Coronary artery disease is a major cause of death and disability among women, worldwide, particularly during the postmenopausal state, with the caveat that their presenting symptoms are atypical compared to men. Also, there is conflicting data regarding the ideal method to determine their risk, particularly for primary prevention. During this review, we sought to determine the utility of the coronary calcium score for assessing such risk in asymptomatic women, by reviewing the most representative current data and analyzing additional studies to help improve the understanding of mechanisms of coronary artery calcification and determine better strategies of prevention and treatment in women with a positive CCS.
La enfermedad arterial coronaria es una causa importante de mortalidad y discapacidad en mujeres en el mundo, especialmente durante la postmenopausia, con la salvedad de que presentan síntomas atípicos comparados con los hombres. Además, existen datos contradictorios en cuanto al método para determinar su riesgo, en particular para la prevención primaria. En esta revisión se busca determinar la utilidad del puntaje de calcio coronario para determinar tal riesgo en mujeres asintomáticas, al revisar los datos actuales más representativos y analizar estudios adicionales para ayudar a mejorar la comprensión de los mecanismos de la calcificación de las arterias coronarias, y definir mejores estrategias de prevención y tratamiento en mujeres con un puntaje de calcio coronario positivo.
Coronary artery disease is a major cause of death and disability, worldwide, among women. Despite the fact that mortality rates from cardiovascular (CV) diseases have declined over the past four decades, CV mortality in younger women has plateaued since the year 2000.1,2
In the United States (US), the life-time risk of developing CAD for women aged 40 and 70 years is 32% and 24%, respectively.3,4 In Colombia, female CV mortality was responsible for 48.6% of all deaths between 1998 - 2011; additionally, from 2010 to 2014, the incidence/year in women was 52.948, which represents 23 more new CAD cases/100,000 compared to the prior four years.5
Women with CAD carry a heavier burden of risk factors (RFs), are, on average, 10 years older than men at the time of presentation, and, when diagnosed before age 45, have a worse prognosis.6–9 Nonetheless, women generally do not identify their initial symptoms as possible CAD due to their atypical symptoms, and for that reason initial risk stratification may be delayed. In an era in which the ideal method to assess CAD risk in asymptomatic subjects is still debated, CCS has emerged as a new strategy to stratify patients in both genders.10
CAC basics in cardiovascular assessmentCoronary calcium score imaging is currently performed using a multidetector computed tomography (CT) scanner with electrocardiographic gating capabilities. Non-gated acquisitions have shown a false negative rate of 8.8% with 19.1% underestimation of a high CCS, and a correlation coefficient for agreement of 0.94 with a gated acquisition. CCS imaging is performed without contrast, during a single breathhold, usually in a prospectively triggered axial acquisition, with image reconstruction at 3mm without overlap. Retrospectively gated acquisition can also be obtained, but results in higher radiation doses.11 The effective radiation doses for multidetector CT are 1.0 to 1.5 mSv in men and 1.1 to 1.9 mSv in women, which are equivalent to the effective radiation dose given off by 10-15 and 11-19 Chest X-rays, respectively.
CCS uses a calcium detection algorithm, which defines calcium as pixels of 130 or more Hounsfield Units (HU) of density, covering an area equal to or greater than 1mm2. The two most common methods of calcium quantification are the Agatston method and the volume method, which perform comparably. The Agatston method, the best studied and established CCS, uses a scaled system depending on the density of the calcifications (1=130-199 HU, 2=200-299 HU, 3=300-399 HU and 4=>400 HU) and multiplies this weighting factor times the area of coronary calcification (square millimeters).12 The volume method uses the area of the calcium and the slice thickness to estimate a volume of calcified plaque.13
The presence and extent of CAC is a marker of the extent of CAD, and can predict the presence of coronary artery stenosis. Extensive CAC may be present before the plaque burden overwhelms vascular remodeling, occludes the lumen, and leads to clinically apparent CAD.14 To relate the CCS to the coronary plaque burden, different studies have classified CAD severity by categories according CCS, as follows: 0=no disease, 1-99=mild, 100-399=moderate and ≥400=severe disease. Furthermore, there are direct relationships between CCS and histologic, intracoronary vascular ultrasound, and angiographic findings in patients with CAD.15–17
Early studies examining the diagnostic and prognostic value of CAC were conducted with electron beam CT based on the Agatston score. During the last 16 years, technology quickly evolved from electron beam to multidetector CT; however, it is reported that CCS using both volume score and Agatston score was equivalent to electron beam CT and 64 slice – multidetector CT.18
CCS significance relies not only on its diagnostic value but on the impact of potential interventions that may prevent future major CV events. As a matter of fact, a high CCS in asymptomatic patients with CAD is associated with major adverse CV events. Nonetheless, CCS was not approved as a reasonable screening test for asymptomatic patients until 2010, when the American College of Cardiology/American Heart Association (ACC/AHA) guidelines on screening for CAD indicated that CCS was reasonable for CV risk assessment in asymptomatic adults with an intermediate Framingham risk-score (Evidence level B), and noted that it might be indicated in selected patients with low-to-intermediate risk.10
CCS accuracyCoronary artery calcification by electron beam CT is a highly sensitive indicator of CAD, as compared to invasive angiography. Compared with angiography, initial “false positive” CAC studies were noted to be true positives for coronary atheromatous plaque, since CAC is a specific marker for coronary plaque.21 Diagnostic accuracy can be improved using gender- and age-specific threshold values.19 In both genders, the sensitivity (SS) and specificity (SP) vary with the degree of CAC; with higher CCS, SS decreases but SP increases, as illustrated by the ACCURACY trial, which reported the following SS/SP: for CCS>0, 98%/ 42%; CCS>100, 88%/ 71% and for CCS>400, 60%/ 88%.20
Studies using a negative CCS to assess accuracy for CAD detection have demonstrated very high SS (generally>95%) and an even higher negative predictive value, 99%, in most studies. On the other hand, the clinical implication of a negative CCS in patients with chest pain syndrome has been under debate, since it might not be an optimal strategy to rule out CAD due to the low prevalence of a negative CCS among symptomatic patients (12 -28% in most studies). In this scenario, CCS provides useful data only in 20% of the cases, which is not desirable for stratification in this selected population.22
CCS compared to other methods used for risk stratificationNoninvasive ImagingThere is a suggestion that CAD may be diagnosed more accurately using CCS compared to radionuclide myocardial perfusion imaging (rMPI). A study of 1,197 patients without clinical CAD who were assessed with both methods, reported a CCS in those with stress-induced ischemia compared to those without, as follows>0 in 95%/ 78%, ≥100 in 88%/ 56%, and ≥400 in 68%/ 31%. Among those with a CCS<100, the frequency of ischemia on rMPI was less than 2%.16
Another study of 411 patients who underwent exercise rMPI, reported that 78% of those with CAC on electron beam CT did not have inducible ischemia with exercise testing, although the likelihood of ischemia increased proportionally with the CCS. There were no women with a CCS<10 who had ischemia, but ischemia was present in 2.6%, 11.3% and 46% of those with a score of 11-100, 101-399 and ≥400, respectively.23 This data shows that there is a modest correlation between silent ischemia detected by stress testing and that detected by CCS, and suggests the ability of CCS to detect CAD despite negative nuclear stress testing.
BiomarkersThe association of inflammatory biomarkers with CAD presence and progression is well known. The SWAN cohort included 372 asymptomatic women (mean age 51.3, 35.2% were African-American) in whom CCS and high-sensitivity C-reactive protein (hsCRP), fibrinogen, plasminogen activator inhibitor 1 (PAI-1), tissue plasminogen activator antigen, and circulating factor VII (factor VIIc) were evaluated separately. All biomarkers were positively associated with the presence and extent of CAC, after adjusting for Framingham Risk Score, site, ethnicity, menopause status, economic strata, and education level. Among African-American women only, hsCRP was independently associated with CAC after multivariate analysis, suggesting that hsCRP and the inflammatory cascade may have an even stronger role in CAD among African-American middle-aged women.24 In a similar population, Wang et al. analyzed 252 women (age 51.2±2.6, 67.5% were white, 56.4% were pre- or early peri-menopausal) in whom biomarkers were measured at baseline, and CCS was quantified at baseline, and after 2.3 years of follow-up; PAI-1 was reported to be associated with CAC progression in middle-aged women, which makes it a potential target to decrease atherogenesis beyond conventional CV-RFs.25
The value of CCS in womenThe effect of gender on assessing the presence and extent of CAC has been addressed extensively. Bellasi et al. performed a meta- and pooled analysis to determine the CCS prognostic value. From the studies which met criteria (outcome data for women and men, sufficient detail on collection of prognostic evidence and CV RFs); three studies in 6,481 women and 13,697 men, and two observational registries for annual all-cause death rates by CCS in women (n=17,779) and men (n=17,850) were included; the registries involved asymptomatic patients referred by their primary physicians for CAC measurement. Analysis showed a high degree of risk discrimination for all-cause death by extent of CAC, and reported no significant differences between genders for mild-to high-risk CCS, suggesting CAC screening is equally accurate in stratifying risk in both genders.26
Likewise, a study of 50 women (age 56+/- 11) and 89 men (age 47+/- 7), comparing CCS to angiography showed that SS, SP and positive and negative predictive-value for CCS were nearly identical for both genders, regardless of CCS. Negative predictive value in men and women ranged from 79%-91% for any CAD and from 95%-100% for significant CAD, respectively. The CCS was significantly lower in subjects with normal angiography compared to those with significant CAD; however, CCS had limited ability to separate trivial, moderate, and significant CAD.21
Haberl et al. analyzed the data from a population of 1,764 patients (1,225 men aged 56±14, and 539 women aged 60±16) with suspected CAD (65% had angina) who underwent electron beam CT and angiography. Angiography revealed, in men and women respectively, as follows: ≥50% coronary stenosis (significant CAD) in 56% and 47%; ≥75% stenosis in 37% and 30%, and normality in 25% and 41%. In terms of the SS at different percentiles of age groups, SS to detect stenosis in men and women, respectively, fell to 97% and 98% at the 20th, to 93% and 82% at the 100th and to 81% and 76% at the 75th. However, the SP increased to 77% in both genders, confirming what has been noted before. Negative CAC was found in 23.7% of men and 40.8% of women without significant CAD. Compared with angiography, among women with negative CAC there was no significant CAD; nonetheless, 0.7% (n=5) of men with negative CAC were identified as having significant CAD, and two of them required intervention. Despite this rare finding, exclusion of CAC was associated with a probability of stenosis (<1%), even in symptomatic patients.19
The most interesting data comes from the ELSA Brazil Study which noted that, after adjusting for age and sex, low-risk individuals (n=3,616, 54% women; mean age 50 years) had significantly lower CAC prevalence and burden compared with other low-risk individuals worldwide. This is the first study to suggest that current CCS strata by ethnicity may not be applied universally to all Latin-American patients, according to the current and validated method used routinely in clinical practice.27
Influence of age on CAC in womenThere is robust evidence that women have a lower CCS than men, and that the amount and prevalence of calcium increases steadily with age in both genders.28
Haberl et al. showed a remarkable difference in CCS between men and women in all age groups, with mean CCS significantly higher in men, a tendency that was maintained with older age; the wide CCS variability noticed within each age strata was caused by a minority of individuals with very high CCSs. Nonetheless, the reliability of CCS in predicting significant stenosis was equally effective in both genders and the diagnostic benefit was uniform across all age groups.19
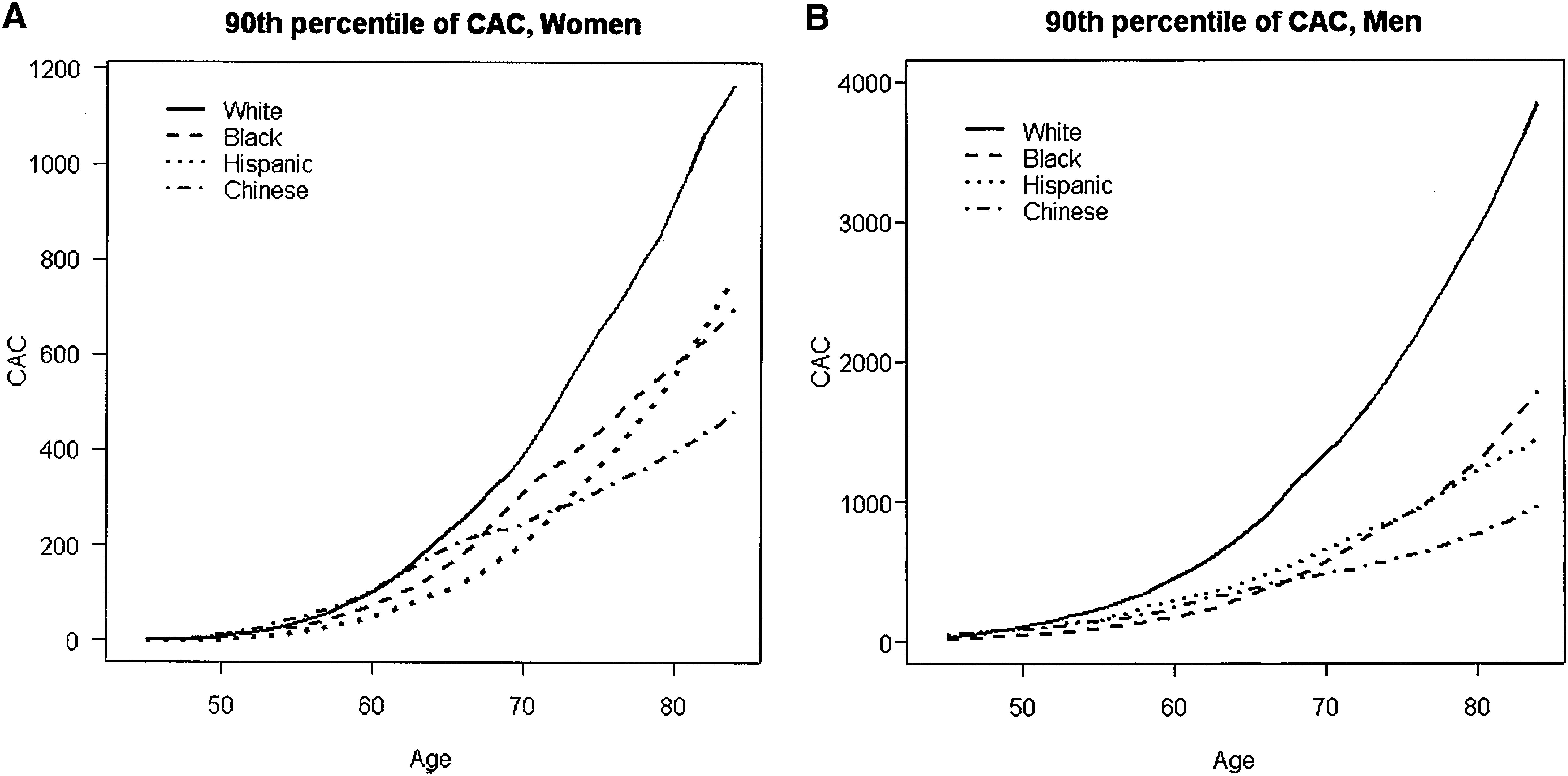
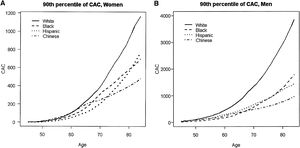
Additionally, the MESA study showed that the relationship between the probability of any detectable calcium and age was linear, with certain gender and race subgroups for which there was discernible nonlinearity. Figure 1A below, taken from the MESA study report, illustrates how age relationships for women tended to be concave up, increasing more slowly, which was particularly true for Hispanic women, who had the lowest rate of positive CCS; graphics also show that these two plots are very different between genders: the scale in men (Figure 1B) is three times as large in the y-axis compared to women.28
(A and B) Estimated 90th percentile of the CAC distribution by gender, age, and race/ethnicity. CAC: coronary artery calcification.
McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113(1):30-7.
In older adults, studies have illustrated how CCS reclassifies their risk. Raggi et al. followed 1,795 asymptomatic patients with a mean age of 69.2 during 9.2 years, and reported that in the intermediate risk group by Framingham Risk Score, CCS reclassified 52% of the population, using cut-offs of>615 Agatston units as high-risk and<50 as low-risk.29 Additional data showed that up to 40% of patients over the age of 70 can be reclassified as low-risk for CAD with a negative or low CCS, despite having multiple RFs.30
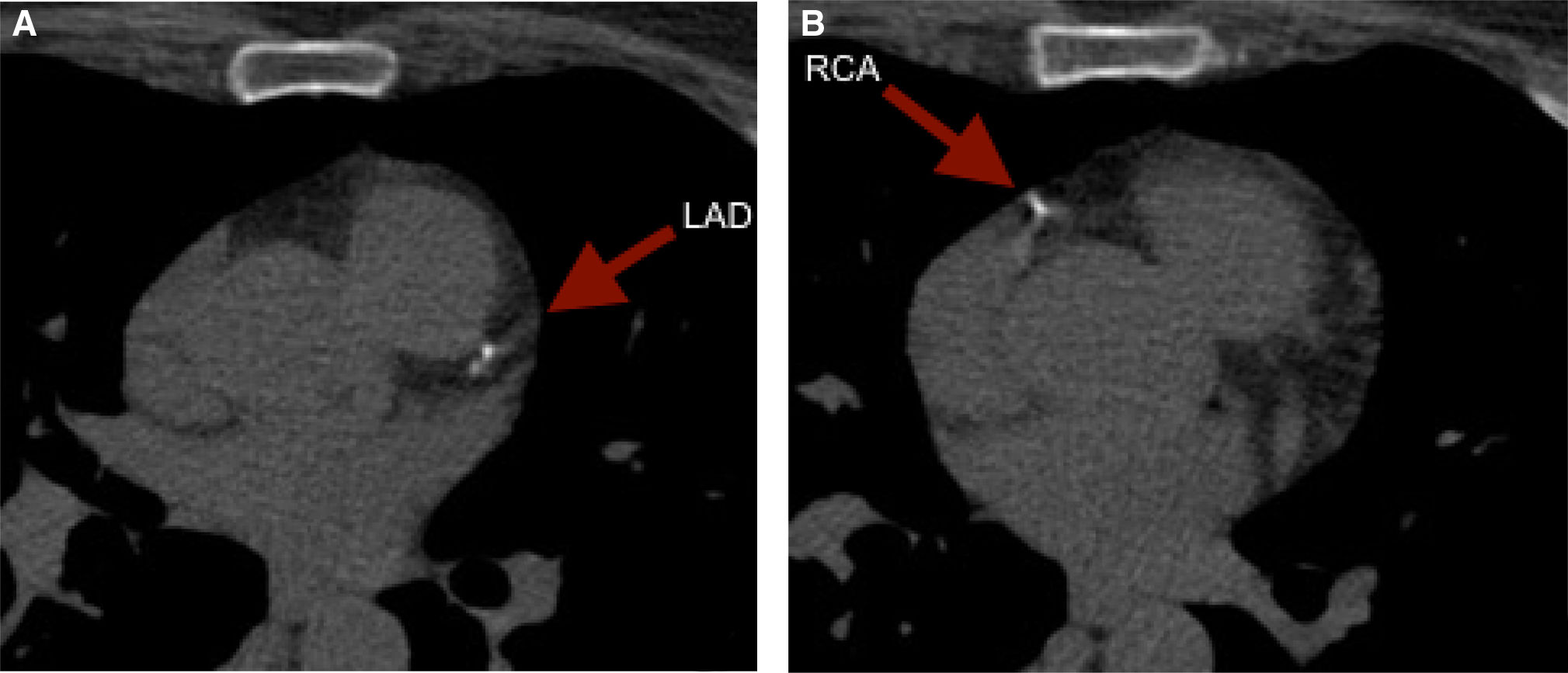
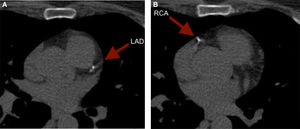
It is of note that although less reported, CCS can also reclassify younger adults. To illustrate, we present the case of a 59 year old female with an intermediate risk profile using the Framingham Risk Score, who was found to have a CCS of 52. The images revealed punctuate calcium deposits in the left anterior descending (LAD) (Figure 2A) and right coronary artery (RCA) (Figure 2B).
With regard to the age effect on RF-based prediction of CAC in symptomatic patients, a retrospective study of 6,309 symptomatic patients (62% male) reported that while in patients aged<70 in either gender, RFs (including diabetes (DM), hypertension and dyslipidemia) had an equal predictive ability for CAC; in patients aged>70, dyslipidemia in men, and smoking and DM in women, predicted a positive CAC. This illustrates the differences in the ability of conventional RFs to predict CAC between genders and patients<and ≥70 years of age.31
Influence of ethnicity on CAC among womenReports from the MESA study have shown significant differences in CAC by race, across genders and different age-groups. Bild et al. reported that CAC prevalence was higher in Caucasians compared to Chinese, African American and Hispanic women (45% vs 42%, 37%, and 35%, respectively).32 McClelland et al. noted that among women, CCS was highest in Caucasians and lowest in Hispanics; however, Chinese women had the lowest CCS in the oldest strata.28 Diez et al., after adjusting for age, sex, and income, reported that country of origin other than the US was associated with a lower CAC prevalence in African Americans and Hispanics (adjusted relative prevalence 0.75 and 0.89, respectively); and for Chinese not born in the US, the CAC prevalence increased with the duration of their residence in the US (adjusted RP 1.06 for each 10 years of residence). Also, among Caucasians, low education was associated with a higher CAC prevalence, and a trend towards a lower CAC prevalence in Hispanics.33 Evidence suggests that among women, ethnicity affects the likelihood of having CAC, and its extent, but in general terms, its predictive value for CAD and CV events remains the same.
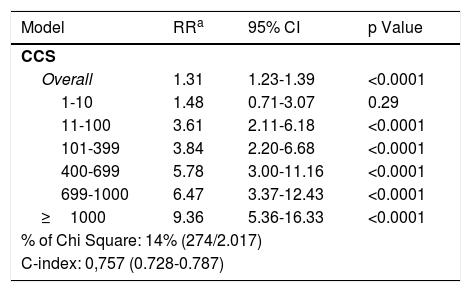
CCS prognostic value additional to current stratification methodsThe CCS adds independent prognostic information to that determined by the Framingham Risk Score. Large observational data series have shown that CCS provides independent incremental information in addition to traditional risk factors in the prediction of all-cause mortality. Budoff et al. studied a cohort of 25,253 asymptomatic individuals (mean age 56+/-11; 54% males) using the National Cholesterol Education Program Guidelines and CCS; this method provided independent ability, compared to traditional RFs, for predicting all-cause mortality. The table shows the risk-adjusted models for all-cause death; CCS remained independently predictive of all-cause mortality even after adjusting for age, gender, ethnicity, hypertension, hyperlipidemia, DM, smoking, and family history of premature CAD.34
Risk-adjusted models for all-cause death.
| Model | RRa | 95% CI | p Value |
|---|---|---|---|
| CCS | |||
| Overall | 1.31 | 1.23-1.39 | <0.0001 |
| 1-10 | 1.48 | 0.71-3.07 | 0.29 |
| 11-100 | 3.61 | 2.11-6.18 | <0.0001 |
| 101-399 | 3.84 | 2.20-6.68 | <0.0001 |
| 400-699 | 5.78 | 3.00-11.16 | <0.0001 |
| 699-1000 | 6.47 | 3.37-12.43 | <0.0001 |
| ≥1000 | 9.36 | 5.36-16.33 | <0.0001 |
| % of Chi Square: 14% (274/2.017) | |||
| C-index: 0,757 (0.728-0.787) | |||
| CAD | |||
| Overall | 1.58 | 1.28-1.96 | <0.0001 |
| 1-vessel | 1.39 | 0.95-2.03 | 0.086 |
| 2-vessel | 1.85 | 1.03-3.30 | 0.038 |
| 3-vesselb | 2.44 | 1.58-3.78 | <0.0001 |
| % of Chi Square: 4% (25/677) | |||
| C-index: 0.552 (0.511-0.592) | |||
Risk-adjusted controlling for age, gender, hypertension, hyperlipidemia, diabetes, family history of premature coronary disease, smoking, and ethnicity. C-index for risk factor data 0.354 (0.312–0.395).
or Left Main.
CAD: coronary artery disease; CCS: coronary calcium score; CI: confidence interval; RR: relative risk.
Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. Journal of the American College of Cardiology. 2007;49(18):1860-70.
A systematic review to evaluate the predictive accuracy of CCS in women26 concluded that CCS was equally accurate for risk stratification, independent of gender. The study reported that all-cause mortality rates in women were 0.1%/year for those with a CCS 0-10 vs. 1.6%/year for those with a CCS ≥1000. The RR ratios for CAD death or myocardial infarction (MI) in women increased 4.9-fold for mild risk CCS, 5.5-fold for moderate, and 8.7-fold for high risk. Furthermore, a meta-analysis of four major studies16 reported an adjusted RR of death or MI of 2.1 comparing a CCS of 1-100 vs a CCS of 0, for both genders. The RRs of death and MI estimated for CCSs>100 ranged from 3.0 to 17.0, but varied widely among studies. The mean CCS for those with and without a cardiac event was reported at 764 and 135, respectively, and a CCS ≥160 was associated with an odds ratio of 15.8 for a cardiac event. Additionally, there is evidence that CCS remains predictive of a cardiac event after an average follow up of 3.6 years.35
Similarly, the absence of CAC in asymptomatic individuals confers a very low risk of CV events and is associated with a low CAD risk, despite significant CV RF profiles. Patients with a negative CCS have a<1% probability of significant CAD.19 To prove that CCS provides incremental prognostic information after adjustment for traditional RFs in asymptomatic women, data from the National Death Index in 10,377 asymptomatic individuals (40% women) referred by primary care physicians for CAC screening was obtained with mean follow-up of 5+/-3.5 years. Women had a greater probability of death than men in each strata of CCS, and RR ratios were increased by 3 with a CCS of 101-399, by 5.5 with a CCS of 400-1000, and by 5.5 with a CCS>1000, for women compared to men.36
Low and intermediate risk womenCCS has prognostic value in asymptomatic women with intermediate risk. It is reported that a positive CCS in asymptomatic, low-intermediate risk women is associated with a significantly higher event rate, including death, MI, bypass surgery and percutaneous coronary intervention (PCI) (3.3% vs. 1.0% in those with any CAC vs. none, after 37 months’ follow-up).37
It has also been illustrated how the degree of risk correlates with the extent of CAC. A study of asymptomatic patients (mean age 53, during 19 months’ follow-up) reported the following SS/SP for a CCS threshold of 100: 89%/ 77%; 160: 89%/ 82% and 680: 53%/ 95%, with an odds ratio for the development of symptomatic CAD from 22:1 to 36:1.38 Of note, most studies evaluating the predictive value of CCS in asymptomatic, low-intermediate risk subjects used Agatston score absolute values, with a suggestion that those individuals above the 75th percentile have a higher risk for future CV events.15
High-risk womenCurrently, most providers use the modified NCEP/ATP III Framingham Risk Score for stratifying patients and making crucial decisions in terms of primary or secondary prevention, such as the prescription of lipid-lowering therapy (LLT). However, this method does not perform optimally in women, especially in younger age strata; CCS may be considered to be an alternative to weigh this known weakness.17
The MESA study reported that among participants in whom LLT was not indicated by the ATP-III guidelines, the CCS was>400 in 6.8% of patients at intermediate risk and in 19.7% of patients at high risk. Overall, 40% of eligible individuals with a CCS>400 were not receiving LLT.39 Similarly, a report of 1,611 asymptomatic subjects (mean age 53) who underwent CCS reported that despite the fact that no women were at high risk according to international guidelines, severe disease (CCS ≥400) was present in 5% of women.17
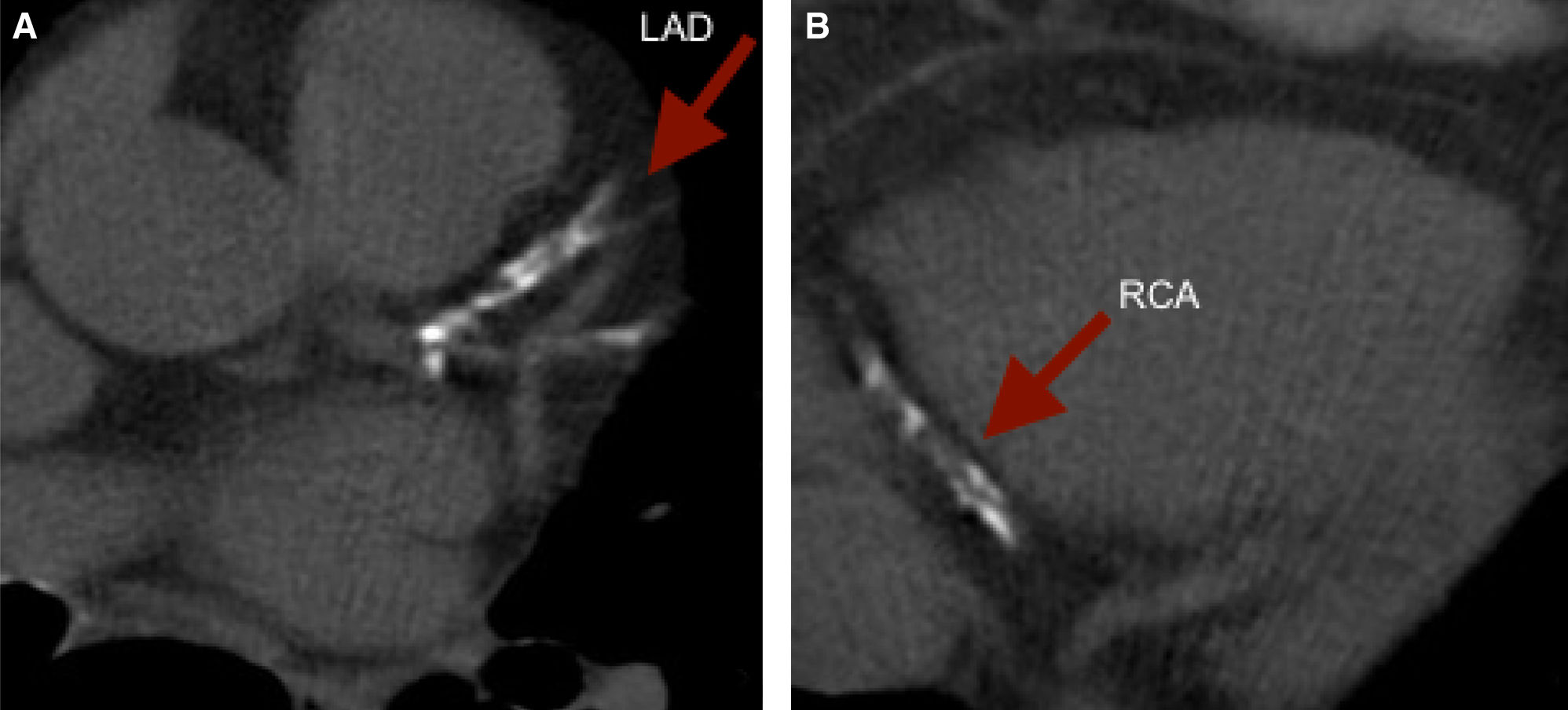
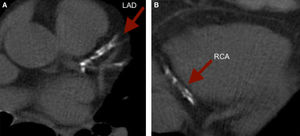
To illustrate, we present a case of a 61 year old female with a strong family history of CAD and a low Framingham Risk Score, who was found to have a CCS of 1,171. The images revealed extensive calcification of the proximal LAD and distal RCA. (Figure 3).
These key observations demonstrate that global RF scores used routinely in clinical practice lack accuracy for identifying women with a positive and even high CCS. The current evidence warrants more studies to define the exact role of preventive measures based solely upon the CCS for an improvement in CV outcomes, or to define the setting, in terms of RF identification, where CCS should be added to routine stratification methods such as the Framingham Risk Score.
Special female conditions and CACMenopauseEstrogen deficiency is a known RF for CAD. Petisco et al. determined the prevalence of subclinical CAD in Brazilian postmenopausal women with a low-to-intermediate Framingham Risk Score. CT and ultrasound were performed in 138 asymptomatic women (mean age 56.1±4.9). Subclinical CAD, defined as at least one abnormal imaging test, was associated with age, Framingham Risk Score, waist-to-hip ratio, systolic and diastolic blood pressure and HDL-c. The reported prevalences accounted for 45.7% increased carotid intima-media thickness (CIMT), 37.7% carotid plaques, 62.3% increased CIMT and/or plaques, 23.9% CCS>0 and 45.7% aortic calcification (AC). Normal imaging tests were found in 22.4%; with carotid atherosclerosis being the most common presentation. Subclinical CAD was prevalent in this population.40
In the Healthy Women Study, the relationship between CV-RF measured before menopause at age 48, CCS and AC by electron beam CT at age 58, as well as the influence of postmenopausal hormone replacement therapy (HRT) on that relationship, was evaluated among 169 women. A very strong association between LDL and CAC was reported; among women with premenopausal levels of LDL<100mg/dL vs LDL>160mg/dL, 9% and 30% had a CCS>/=101, respectively. That relationship was modified by HRT; women on HRT (during at least three to four years) at their eighth postmenopausal examination (mean age 59) had a lower prevalence of extensive CAC; it was significantly related to their LDL levels at pre-menopause. Among women with a baseline premenopausal LDL<130mg/dL, only 4% of those on HRT had a CCS>=101 vs. 12% of those not on HRT. Similarly, among women with a baseline LDL>130mg/dL, 22% of those on HRT had a CCS>=101 vs. 40% of those not on HRT.
It was also noticed that the combination of LDL and HDL identified groups of women with a>nine-fold difference in the risk of a high CCS, and an almost four-fold difference in the likelihood of having no CAC at all. Other RFs associated with a greater CCS were smoking, higher systolic blood pressure, triglyceride levels, and blood glucose; HDL was strongly inversely related to CCS. This study demonstrates that premenopausal RFs are determinants of the risk of subclinical CAD measured by electron beam CT in postmenopausal women, which could help to identify these women, to prevent CAC; it was also concluded that most women on HRT were at low risk of either CAC or AC.41
A later study including 363 women validated prior results, concluding that premenopausal RFs are strong predictors of postmenopausal CAC and AC, which warrants clinical trials for testing if reduction of premenopausal RFs reduces the risk of early calcification. In that study, RFs were assessed when women were premenopausal and five years after menopause; CAC and AC were assessed by electron beam CT on average 14.6 years after study entry, and 267 had a second electron beam CT 6.4 years later.42
Gudmundsson et al. also assessed the relationship between HRT and CAC in a cross-sectional study that included 2,867 women (mean age 76±5). Electron beam CT-CCS was compared between women with a history of HRT and those who had never used HRT. There were significant negative associations between CAC and history and length of HRT use, in all age categories. When HRT had been used>15 years, the median CAC level was less than 50% compared to controls. The prevalence of CV disease was similar in both groups.43
CAC association with non-cardiovascular comorbidities among womenDiabetes mellitusDiabetic patients experience higher CV disease incidence and mortality. In patients with type 1 DM, CAD occurs earlier in life and affects women almost as often as men; the loss of relative protection from CAD in pre-menopausal diabetic women is not well explained by differences in established RFs. A study that examined whether estimated insulin resistance and insulin resistance related factors were associated with CAC, concluded that insulin resistance was associated with CAC independent of CAD-RFs, and that gender differences in insulin resistance-associated fat distribution may explain why type 1 DM increases CAC in women relatively more than in men.44
Olson et al. sought to determine the relationship between CAC detected by electron beam CT, clinical CAD (defined as a confirmed history of MI, significant CAD, Pittsburg Study physician-diagnosed angina, or ischemic electrocardiogram) and established CV-RFs in type 1 DM. The study included 302 patients with type 1 DM (mean age 38.1+/-7.8); CAC had an SS of 84% in men and 71% in women for clinical CAD; and 100% SS for MI or obstructive CAD. A CCS of 400 was the most efficient, correlated to clinical CAD. Among subjects with angina only, CAC SS was 83% in men and 46% in women. They concluded that CAC was independently correlated with MI and clinical CAD in both sexes, but was not independently associated with angina and ischemic ECG in either sex.45
On the other hand, type 2 DM, known in cardiology due its widely debated role as a CAD risk equivalent, is associated with a two-fold increased risk of CAD, stroke, and CV mortality.46 A systematic review and meta-analysis of eight studies (n=6,521; 802 events; mean follow-up 5.18 years) to evaluate the baseline CCS in type 2 DM patients and subsequent all-cause mortality or CV events (fatal and non-fatal), reported the RR for all-cause mortality or CV events, or both, comparing a total CCS ≥ 10 with a CCS<10 was 5.47. The overall SS of a CCS≥10 for this composite outcome was 94%, with an SP of 34% (24% to 44%). The positive and negative likelihood ratios were 1.41 and 0.18 (0.10 to 0.30), respectively. For subjects with a CCS<10, the post-test probability of the composite outcome was about 1.8%, representing a 6.8-fold reduction from the pre-test probability. This proved that the CCS predicts all-cause mortality in patients with type 2 DM, with an increase in mortality for every increment in CCS.47
In terms of primary prevention, it should be emphasized that current evidence proves aspirin appears to produce a modest-sized reduction in MI and stroke in patients with DM, but is not conclusive because of the few events in the available trials to precisely estimate its effects, and because most analyses have been made based on subgroups within larger trials, which have more potential for bias. Assessment of CAD by CCS also may be relevant due to the fact that patients without CAD will not benefit from low dose aspirin, which is clinically important since>30% of asymptomatic diabetic patients may not have CAD. The currently available data also reinforce that the possible differences in outcomes for men and women require further studies.48,49
The FACTOR-64 trial included 900 asymptomatic patients with type 1 and 2 DM of at least three to five years¿ duration, who were randomized to CAD screening with coronary CT angiography (n=452) or to routine clinical care (n=448). After a mean follow-up of four years, they concluded that there was no significant difference in the primary endpoint (composite of all-cause mortality, non-fatal MI, or unstable angina) following screening with coronary CT angiography (6.2% versus 7.6% without screening); thus it is not recommended as screening in this population.50
Based on the current evidence, in the 2014 Standards of Medical Care in Diabetes, the American Diabetes Association did not recommend routine screening for CAD in asymptomatic patients with type 2 DM, considering that outcomes are not improved if CV RFs are treated.51 Until now, other than the higher level of suspicion given by the significantly increased CV risk in type 1 DM, there is no evidence that warrants special considerations among asymptomatic women with type 1 DM with respect to early CV screening based upon CCS. Nonetheless, a better definition of the characteristics of diabetic patients who may benefit from CAC is really needed to decrease hard outcomes in this population.
Therapy effect on CACPharmacologic therapyCertainty of the risk for CV mortality in patients with a positive CCS has raised the question regarding the potential benefits of starting LLT and aspirin in such patients. Results from randomized clinical trials show controversial evidence regarding the optimal pharmacologic therapy to prevent symptomatic CAD, based on the presence of CAC, in both genders.
The St. Francis Heart Study sought to determine whether LLT and antioxidants retard the progression of CAC and prevent CV events. This was a double-blind placebo-controlled trial of asymptomatic subjects with a CCS ≥80th percentile for age and gender, (n=1,005; 74% males aged 59+/-6; follow-up of 4.3 years). Treatment with low dose aspirin alone or aspirin with LLT (atorvastatin 20mg/day), vitamin C (1g/day) and vitamin E (1,000 U/day), reduced total cholesterol, LDL and triglycerides, but had no effect on progression of the CCS; however, it was associated with a non-significant trend toward a lower rate of all CV events (6.9% versus 9.9%), especially in those with a CCS>400.52
The BELLES trial evaluated the effect of different intensity LLT (atorvastatin 80mg/day vs pravastatin 40mg/day) on CAC in hyperlipidemic postmenopausal women (615 were randomized, 475 completed the study), most with no history of CV disease, with moderate coronary atherosclerosis (calcium volume score ≥30). The degree of LDL lowering was greater in the intensive arm (47%) compared to the moderate arm (25%). Nonetheless, there was no significant difference between the two groups in the degree of CAC progression (15.1 versus 14.3), with the caveat that the follow-up was just one year.53 A similar lack of benefit with more intensive statin therapy was noted in other randomized clinical trials (RCTs) that also included patients with no history of CAD.54
In terms of HRT, a sub-study of the Women's Health Initiative reported a reduced CCS after 7.4 years of estrogen in younger (50 to 59 years old) post-menopausal women, compared with placebo in women who had undergone hysterectomy. Although a subsequent study found no benefit, it was limited by a smaller sample size and short duration of treatment.55
Potential limitations of these trials include the short follow-up to demonstrate an effect on CAC progression, particularly when given for primary prevention, and the fact that outcomes have been centered on the progression of CAC more than on clinical outcomes. More conclusive evidence, based on large RCTs with routine clinical outcomes used in cardiology trials, is needed to determine the impact of LLT and anti-platelet agents in patients with a positive CCS. At this time, the use of both of these therapies in patients with abnormal studies still remains to be individualized, and clinical judgement is recommended.
Cardiovascular proceduresCAC is associated with increased complications, irrespective of whether patients are treated with bypass surgery, PCI, vascular surgery, valve surgery or transcatheter aortic valve replacement. Percutaneous coronary intervention of calcified vessels results in a lower success rate and an increased incidence of adverse events such as coronary dissection, perforation, periprocedural MI, asymmetric and underexpanded stents, stent thrombosis, and target lesion revascularization (TLR).56
To determine the clinical correlation and prognostic impact of CAC in women undergoing PCI with drug eluting stents (DES), data from female participants in 26 randomized trials of DES was pooled. At three years, women with moderate or severe CAC were at higher risk for death, MI, TLR and stent thrombosis compared with those with mild or no lesion CAC, assessed through coronary IA. It was concluded that women undergoing PCI of calcified lesions tend to have a worse clinical profile and remain at increased ischemic risk, irrespective of new generation DESs.57
This leads us to think that plaque modification could be advantageous, but there is only one randomized trial about rotational atherectomy of calcified lesions that showed a higher procedural success rate. It is also reported that orbital atherectomy in a large registry of calcified lesions appeared to have high success, with low rates of TLR. Despite this evidence, more clinical trials are still needed to further consider the benefits of this strategy among patients with CAC.58,59
CCS measurement benefits on complianceThere is evidence about the benefits on compliance from knowing the CCS. Studies with a follow up of three years have demonstrated that knowing baseline CCS was strongly associated with statin compliance; percentages account for 44% in the lowest tertile and 91% in the highest, in individuals on statin therapy at baseline.60 It is also demonstrated that CAC was related to aspirin initiation, dietary improvement and increased exercise, which would improve the CV status.61,62
When and why use CAC scoring in women?- -
Current AHA/ACC guidelines recommend using CCS to stratify patients classified as intermediate risk using the Framingham Risk Score, irrespective of gender. It is suggested that in this particular group, CCS can play a pivotal role in determining if primary vs secondary prevention is needed in asymptomatic individuals.
- -
In women with a high risk for CV events by the Framingham Risk Score, utilization of CCS is not recommended and they should be started on LLT+/- aspirin (or other anti-platelet agents), based on clinical judgment.10,13,63
- -
Stratifying intermediate-risk women presenting with chest pain with CCS is not recommended. In this population, coronary CT scan with contrast can be used, and has a large amount of randomized data to support its utilization, compared to other non-invasive tests.64–69
- -
For those women with particular RFs that make them prone to having a higher risk for CAD despite the Framingham Risk Score stratification, such as post-menopause state and diabetes, advice is given to individualize the decision of pursuing CCS. This strategy, despite the lack of randomized data, can potentially add significant benefits in selected patients.41,42,70–72
- -
There are no local (Colombian) or regional (Latin American) large cohorts that describe the pattern of CAC in asymptomatic individuals. In fact, there is data that the current CCS based on a US population does not perform equally when applied to other ethnicities, as shown by the ESLA study based in Brazil. Further cohorts need to be assembled both locally and regionally to determine the population-specific CAC patterns.
- -
CAC measurement and awareness have demonstrated its beneficial role in stabilizing the Framingham Risk Score as well as improving CV-RF control, LLT adherence, aspirin initiation, dietary habits and increased exercise. Randomized data are needed, with longer than usual follow-ups, to demonstrate the impact of LLT on patients with a positive CCS, for secondary prevention.
The authors declare no conflicts of interest.