data supporting their positive impact on the treatment of patients with chronic pain and they are widely recommended,15 they should be used as adjuvants in multimodal therapeutic approaches because, used alone, they are not sufficiently effective for the treatment of severe pain.16 Spinal cord stimulation has been shown to be a cost-effective alternative as compared with conventional modalities, particularly in situations of intractable neuropathic pain such as the failed back syndrome and the complex regional pain syndrome Type I.17,18 Used early on, it may result in greater therapeutic efficacy.19
In conclusion, the normal proprioception in the lower limbs of our patient led us to assume that pain pathways had been injured separately, while the dorsal horns remained intact. This clinical case shows clearly that spinal cord stimulation is a viable option for the treatment of central neuropathic pain following partial spinal cord injury.
FundingNone
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Benedetti EM. La estimulación medular torácica es útil en el tratamiento del dolor post lesión medular cervical incompleta. Rev Colomb Anestesiol. 2013;41:146–9.