To analyse the use, indications and potential risks of tricyclic antidepressants (TCAs), using a technological system of clinical alerts at the time of prescription.
MethodsObservational, descriptive, retrospective study on a population covered by a Colombian health insurance plan with an average of 2,333,582 members/month. The information was generated in the PBM (Pharmacy Benefit Management) MC21 Colombia technological platform.
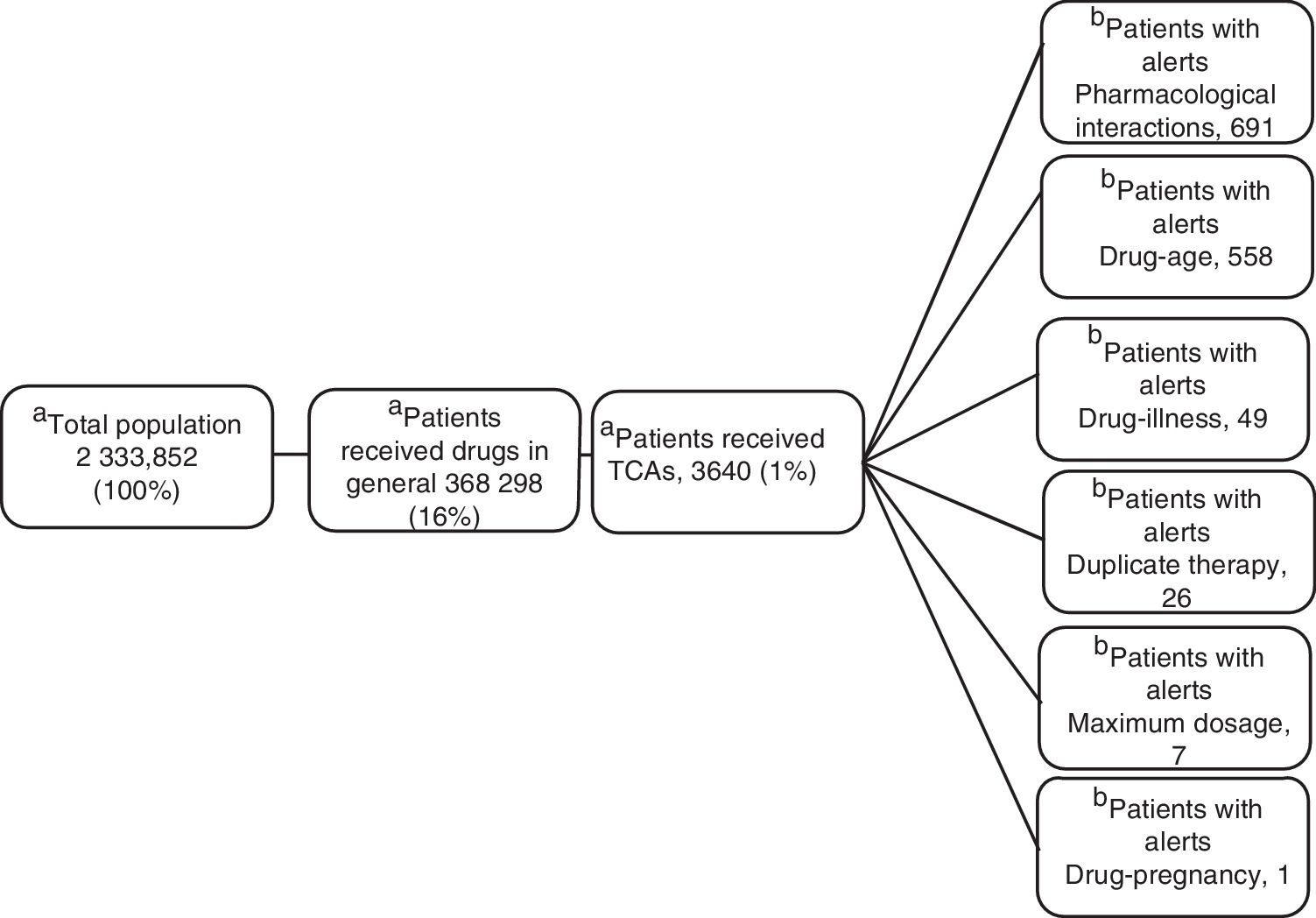
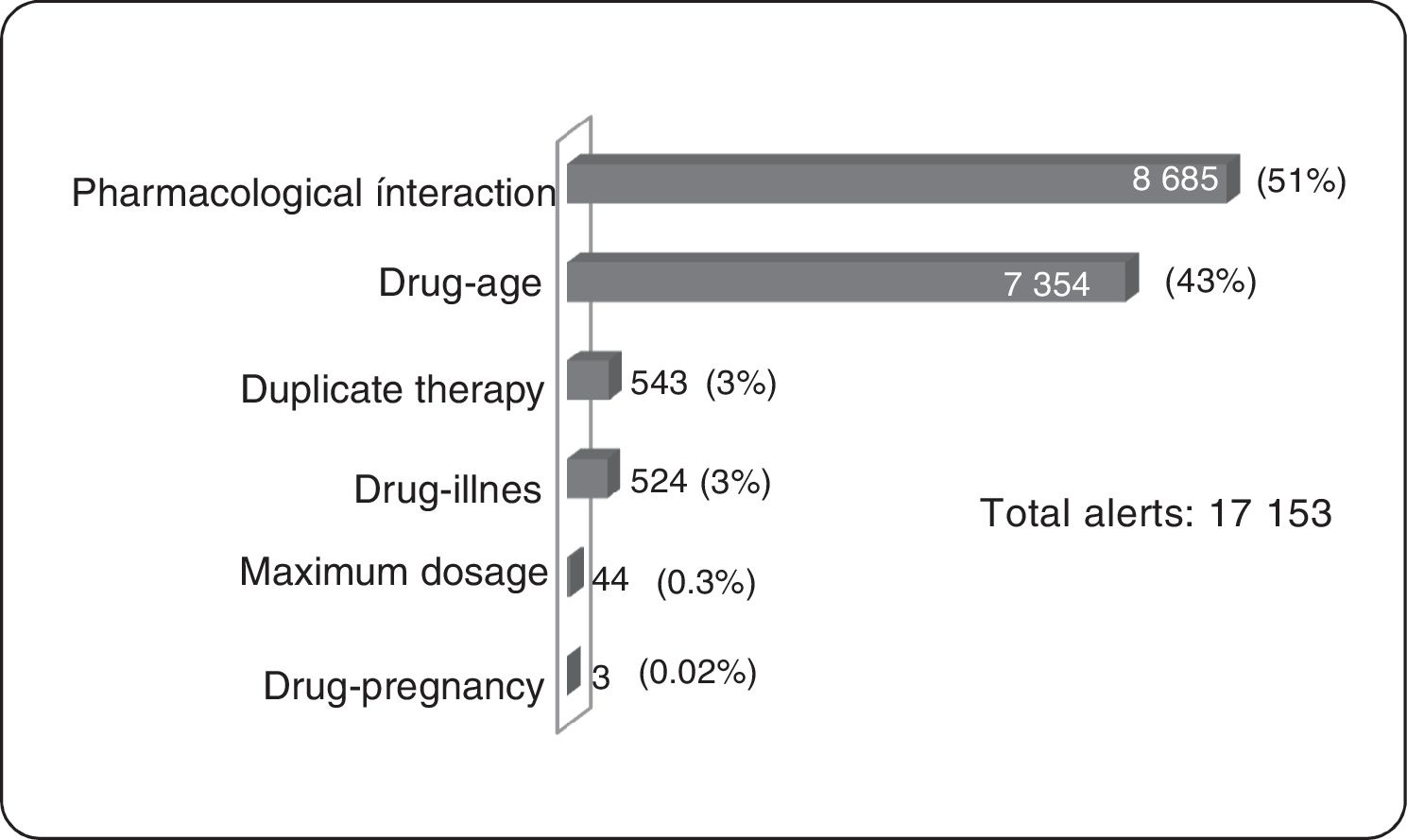
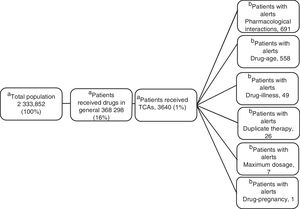
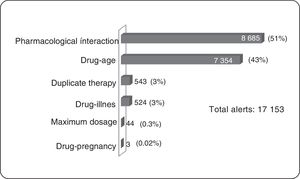
ResultsOf the total members, 368,298 (16%) patients/month on average were prescribed medicines; 3,640 (1%) were prescribed TCAs: 2,573 amitriptyline (70%) and 1.062 imipramine (29%); 817 (22.5%) were over 65 years of age. The median daily dose of amitriptyline and imipramine was 25 mg. A total of 17,153 alerts were reported: 8,685 (51%) for drug-drug interactions, 7,354 (43%) for drug-age interactions and 543 (3%) for duplicate therapy.
ConclusionsRisks were identified in the prescription of tricyclic antidepressants, especially in the over-65 population, where these drugs are used in particular for the management of neuropathic pain. The clinical alert system at the time of medicinal product formulation can make an important contribution to the prevention of potential adverse events associated with the use of medicinal products.
Analizar el uso, las indicaciones y los riesgos potenciales de los antidepresivos tricíclicos empleando un sistema tecnológico de alertas clínicas en el momento de la prescripción.
MétodosEstudio observacional descriptivo y retrospectivo en una población de una aseguradora colombiana con un promedio de 2.333.582 afiliados/mes. La información se generó en la plataforma tecnológica del PBM (Pharmacy Benefit Management) MC21 Colombia.
ResultadosDel total de afiliados, se prescribieron medicamentos a una media de 368.298 (16%) por mes; a 3.640 (1%), antidepresivos tricíclicos: amitriptilina a 2.573 (70%) e imipramina a 1.062 (29%); 817 (22,5%) eran mayores de 65 años. La mediana de dosis diarias de amitriptilina e imipramina fue 25 mg. En total se reportaron 17.153 alertas de antidepresivos tricíclicos: 8.685 (51%) de interacción farmacológica; 7.354 (43%) de interacción fármaco-edad (pacientes mayores de 65 años) y 543 (3%) de terapia duplicada.
ConclusionesSe identificaron riesgos en la prescripción de antidepresivos tricíclicos especialmente en la población mayor de 65 años, que usan estos medicamentos principalmente para tratar el dolor neuropático. El sistema de alertas clínicas en el momento de la formulación de medicamentos hace un aporte importante en la prevención de potenciales eventos adversos asociados con el uso de medicamentos.
Tricyclic antidepressants (TCAs) were described in 1957 in Switzerland by Huhn,1 who documented that imipramine was effective in treating major depression; this marked a highly significant therapeutic advance in psychiatry. These medications began to be used in the mid-twentieth Century, generating controversy for their therapeutic effects when acting on the main central nervous system neurotransmitters (norepinephrine, serotonin, acetylcholine, dopamine, and antagonism of H1 and H2 receptors), which result in a large number of adverse reactions such as constipation, urinary retention, blurred vision, orthostatic hypotension, sedation, memory impairment, dizziness and cardiovascular side effects such as 2nd level cardiac arrhythmia.2
Since entering the market in 1957, tricyclic antidepressant drugs have been widely used, initially in the field of psychiatry, with subsequent expansion into other medical specialties, thereby further increasing the number of patients who are prescribed TCAs.
The mechanism of action of TCAs is to block the reuptake of norepinephrine and serotonin amines in addition to antagonizing muscarinic receptors and histamine H1 and H2, which translates into increased concentration and increased availability of these neurotransmitters in the synapse, which also explains most of its adverse effects.
Due to its mechanism of action with the variability in its pharmacokinetics and pharmacodynamics, TCAs have been converted into a group of drugs for a broad spectrum of indications. In addition to major depression and dysthymia, they are effective in panic disorder, social phobia, other anxiety disorders, bulimia nervosa, post-traumatic stress disorder, chronic pain and, in young children, enuresis.3 For this reason, TCAs are used in a wide range of specialties including neurology, psychiatry, internal medicine, physical medicine, pain medicine, general medicine, family medicine and pediatrics, leading to increased use and therefore the risk of potential adverse reactions.
Our research analyzes the alerts generated in the doctor's office right at the time of making the prescription; however, there is research in the literature on the importance of clinical alerts in the pharmacy at the time of dispensing the medication to the patient. For example, the study conducted by Mette Heringa et al.4 in which they analyze several families of medicines, found that of all the processed prescriptions, 43% led to one or more drug safety alerts, more frequently drug and drug interaction alerts (15% of all prescriptions), drug interaction-disease alerts (14%), duplicate medication alerts (13%), and dosing alerts (7%). In this study, 5.7% of drug interaction alerts included an antidepressant. It is for this reason that we want to analyze the clinical alerts of a particular group of antidepressants such as TCAs, but not in the pharmacy, but at the exact time that doctors in their office make the prescription, which could have a greater impact.
ObjectiveAnalyze the use, indications, and risks of tricyclic antidepressants using a system of clinical alerts in the prescription of drugs.
MethodsA retrospective longitudinal descriptive about the uses and risks of TCAs in Colombia was held in a period of 6 months (January to June year 2014) in an average population of 2.333.582/month affiliated to a health insurance plan. All patients in the insurance affiliates in that period who attended medical consultation and prescribed TCAs.
The information was obtained from the of PBM (Pharmacy Benefit Management) MC21 Colombia technological platform, which registers the prescribing and dispensing of outpatient medications and performs testing for clinical alerts. From this platform, these variables were obtained: a) socio-demographic (age), and b) tricyclic antidepressant drugs with their respective dosage and duration of treatment. For a description of the risks in the study population information from different parameterized clinical alerts was used on the MC21 Colombia, it understood as a clinical alert security information that appears on the computer screen when you make a doctor's prescription can lead to potential risks; the clinical alerts are divided into: maximum dose, duplicate therapy, drug age, disease drugs, drug pregnancy and drug interaction. The statistical software SAS (Statistical Analysis System) was used to organize and analyze the information. Univariate analyses of association using statistical measures as medium, absolute frequencies and relative frequencies with exploratory association analyses where bivariate contingency tables for the respective clinical and pharmacological analyses were generated. The information obtained was analyzed with the most current scientific evidence including drug database Micromedex 2.0. The analysis of this information was followed by recommendations to promote proper use of tricyclic antidepressants.
ResultsOf the 2 333 852 members, an average of 16% (368 298) received monthly outpatient drugs, and of these, 1% (3640) received TCAs (figure 1).
In terms of age distribution, 1.3% (47 patients) is in the age group of 0-14 years, 42% (1530 patients) is between 14-44 years, 34.2% (1246 patients) is in the 45-64 years group, and 22.5% (817 patients) is concentrated in the group over 65 years. The average age of prescription of TCAs was 48 years.
Regarding the use of TCAs, the drugs most used were amitriptyline, imipramine, clomipramine, doxepin, and maproptiline, which are those that currently have authorization for marketing in Colombia.
The median dose of TCAs with median absolute deviation (MAD) where the “zero” interval indicates that the data are concentrated around the median described; most of TCAs have no antidepressant doses, except maproptiline median dose which presents with indication for depression.5
The most common use of TCAs was for neuropathic pain with 13% (2804 patients); in an average daily dose of 27 mg daily, this diagnosis being the primary indication for prescribing amitriptyline. TCAs prescription for depression was also observed in 2.3% (494 patients), the average daily dose of amitriptyline for depression was lower than 75 mg daily in 94% (257 patients) and imipramine average daily dose was less than 100 mg day in 95% (184 patients).
As for the diagnosis of enuresis, the majority of TCAs prescriptions were for imipramine, 190 patients (3%).
Regarding the medical specialty, we found that: 13.7% of prescriptions with clinical alert corresponded to family medicine, 12.5% to internal medicine, 7% to neurology and only 1.9% of prescriptions with clinical alerts corresponded to psychiatrists, the rest of prescriptions corresponded to general medicine.
Clinical Alerts AnalysisIn the average of 3640 patients per month who received TCAs, an average monthly rate of 79 alerts was observed for each 100 patients. A patient could present one or more alerts (figure 2).
Pharmacological InteractionThe greatest concentration of patients with drug interaction alerts occurred in the age group over 65 years (25%, 206 patients) of all patients over 65 years (817). According to the classification of risk of drug interactions, 311 pharmacological interaction alerts contraindicated presented in 156 patients were observed, alerts of this type in their entirety were generated by combining metoclopramide with a TCA within these the most frequent was amitriptyline (91.1%). Two side effect alerts per 100 patients prescribed TCAs were generated. No side effect alerts of pharmacological interaction for clomipramine, doxepin, maproptilina were observed.
6814 high-risk alerts occurred in 3365 patients; 52% of alerts of this type appeared with the combination of 2 antidepressants (1974 patients). The first 2 places are given by the combination of fluoxetine-amitriptyline (26%, 1002 patients), the remaining 48% was generated by the combination of a tricyclic antidepressant with other medicines such as analgesics opioid (tramadol-amitriptyline: 16%, 723 patients). One patient had a simultaneous prescription for fluoxetine, sertraline, amitriptyline, tramadol, lorazepam, and alprazolam, event that was reported in a timely manner to the insurer due to the potential risk of adverse drug reaction (ADR) for this patient.
As for moderate risk of drug interactions, 2065 alerts were presented in 965 patients, 62% are interaction between anticonvulsants and TCAs (the interaction carbamazepine-amitriptyline was the most frequent, occurring in 422 patients) and 9% corresponds to the interaction between anticoagulants and TCAs, all of these combinations involving drugs with narrow therapeutic windows with risks of significant adverse reactions.
Duplicate TherapyWere presented in total 543 alerts duplicate therapy, these the 99.5% of alerts correspond to simultaneous prescribing amitriptyline and imipramine, occurring in 185 patients. One patient had simultaneous prescription clomipramine with imipramine.
Maximum DosageAlerts maximum dose in 7 patients average/month were presented and considering that the maximum dose of amitriptyline is 300 mg/day,3 alerts for maximum dosage with this medication occurred in four patients average/month, were above the prescribed maximum recommended dose with potential consequent risk of central nervous system depression, seizures, tachycardia, hallucinations. In the same way, with imipramine three patients average/month had maximum dose alerts.
Drug-ageAll alerts of this type occurred in patients older than 65 years, with significant involvement of amitriptyline being the most frequent prescription.
Drug-diseaseAlerts of this type were concentrated in patients with prostatic hyperplasia (40.5%) and in patients with cardiac arrhythmia (15.3%).
Drug-pregnancyConsidering that TCAs are category C according to the Food and Drug Administration (FDA) (animal studies have shown adverse events in the fetus, there are no adequate studies in pregnant women), 1 patient with diagnoses related to pregnancy presented ADT prescription with the potential risks of teratogenic effects.
DiscussionTCAs have been shown to be effective for multiple indications, actually are still used in both outpatient and Hospital patients.2 That is why in our study, we found that of all the patients who were prescribed drugs, 1% received TCAs.
In a study by Machado et al., it was found to be an antidepressant medication dispensed to about 0.9% of patients,6 close to that found in our study to ADT prescription.
Analysing the distribution by age, we found that 42% of patients with prescription of TCAs are between fourteen and forty-four years old, which is in accordance with what is reported in the literature for the use of antidepressant for depressed people, given that the beginning of the symptoms generally appear towards the final three decades of life.7 However, it is important to note that the patients over 65 represent 22% of patients prescribed with TCAs in our study, a relevant fact if we consider that these patients are at the greatest risk of adverse drug reactions due to age related physiological changes the coexistence of chronic diseases such as hypertension and diabetes for example and the high prevalence of polypharmacy; it is estimated that the adverse drug reactions occurs in 41% of outpatients, of which 59-81% are preventable or avoidable; 23% may require hospitalization for treatment and that in many cases are associated with the various interactions between different drugs, so the identification of potential adverse events associated with the use of drugs becomes transcendental.6
The most commonly prescribed TCAs in our study were amitriptyline and imipramine. The use of other TCAs was very rare, less than 1% of the total (doxepin, 2 patients; maprotiline, 8 patients; clomipramine, 16 patients), this could be due to amitriptyline and imipramine are the oldest TCAs and that more information can be found by reviewing the literature and which are approved for several indications.8 On the other hand, the use of doxepin and maproptiline is very low due to its negative safety profile.
TCAs are not considered first line drugs for the treatment of depression9 due to the adverse drug reactions that they can cause, especially in the elderly, for whom other options are considered more effective and safe. We believe this is the main reason why in our study we found that TCAs were prescribed for the treatment of depression in only 2.3%. Gillman believes that the adverse effects and risks associated with TCAs have been exaggerated, probably due to the intervention of pharmaceutical houses in studies for new antidepressives.10 Similarly, Thiwan's study11 showed that most of the symptoms associated with the use of TCAs were already present before starting treatment, and only a few, predominantly anticholinergic side effects, worse after two weeks TCAs treatment. Given this, new clinical studies in which the risks and benefits of TCAs are evaluated are necessary.
Neuropathic pain is caused by injury or illness that directly affects the somatosensory system, its treatment is often difficult and ineffective. At this point, the TCAs represent the first-line drugs, as inhibitors of serotonin and noradrenaline reuptake.12 Pain relief by amitriptyline produced is similar in magnitude and quality of gabapentin, with much lower costs.13 It is for this reason that in our study we found that this is the indication for which the TCAs were prescribed more frequently, with 13% of all prescriptions. Similarly, the management of migraine and chronic tension headache occupied an important place in our study as to the indications for which it was prescribed a TCAs, with 12% of prescriptions. The efficacy of TCAs in this type of pathology is well documented. Jackson et al. conducted a meta-analysis comparing the efficacy.14 The analgesic effect of TCAs is independent of changes in mood and they appear doses and treatment times much lower than those used for depression.15 Several studies show that TCAs are highly effective as analgesics, needed to treat diseases such as chronic pain, including neuropathic pain, fibromyalgia, low back pain, and chronic headache or migraine.16 Findings related to our results where that neuropathic pain diagnosis was the main reason for prescribing TCAs; however it should evaluate this therapy in patients with diagnoses such as cardiac arrhythmias (47 patients) where may be more risk than benefit.
Drug interactions have been identified as a significant problem of drug therapy, with a significant impact on morbidity and mortality patients.17 Our study also evaluated the clinical alerts that occurred among patients who were prescribed with TCAs. The main warning was detected by drug interaction with 51% of alerts. This is because different groups of medications interact with these. Of all patients who were prescribed ATC, drug interaction alerts contraindicated in 156 patients, all by the interaction between metoclopramide and TCAs were presented. A study by Gaertner18 classified both metoclopramide and amitriptyline as very high risk for drug interactions presentation also stressing that joint use is contraindicated for the very important anticholinergic activity of both drugs, raising the incidence adverse drug reaction as sedation, dry mouth, difficulty in urination, and delirium. As for high-risk alerts, most came by interaction of amitriptyline-fluoxetine; interaction increases the risk of adverse drug reaction for TCAs due to inhibition of CYP2D6 and CYP3A4 enzymes responsible for metabolize amitriptyline.19
In clinical alerts reported among users of TCAs prescription striking alert drug-pregnancy, which occurred in 3 patients. The FDA classifies TCAs as category C. Epidemiological studies to evaluate the effect of exposure to TCAs during pregnancy have produced inconsistent results. A meta-analysis of 414 cases of exposure to TCAs during the first trimester of pregnancy showed no increased risk of major congenital abnormalities. On the other hand, it is reported that women exposed to tricyclic antidepressants have increased rates of abortions and neonatal complications, including minor malformations.20 Therefore, it is necessary that the use of TCAs during pregnancy is prudent, always weighing the benefit/risk for both mother and fetus, conducting clinical and paraclinical monitoring or attempting to minimize the risks of early detection morbidity and maternal/fetal mortality. For the case identified during the study were notified to the medical directors of the insurer and some alerts as restrictive is parameterized during pregnancy, especially categories X and C.
ConclusionsOnce the patient requests their medications at pharmacies, it is not easy to generate interventions to prevent the presence of possible adverse events associated with the use of medications. That is why the system of clinical alerts at the time of formulating medications can generate an important contribution to the prevention of potential adverse events associated with the use of these. The integration of the use of information on medicines in a technological platform can serve as an input to identify and suggest management sources, in this case, for example, pharmaceutical care and active pharmacovigilance for patients over 65 years in whom potential risks were identified associated with the use of tricyclic antidepressants.
Recommendations- •
The potential risk is evident in patients older than 65 with the use of TCAs; in this study this age group has the highest rate of drug interactions, interactions drug-age and drug-disease interactions; so it is critical in this population to implement strategies of pharmaceutical care and active pharmacovigilance.
- •
It is important to support the prescription offered by clinical alerts in the detection of the possible adverse reactions associated with the use of TCA.
- •
Given the risks identified with the use of TCAs, education programs for doctors play a major role as an intervention strategy for the proper use of these drugs.
MC21 Colombia S.A.S funded this project.
Conflicts of interestsAlvaro Vallejos, Dieric Diaz, Sandra Torres, William Hernandez, and Laura Maldonado report working relationships with MC21 Colombia. The other participants do not declare conflicts.
We thank MC21 Colombia technology department, for facilitating the information for the development of this research.
This publication was presented as a poster at the XI International Pharmacovigilance Meeting of the Americas in Lima - Peru (November 6-7, 2014).