Initial interpretation of the process of coagulation refers to two pathways: (1) the extrinsic pathway, consisting of tissue factor (TF) and factor VII, and (2) the intrinsic pathway, in which factors XII, XI, IX, VIII and V are involved. Currently, this concept has changed and it is accepted that the main initiating event in blood coagulation is TF exposure. In this article, we review the new concepts of the coagulation cascade based on thromboelastography findings and the different mechanisms involved in trauma-associated coagulopathy. For this purpose, a systematic research was carried out using the main databases, including Medline, Embase and Lilacs between 2000 and 2011. One hundred and fourteen articles were found, and 50 were selected for the review. A relevant finding was that thromboelastography allows a precise detection of the underlying flaw in the coagulation cascade. Therefore, this procedure has become an essential tool and a guide for the management of trauma-associated coagulopathy.
La interpretación inicial del proceso de coagulación mencionaba la presencia de 2 vías: la extrínseca, formada por el factor tisular (FT) y el factor VII, y la intrínseca, en la que participan los factores XII, XI, IX, VIII y V. Hoy en día este concepto ha cambiado y se acepta de forma categórica que el evento iniciador principal de la coagulación sanguínea es la exposición del FT. En este artículo revisamos los nuevos conceptos de la cascada de coagulación con los hallazgos de la tromboelastografía y los diferentes mecanismos que precipitan la coagulopatía asociada al trauma. Para tal fin se realizó una búsqueda sistemática en las principales bases de datos, como Medline, Embase y Lilacs, en el periodo comprendido entre 2000 y 2011. Se encontraron 114 artículos, de los cuales se tomaron 50 para realizar la revisión. Como hallazgo relevante se encontró que la tromboelastografía permite detectar con precisión el defecto subyacente en la cascada de coagulación, y de esta manera se ha convertido en una herramienta útil e indispensable para guiar el manejo de la coagulopatía asociada al trauma.
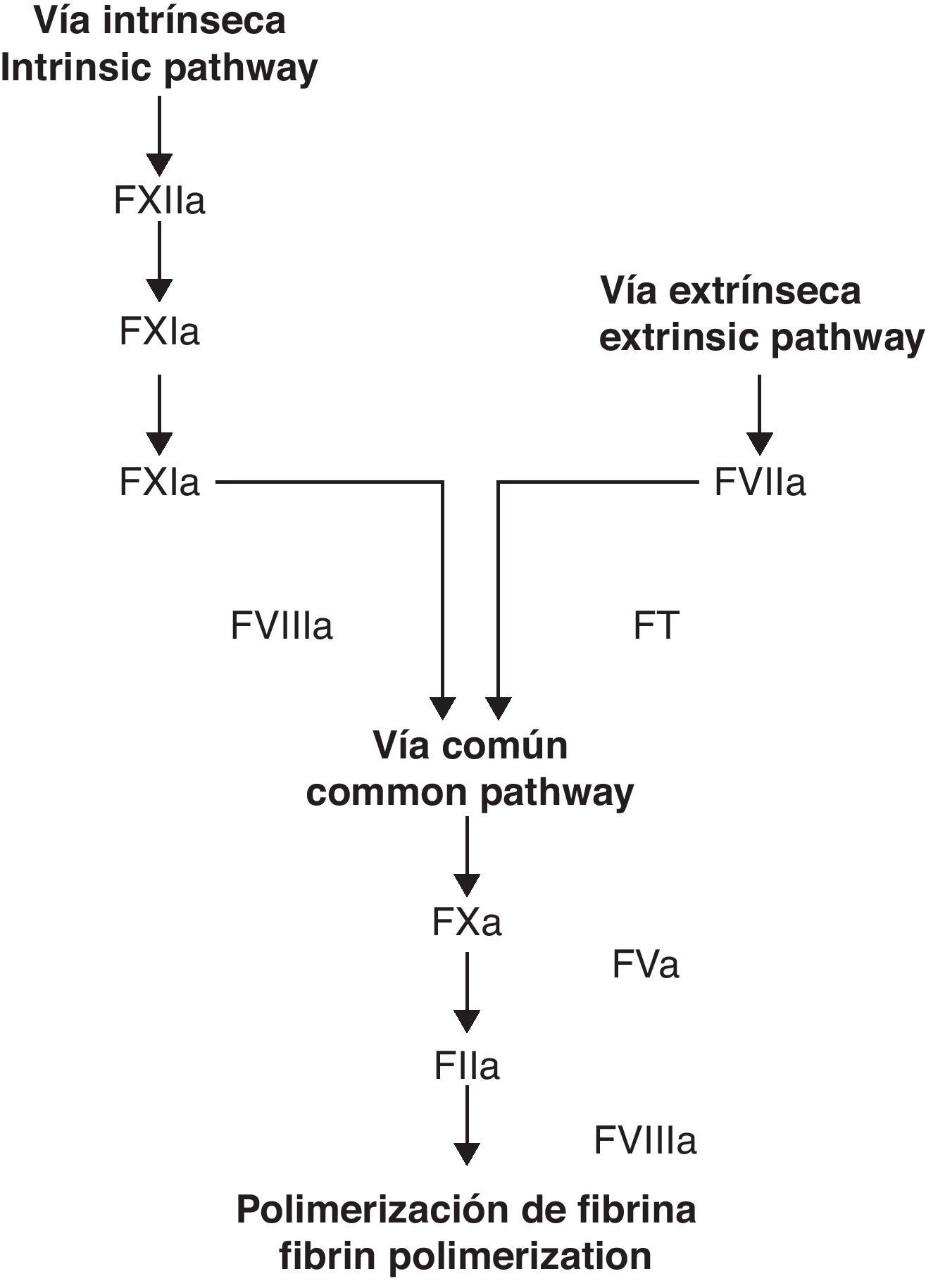
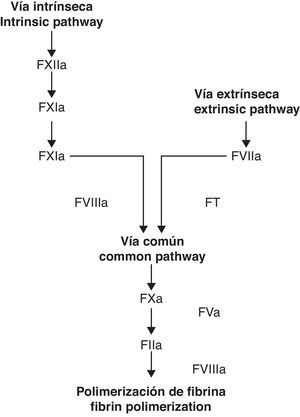
For decades, the coagulation cascade has been considered to have two different starting points, which are commonly labeled as extrinsic and intrinsic pathways. In time, however, it has become clear that these pathways do not function as parallel and/or independent systems.1 It has been proven that the TF-factor VIIa (FT/FVIIa) complex of the extrinsic pathway activates both systems, implying a correlation among them.2 These findings, in addition to an increasingly growing understanding of platelet function have given way to the cellular coagulation model.3 In contrast to the older model (extrinsic/intrinsic pathways), the celular model includes important reactions between cells directly implied in haemostasis (TF receptor cells such as the monocyte and fibroblast) and the coagulation factors.4
MethodsA systematic search on the main databases such as Medline, Embase and Lilacs was carried out. The following key words were chosen: haemostasis, coagulation, thromboelastography, coagulopathy and trauma. The search was aimed at data from the time period between 2000 and 2011. Research yielded a total 114 articles. Each was analyzed exhaustively and 50 documents were finally selected for revision.
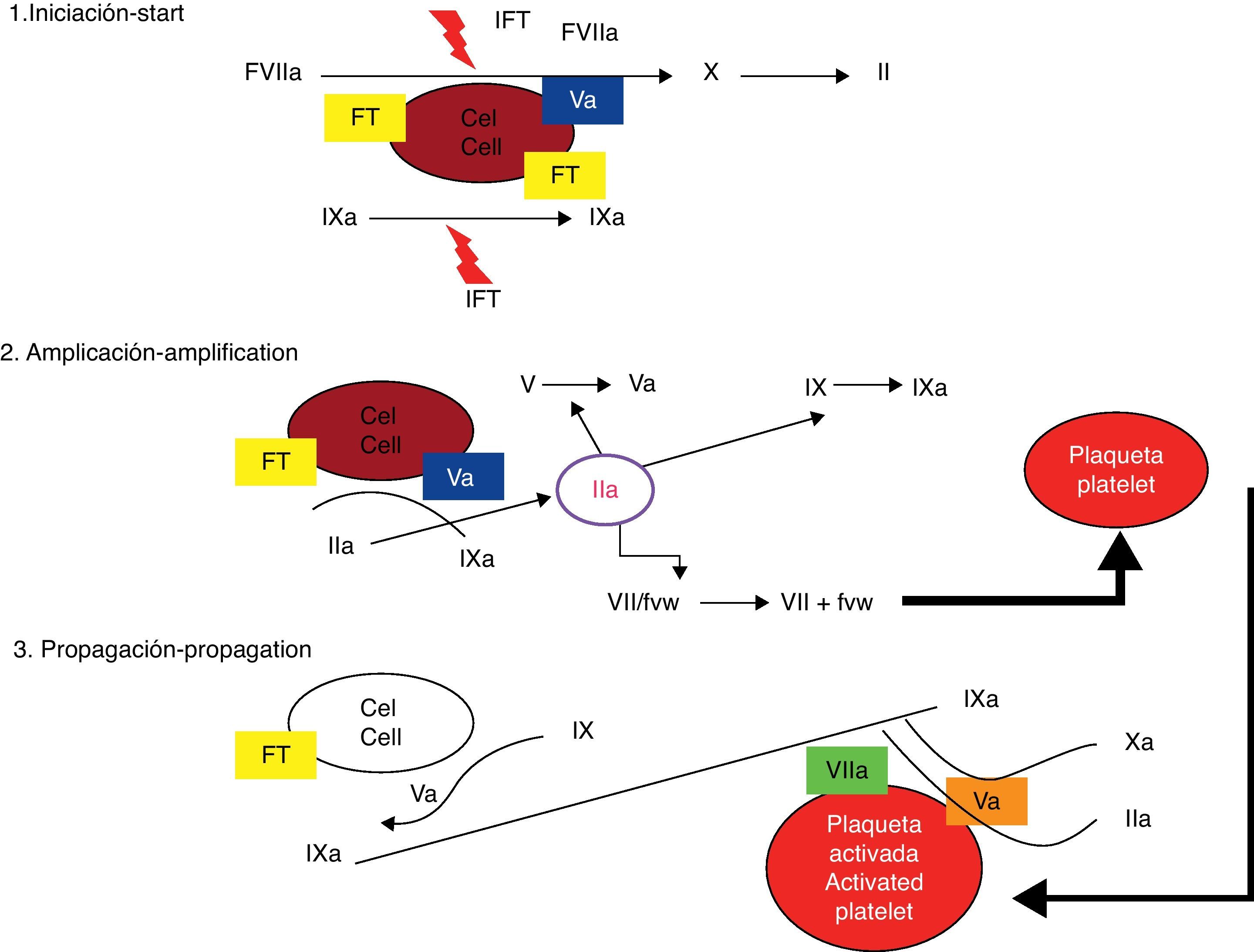
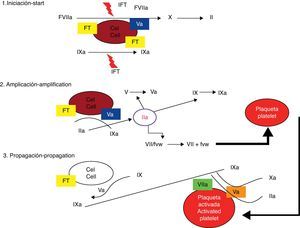
The cellular modelThe celular model identifies the membrane of the TF receptor cells and platelets as the sites where coagulation factor activation occurs.5 The traditional model, posed 40 years ago, separated the cellular and humoral phases of coagulation and considered that the process of haemostasis was achieved through sequential activation of effector enzymes in two different pathways.6 Recently, a new model that allows greater understanding of how the haemostasis process really functions in vivo (Figs. 1 and 2) has been conceived. This new theory is known as the cellular coagulation model. It emphasizes on interactions between soluble factors and cell membranes and cells are considered essential elements that determine the haemostatic process.7 The new model highlights the importance of the TF/FVIIa complex in the activation phase of the system and considers coagulation to be a process of three different phases that involve different cell membranes and occur simultaneously: initiation, amplification and spreading.8,9
Classic coagulation cascade model. From Jiménez et al.49
Cellular model (modern model) of coagulation cascade. From Jiménez et al.49
The TF/FVIIa complex activates factor X directly and indirectly through factor IX, transforming small quantities of prothrombin into thrombin, which are still insufficient for completion of the fibrin formation process.10 Factor VII in the bloodstream is mainly an inactive molecule, and its physiologic function are virtually nonexistent if its cofactor is not present.11,12 TF is not in contact with blood elements. The cell that holds its receptor (fibroblast, myocyte, mononuclear cell, macrophage) is outside the vascular system so long as it holds its integrity.13 The interaction between TF and FVIIa is the fundamental step in coagulation initiation; it increases factor VII activity by 1×107.14
Amplification phaseThrombin, altogether with calcium and acid phospholipids form the platelet, has an active role in a feedback process for activation of factors XI, IX, VIII and V, as well as accelerating platelet activation.15 Through chemotactic mechanisms, the factors mentioned are simultaneously drawn to the platelet's surface, where very important processes of activation and multiplying occur rapidly.16 The amplification phase depends on the presence of activated platelet membranes and the interaction of these with the coagulation factors, especially with the low amounts of thrombin generated near the cell transporting TF.17 Platelets are activated and then degranulated, while they adhere and form a clot in the injured vessel. A very important characteristic of platelet activation is the polarity change of the negatively charged ends of the phospholipids, which allows interaction with coagulation factors.18 The small amount of thrombin yielded by the TF/factor VIIa pathway is essential for amplification, even though it is insufficient for the formation of a clot.19 Thrombin is a very active platelet recruiting agent and gives positive feedback because it can also activate factors V, VIII and XI. Amplification is also characterized by activation of the negative feedback system through natural anticoagulants: TFPI (TF/FVIIa complex inhibitor), antithrombin and C protein, which carry out an important role in regulating procoagulation.20
Spreading phaseAmplification of coagulation through feedback mechanisms involving thrombin, platelets and activation of factors allow factor X to become very active and form the prothrombinase complex in order to convert prothrombin into thrombin and consequently turn fibrinogen to fibrin.21 The final process always takes place in the platelets’ surface, and it is accelerated to quickly generate great amounts of thrombin and fibrin. The spreading phase takes the processes that lead to thrombin generation from the TF transporting cell onto the active platelet.22 Phospholipids in the active platelet's membrane allow the IXa/VIIIa complex to assemble and enhance its activity 1×108 fold. Great amounts of thrombin are produced during this phase, resulting in proteolytic escision of fibrinogen and formation of fibrin monomers that polymerize to solidify the initial platelet clot into a firm, organized fibrin clot. Thrombin simultaneously activates factor XIII and TAFI with positive additional effects on the stability of the clot and plasmin effect resistance.23
Role of plateletsPlatelet activation alters permeability of the membrane, allowing calcium entry and chemotactic substance liberation that draws coagulation factors to it. Factor V and acid phospholipids are released at the same time, providing the complement necessary for coagulation.24
In conclusion, the new coagulation cascade holds fibrin formation as a joint result of two processes: coagulation (represented by thrombin) and platelet activity, which are mutually complementary.
FibrinolysisFibrinolysis is another fundamental process in coagulation. Its purpose is to eliminate fibrin clots during the healing process, and to remove intravascular clots to prevent thrombosis.25 The final effector is the plasmin system, which causes fibrin breakdown into degrading products (PDF and D dimer). Plasmin is synthesized from plasminogen, an inactive precursor, by two activating agents of plasminogen: the tissue activator (t-PA) and the urokynase type activator (u-PA). Regulation of these activators depends on the activity of inhibitor agents (PAI), of which PAI-1 is the most important. Plasmin itself is quickly inhibited by α2-antiplasmin, thus preventing systemic fibrinolyisis.26 Fibrinolysis is initially stimulated by t-PA released by endothelium cells as a reaction to several stimuli (thrombin, venous occlusion, exercise, etc.). Once in the bloodstream, it binds with fibrin and activates conversion of plasminogen into plasmin, and finally the fibrin clot is broken down.27 Thrombin can actívate another fibrinolytic inhibitor, TAFI. This agent eliminates residual lysine from degraded fibrin, inhibiting plasminogen and clot breakdown.28
Coagulation monitoring in patients with massive hemorrhageThere are several tests for assessment of the coagulation process. However, thromboelastography is the most useful for coagulopathy in trauma patients.29 This test measures the physical properties of blood as a fluid from a dynamic standpoint. It allows proper assessment of the different phases of coagulation and fibrinolysis, thus providing pertinent data aimed at detection of defficiencies in the haemostatic system.30
ThromboelastographyThromboelastography is a tool for measurement of the adhesiveness, elasticity and other physical properties of the blood from a dynamic and global perspective.31 It was developed in Germany in 1948 by Hartert; yet it remained fairly unused until the mid 1980s, when Dr. Kang et al. picked it up for coagulation management during liver transplant and heart surgery with cardiopulmonary bypass.32 From that moment on, it has gained more and more importance in several fields of medicine, including obstetric anesthesia, trauma patient anesthesia and management of critical patients with coagulopathy.22
Samples for thromboelastogram (TEG) are taken from unmodified blood and they are generally extracted from central catheters or arterial lines since TEG is often performed in surgical and intensive care unit patients.33 The amount needed for testing is 3cc and it can be obtained with a regular needle or a citrate tube (blue tube). The citrate tube provides a much larger time window for processing (up to 2h). Nevertheless, a standard time of 15minutes is recommended to begin processing. This test is carried out by placing 0.36cc of blood in a cup that must be synchronized with the patient's body temperatura. At this point, a pin is introduced into the sample at a 4°45″ angle. This pin decodes the physical properties of clot formation. The cup spins as the sample's physical properties change and an electronic device keeps a record of these variations and draw a curve that shows the results and absolute values of the pertinent paramteres.34
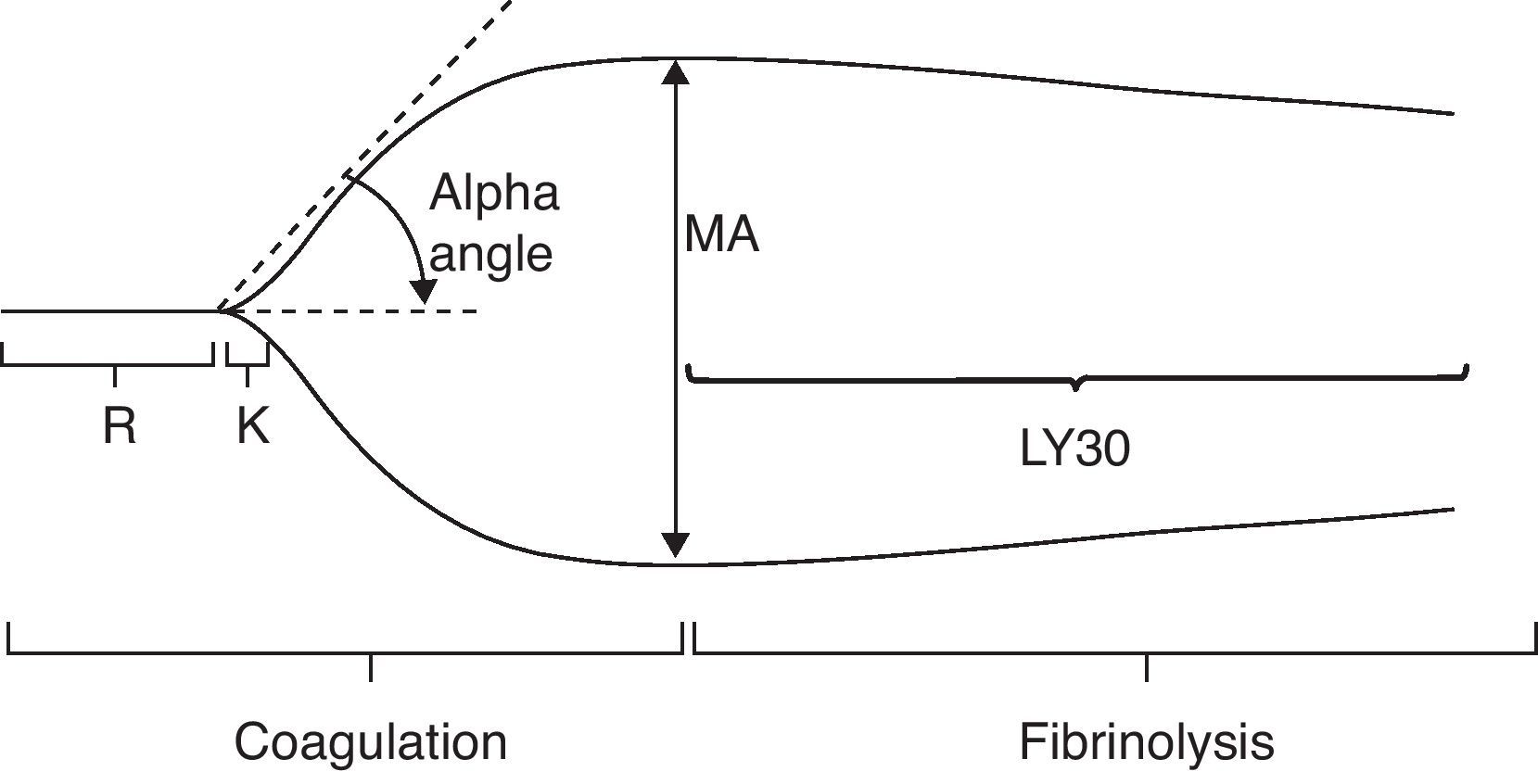
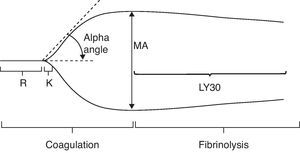
These are the parameters and the conventional values for the different stages of TEG (Fig. 3).35
Normal thromboelastography. From Gempeler et al.50
R. Reaction time (minutes): this is the time interval between the start of coagulation and the point where the TEG reaches a 2-mm amplitude. It shows the rate at which thromboplastin is generated and the function of the intrinsic pathway, especially the activity factors XII, XI and VIII. It is delayed by coagulation factor defficiencies and anticoagulant drugs (warfarin, heparin). If shortened, it indicates the presence of hypercoagulability regardless of its origin. The normal interval is 4–8min.
R+K. Coagulation time (minutes): this is the time interval between the start of coagulation and the point where TEG amplitude reaches 20mm. It shows the rate at which a relatively firm clot is formed. It also measures the function of the intrinsic pathway, platelets and fibrinogen. In this phase, platelet activity reaches its peak and fibrinogen activity is prolongued if there is coagulation factor defficiency or platelet inhibiting drugs. Time shortening indicates an increased platelet activity. Normal duration is 1–4min.
Alpha angle: this is the angle created by the R arm and the K inclination. It shows the rate at which a solid clot is formed. It is an indicator of the quality of platelets and fibrinogen. The angle is greater if there is higher platelet activity or blood fibrinogen, and it is smaller if anticoagulants are or platelet inhibitors are present. The normal alpha angle ranges from 47° to 74°.
MA. Maximum amplitude (mm): MA is the greatest diameter of the clot and a measure of the clot's elasticity. It increases with higher quality of platelet, fibrinogen and factor XIII. It shows the greatest measure of the clot and it relies fundamentally on the interaction of fibrin and platelets. Normal values range from 55 to 73mm.
A60: this is the maximum amplitude at 60min (mm).
CLI. Clot Lysis Index (%), A60/MA: this is a quotient measure that indicates the amount of the clot that shows fibrinolysis in a set time (30min). Its normal value ranges from 0% to 8%. If values above 8% are registered, primary and/or secondary hyperfibrinolysis must be considered.
G. It is a part of the maximum amplitude; it is obtained with the following formula: 5000 MA/(100−MA). It is an indicator of how firm the clot is, it is reported in absolute numbers and is very sensitive to changes in maximum amplitude.
CI. Coagulation Index: It is a numerical value that may be positive or negative, ranging from −3 to +3. If lower, it is suggestive of hypocoagulation; whereas it suggests hypercoagulation if higher.
T: Thrombosis.
F: Clot lysis (minutes): it measures the time interval between MA and 0 amplitude in the TEG. It is a marker of fibrin lysis activity.
Clinical use of thromboelastographyThromboelastography is a test that can be carried out beside the patient, providing valuable information on the state of coagulation. It allows blood transfusion therapy to begin earlier and directed towards more specific disorders such as coagulation factor deficits and/or platelet deficiency or dysfunction.36
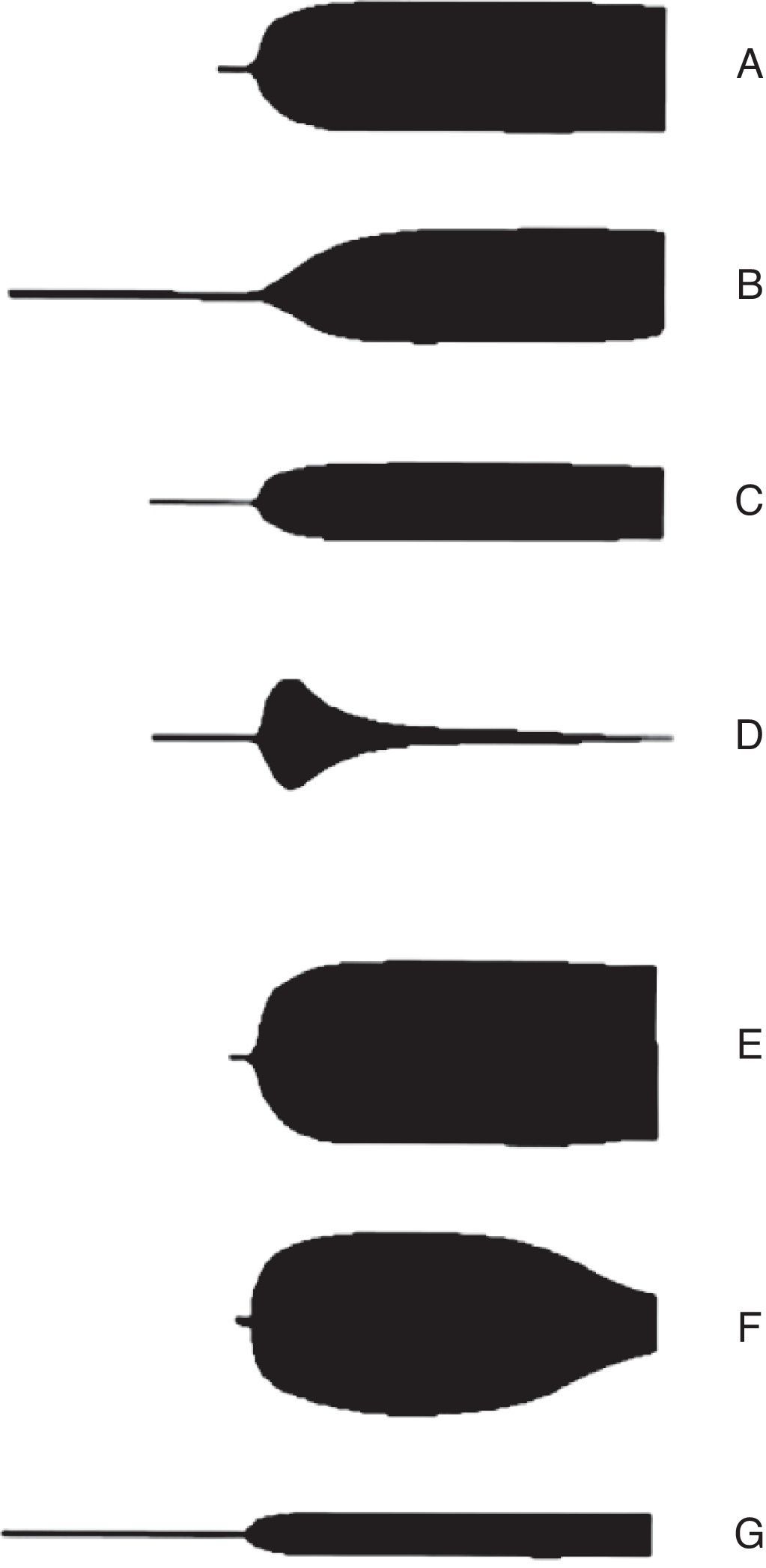
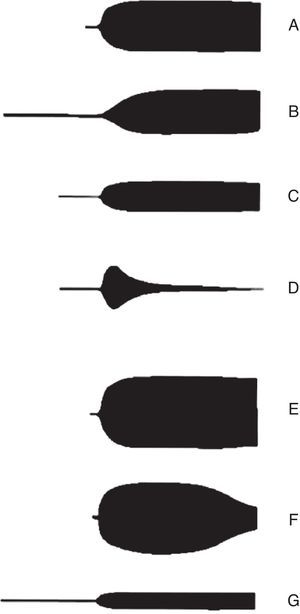
The advantages of thromboelastography include in vitro analysis of the relation between different components of coagulation, and thus observing interactions among platelets, fibrinogen and coagulation proteins from a general standpoint. Opportune and reliable information is indispensable when faced with a patient with bleeding that has quick and complex shifts in haemostasis; if possible, in real time.37 The main utility of TEG is that it allows integration of conventional coagulation tests and platelet function, achieving a larger understanding of the physiology of haemostasis. According to thromboelastography patterns (Fig. 4) it is posible to accurately identify the underlying defect in the coagulation cascade. Therefore, it is possible to know which phase is altered and ease patient treatment decisions.38
Thromboelastography patterns. A: Normal, B: prolongued (anticoagulation and factor deficiency), C: decreased maximum amplitude (thrombocytopenia and platelet function inhibitors), D: fibrinolysis, E: hypercoagulability, F: disseminated intravascular coagulation (DIC), G: end-stage DIC (hypocoagulation). From Raffan-Sanabria et al.30
Coagulopathy in trauma is a complex process; it causes tissue damage and hypoperfusion, which lead to a series of events that result in a state of hypocoagulation.39 Even though hypothermia, acidosis and coagulation factor dilution (secondary to crystaloid reanimation) have an important role; current evidence suggests tissue damage, hypoperfusion, accelerated fibrinolysis and inflammatory are also crucial for the development of coagulopathy.40,28
Coagulopathy etiology in trauma patientsThere a are several causes of bleeding in the trauma patient41:
- 1.
Dilutional coagulopathy: Dilution of coagulation factors and platelets after large volume infusion (coloids, crystaloids) in initial reanimation for preserving circulation volume.42
- 2.
Hypothermia: This is the main cause of coagulopathy in traumatic shock. It induces severe platelet dysfunction and blocks physiologic enzyme reactions of coagulation.43
- 3.
Consumption coagulopathy: Disseminated intravascular coagulation (DIC) is a pathologic process characterized by massive activity of coagulation and procoagulant capacity reduction.44
- 4.
Polytransfusion: repeated stored blood transfusion leads to coagulopathy.45 Whenever the supply of hematic derivates is increased it is possible for “blood bank lesions” to appear. These are characterized by:
- •
Oxygen tissue delivery impairment,
- •
Hypothermia,
- •
Citrate intoxication: low serum calcium,
- •
Hyperkalemia,
- •
Acidosis,
- •
Hiperglycemia.
- 5.
Fibrinolysis: Although infrequent, a hyperfibrinolytic state may appear in the early stages of trauma.46
- 6.
Acidosis: pH is most important factor in coagulopathy prognosis. The activity of factor VIIa, TF/FVIIa complex and Xa/Va complex is progressively reduced as pH values reached 7. Coagulation factor enzyme activity decreases in as much as 90% a pH.47
- 7.
Lesion severity: Brain mass trauma, bone fractures and the presence of amniotic fluid are situations where embolic events are likely. Even more so considering these are sources of thromboplastin, TF and may cause disseminated intravascular coagulation, further rising coagulation factor consumption.48
Recent breakthroughs in coagulation cascade physiology allow larger understanding the mechanisms for bleeding in trauma patients. The use of thromboelastography has led physicians to act on events that occur in the patient with better criteria, thus offering direct medical therapy on the real cause of the base haemostatic alteration. Many uncertainties remain regarding the optimal management of trauma coagulopathy, yet medical investigation has given way for further knowledge to reveal itself on treatment of this pathology.
FundingNone.
Conflict of interestsNone.
Please cite this article as: Galvez K, et al. Tromboelastografía: nuevos conceptos en la fisiología de la hemostasia y su correlación con la coagulopatía asociada al trauma. Rev Colomb Anestesiol. 2012;40:224–30.