To highlight the inflammatory and immunological mechanisms involved in depression and psoriasis.
MethodsA comprehensive literature search was performed in various databases, in total 145 studies were selected.
ResultsDepression and psoriasis have an association. Immune mechanisms —the actions of tumor necrosis factor-α, interleukin 1 (IL-1), IL-2, IL-10, IL-22, IL-17, interferon-γ, IL-1β, prostaglandin E2, C-reactive protein, IL-6, and IL-8 etc.—, and some genetic changes are involved.
ConclusionsA possible bidirectional relationship of psoriasis and major depression exists; i.e. the depression leads to psoriasis, and psoriasis leads to depression. We recommend more studies in the future to get a deeper and better understanding about this relationship.
Poner de relieve los mecanismos inflamatorios e inmunológicos involucrados en la depresión y la psoriasis.
MétodosSe realizó en varias bases de datos una búsqueda bibliográfica completa; en total se incluyeron 145 estudios.
ResultadosHay asociación entre depresión y psoriasis y están involucrados mecanismos inmunitarios —las acciones del factor de necrosis tumoral alfa, las interleucinas (IL) 1, 2, 10, 22 y 17, el interferón gamma, la IL-1β, la prostaglandina E2, la proteína C reactiva, la IL-6 y la IL-8, etc.— y algunos cambios genéticos.
ConclusionesHay una posible relación bidireccional entre psoriasis y depresión, es decir, la depresión lleva a psoriasis y la psoriasis lleva a depresión. Se recomiendan más estudios en el futuro para obtener una comprensión más profunda y mejor sobre esta relación.
“Doctor! I have been treated for major depression for the past so many years and now I have developed this silvery skin.” These kinds of statements are occasionally heard in the psychiatric settings. The similar kinds of complaints are brought to a dermatological clinic, where patients with skin problems complain of depressed moods. These kinds of cases are the classic examples of psycho-dermatological phenomenon,1 which is a sub-branch of psychosomatic medicine.2 The effects of behavioral, social, and psychological factors on the bodily processes and their relationship with each other are studied in psychosomatic medicine.2 In this review article, we emphasize on psycho-dermatological relationship between psoriasis and major depression.
Any disorder that involves an interaction between the brain and the skin is classified as a psycho-dermatological disorder. Three common kinds of psycho-dermatological disorders have been described so far, that includes: psycho-physiologic disorders, primary psychiatric disorders, and secondary psychiatric disorders.3 The subject of the association of skin and brain is well studied in the recent past. Moreover, the relationship between depression and psoriasis is also studied in relation to psycho-dermatology. Today, we do know that psoriasis can cause major depression, its bi-directionality (depression leading to psoriasis) is not fully understood yet. The ideas that “major depression leads to psoriasis, any cutaneous damage associated with major depression that causes psoriasis, or any inflammatory markers or cytokines released in depressed patient's brain or body that can initiate psoriasis” is not much discussed. In this article we will highlight the answers to the above mentioned statements.
The mechanism of depression has been studied in depth, and its association with various neurochemicals has been suggested. Depression is known to have an effect on the integumentary system and other organ-systems, like the cardiovascular system.4–8 Similarly, psoriasis is a chronic, immune mediated inflammatory disease of skin that leads to red, itchy plaques (white/silver) on the skin.9 Naturally, having such a severe cosmetic disease can affect the patient's mental health and may lead to depression. However, it could also be a consequence of major depression, due to an immunological and a neurochemical phenomenon.10
In this review, we will discuss the association of the human brain with the human body's immune system; inflammatory mechanism involved in the pathophysiology of psoriasis, inflammation and its relationship with major depression, and how both of these conditions could be related and augment each other; possibly due to a bidirectional mechanism. We will conclude the article with the future research recommendations.
Immune System and the BrainHeightened keratinocyte proliferation and leukocyte invasion into the uppermost layers of skin during inflammation that characterizes the disease known as psoriasis, causing the formation of physical pathology, has recently been found to be associated with psychological disorders such as depression through the mechanisms revolving the immune system.10 Statistically speaking, significant evidence in the previous decades have suggested an association between clinically diagnosed depression, or depressive factors, and skin diseases including psoriasis on multiple occasions. In a study done by Esposito et al.11 on 2391 patients, depressive indicators were present in 62% of the sample size. The presence of psoriasis in individuals, and its respective severity, has also been found to be linked with increased depressive symptomatology in a study done in 2002, where psoriatic patients were found to have even higher depressive “scores” than non-psoriasis afflicted depressed patients.12 Topping it however, Gupta et al.13,14 on two occasions observed that elevation in psychosocial impairment related to depression and stress strongly correlated to psoriasis morbidity and the onset of physical pathology. Reduction in psoriatic clinical morbidity due to medical or other treatment has also been linked to reduced depressive symptoms, with the converse true as well, in multiple studies.15–21 However, as with any apparent dermatological, or even non dermatological disorder for that matter, the antisocial, emotional, self-esteem lowering, anxiousness, and other factors that affect the psychological health of an individual are strong possibilities as to why depressive symptoms are effected and the data above is observed, but this does not explain the converse and this paper supports the idea that there is an even stronger connection between the immune system and the brain not yet fully explored.
Inflammatory Mechanism of PsoriasisThe presence of keratinocytes, leukocytes, T cells, macrophages, and dendritic cells and their migratory pathways towards the epidermis, the outermost layer of skin, and away from the inner layers, followed by the release of inflammatory cytokines including interleukin (IL) 1β, and IL-6, IL-22, tumor necrosis factor-α, (TNF-α) or a type-1 cytokine profile (IL-2, interferon [IFN] γ, and TNF-α) has been observed to be the cause of psoriasis, or more specifically psoriatic lesions.22,23 IL-6 and other cytokines are thought to influence maturation of T cells into Th17 cells, pulling neutrophils to specified location.24 IFN-γ and TNF-α levels may also be elevated from either Th125 or Th17 cells,26 and further amplify inflammation. The release of the cytokines IL-1, IL-6, and TNF-α due to dendritic and T cell activity initiate inflammatory pathways with trigger further inflammation, and deficiencies in inhibitory or regulatory IL-10 and T cells may be what allows for this to happen.27 Dendritic cells which activate the usage of antigen-specific adaptive immunity by acting as messengers of the innate immune system are thought to act as the first step in the physical formation of psoriatic lesions though these systems28 resulting in inflammatory upregulation that causes hyperproliferation of keratinocytes.29,26,30,31 The hyperproliferation of keratinocytes and respective cell activity is greater than standard cell activity, even during injury or healing,32 and causes 6 to 10 times faster skin replacement, without proper removal through standard practices, i.e. shedding.33 However, although mutations in regulatory cells are thought to be the cause of such phenomena the downstream effects are not particularly distinct from the standard inflammatory response.34
The term “diffuse brain35” has often been used to describe the highly innervated skin coining its strong relation to psychoneurological factors. The skin is known to contain a wide variety of neuropeptides including calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), nerve growth factor (NGF), substance P, and catecholamines, and studies have found a correlation between autonomic nervous system and respective neuropeptide activation with psoriasis.36,37 In fact, in patients with severance of sensory innervation due to trauma in specific areas, psoriatic plaques in those areas receded, coming back after nerve healing had occurred.38 This finding supports the idea that neuropeptides and brain activity relating to the release of said peptides may factor into the longevity of psoriasis or even its onset.38,39 Psoriatic plaques have also been found to have increased nerve fiber density and abnormal expression of various neuropeptides,39–42 and this finding is substantiated due to the fact that increased concentration of NGF and other neuropeptides mediate T cell and keratinocyte proliferation, memory T cell chemotaxis, mast cell migration, and degranulation, all processes known to be related to psoriatic lesions.39–44 Additionally, blood brain-derived neurotropic factor (BDNF) is found to be significantly reduced in psoriatic and depressive patients.45 As such, it can be said that the inflammation pathway that includes TNF-α, IFN-γ, and other type-1 cytokines strongly delineates the mechanism underlying psoriasis,21 however certain other forces may be at play and this mechanism may not just be limited to local skin areas. Furthermore, IL-17 has also been considered relevant with respect to psoriasis pathophysiology.46–48
Inflammatory Mechanism of DepressionExtensive amounts of data now exist that highlight the role of inflammation in individuals suffering from depression or chronic stress including but not limited to activation of cell-mediated immunity, compensatory anti-inflammatory reflex system, negative immunoregulatory processes and chronic inflammatory responses.49,50 IL-21, IL-17, and transforming growth factor β (TGF–β) are found in the people suffering from major depressive disorder.51
Specifically, increased levels of IP-10, or CXCL10, factors known to be involved in the localization of T cells, dendritic cells, and macrophages,52,53 were found in depressed patients, with antidepressants such as SSRIs lowering concentration, and increasing production of anti-inflammatory cytokines.54–56 Interestingly, changes in leukocyte mRNA expression, mRNA coding for cyclooxygenase-2, myeloperoxidase, inducible nitric oxide synthase and secretory phospholipase A2 type IIA, have been observed in chronically depressed patients, with inflammatory gene expression lowering 8 weeks after treatment.57,58 Peripheral blood inflammatory biomarkers have also been shown to have an association with neural plasticity, neurotransmitter metabolism, and neuroendocrine function, in areas directly related to depression.59 Persistently elevated inflammatory biomarkers have been found in depressed patients leading to chronic damage due to increased oxidative and nitrosative stress, and increased stimulation of signal molecules related to inflammation like NF-κB through activation of the sympathetic nervous system and respective outflow pathways.59–62 This chronic inflammation indepedently has also been shown to be associated with the presence of depression and other psychological disorders.63 Remission of clinical depression through antidepressant treatment has shown normalization of inflammation related substances.64,65
An almost direct dose response has been found between the severity of depression/depressive symptoms and the concentration of proinflammatory cytokines such as TNF-α, IL-1β, IL-2, IL-6, prostaglandin E2, and C-reactive protein (CRP).63,66 Additionally, in vivo studies in animals have shown the onset of depressive symptoms and “sickness behavior” after introduction of proinflammatory cytokines, affecting serotonin concentration and availability and even triggering clinical depression.67 Psychological stressors, including inescapable foot shock, immobilization, and tail restraint, have also been shown to cause significant increases in IL-1 (mRNA) levels in the plasma and brain, with downstream production of NF-κB, activation of prostaglandin and cyclooxygenase 2 production, and increased cell apoptosis.68–74 TNF-α has been thought to be linked to greater 5-HTT availability with TNF-α inhibition decreasing 5-HTT availability.10 Many studies have shown increased acute-phase proteins, increased expression of chemokines and adhesion molecules, and increased proinflammatory cytokines in patients with depression, without the existence of any preexisting inflammatory disorders.75–91 Abnormal concentration of IL-1-β and TNF-α can be seen as well in both the cerebrospinal fluid and peripheral blood circulation in patients with the symptoms of depression.99,52,100,101 Moreover, treatment of disorders, such as hepatitis C, with interferon alpha has shown to induce clinically defined major depression in almost 50% of patients highlighting the necessity in understanding the key factors that may be involved in diseases, such as inflammation in depression.92–98
Physiological ConnectionsThe presence of inflammation in both psoriasis and depression and the specificity in the inflammatory pathways and respective biomarkers that has observed clearly marks the existence a physiological relationship.99 Depression and psoriasis can be said to be statistically and biologically linked, and in fact certain studies have found the existence of specific tandem repeat polymorphisms in intron 2 of the 5-HTT gene and serotonin receptors related genes, which may underlie phenomena present in individuals with psoriatic lesions.99 Many dermatological issues have been shown to have a number of comorbidities with psychiatric and other illnesses, with psoriasis specifically having links to anxiety and depression, inflammatory bowel disease, fatty liver, cancer, diabetes, hypertension, dyslipidemia, cardiovascular disease, and other inflammatory or metabolic-related sickness.52 Prevalence of inflammation related diseases was found to be significantly higher in psoriatic patients, and vaccination related immune responses could elicit depressive symptoms regardless of observable physical sickness.100 Specifically in rats, injection of lipopolysaccharide (LPS) and IL-1 (an inflammatory cytokine) caused behavioral changes including decreases in interest exploring, sleep, energy, sexual activity, and appetite, changes that often predate clinical depression.101,102 Additionally, CRP and IL-6 measurements were observed to correlate to the onset of future depression in a 12-year study.102
Direct causal relations between depression and psoriasis have been difficult to ascertain, however, there is evidence that mechanisms leading to each other are at play. In a study by Capuron et al., it was observed that inflammatory cytokine release, associated with psoriasis, directly increased the activation of indoleamine 2,3-dioxygenase, the enzyme responsible for converting tryptophan to kynurenine.103 Tryptophan is a precursor to serotonin, with kynurenine production effectively decreasing serotonin concentration, and this coupled with the effect of tryptophan metabolism, can independently lead to signs of depression, and glucocorticoids associated with inflammation can also activate this pathway.104 Increased breakdown of serotonin may also be caused by the presence of inflammatory cytokines, and serotonin breakdown can lead to depressive symptoms.105 It is also hypothesized that HPA axis hyperactivity associated with clinical depression can be caused by increased concentrations of inflammatory factors, negatively affecting the feedback inhibition pathway of corticosteroids and lowering serotonin/serotonergic neurotransmitter activity, inducing peripheral cell-mediated activation, activating oxidative and nitrosative stress pathways, increasing central microglial activity, decreasing neuron formation, increasing cell death, and effecting melancholy, anxiety, fatigue, and eventually depression.101,106 Interestingly, systemic increases in inflammation/inflammatory response are associated with depression, which often precede onset of psoriasis symptoms, and this inflammation may increase psoriatic morbidity.107–109 Risk of developing psoriasis has also been shown to be significantly higher in depressed patients, corroborating this finding.110–112 Additionally, CRP has been present in depressed patients with levels at times showing a dose response with depressive morbidity113 and predecessors of depression, such as stress, have been shown to have an association with CRP levels in psoriatic women, with stress causing phenotypic changes in patients.114,115
Another link between depression and psoriasis has been found through the substance known as melatonin. Depression is known to disrupt melatonin release in the body, causing it to no longer function as normal, with elevated levels at night peaking around 3 am.116,117 However, aside from the regulation of normal circadian rhythm and sleep, melatonin can also regulate the immune system, and by decreasing concentrations of TNF-α, IL-6, and IL-8 may reduce inflammation or decrease the negative effects of inflammatory cascade byproducts.117–120 Decreased melatonin levels have been observed in a number of inflammatory conditions, with psoriasis included.121,122 Additionally, lowering of melatonin levels could increase symptoms of psoriasis, as absence of melatonin in rats was shown to delay wound healing, with melatonin replacement undoing said phenomena.123 Treatment of depression can also return melatonin to healthy concentrations and decrease psoriasis symptomology.124 Aside from treating depression, phototherapy is currently a useful treatment for psoriasis and although the mechanism has not been studied in detail, it is assumed that it may inhibit keratinocyte production, increase immunomodulation via altered receptor expression, and cause apoptosis of lymphocytes.125–128 Phototherapy can regulate melatonin levels, decrease psoriasis symptoms, and lessen depressive symptoms that further aggravate psoriasis.129 Studies have shown that a significant number of individuals with psoriasis liken stress and depressive tendencies to their psoriasis, and a significant amount are found with clinical depression, chronic anxiety, and suicidal thoughts, and as such phototherapy may be a useful holistic treatment, along with TNF-α blockers that have found use in other inflammatory diseases.130–132
Aside from biomarkers, a study done in 2012 indicates the potential of a genetic connection between depression and psoriasis.133 Utilizing methods such as G-banding and cell cultivation, changes in chromosomes 8, 15, 21, 22, and the sex chromosomes were observed in a family with both psoriasis and manic depression. Del(1)(q12-q23), del(1)(q21.1-q24), del(1)(q21.1-q23), del(10)(p11.2-pter), der(2)t(2;4)(p25;p12), t(2;22)(p14;p13), t(19;Y)?, and dup(10)(q26) were found as compared to healthy individuals indicating the potential presence of psoriasis/depression genes. Additionally chromosomal changes of 4 with 45,X (5.8%), 3 with 47,XXY (4.3%), and 4 with structural chromosome X (5.8%): del(X)(q13), del(X)(p11-pter), del(X)(q21.3), and inv(Y)(q11.2), were found. These changes were also linked to increased amounts of CD2+, CD4+, and CD8+ in the father, CD4+ reductions in the mother, and CD4+ reductions in the son with increased CD8+. Compared to average the CD4/CD8 ratio of the son and the father was significantly higher than normal implying either a genetic disposition or the damaging effect of psoriasis and depression on genes/inflammatory biomarkers.134
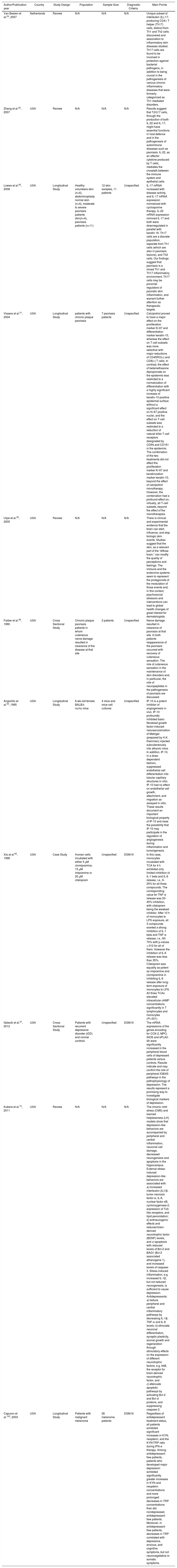
Clinical depression is known to have a strong connection with suicidal thoughts, tendencies, and actions, however, controversy exists surrounding the potential association of psoriasis and suicide.135,136 Previously, it has been assumed that interleukin-17R blockade utilized in the treatment of psoriasis may have an effect on suicidal ideation, but empirical evidence supporting this claim is limited.137 Psoriasis is also associated with alexithymia and anxiety.138 However, this subject warrants its own article. Moreover, the treatment of psoriasis especially with the co-existent depression can be challenging to treat. Because some medications do either worsen or trigger psoriasis. Medication used in the treatment of a wide variety of illnesses including blood pressure medications, anti-malarial drugs, NSAIDs, lithium, fluoxetine, amiodarone and other heart related medicine, however, has been found to increase psoriatic morbidity.139–141 As such, it is essential for healthcare professionals to consider the side effects of such treatment associated with some of the common comorbidities of psoriasis, and the potential relationship between psoriasis and other phenomena when dealing with psoriatic patients. (Table 1)
Showing some relevant studies about the association of depression and psoriasis.
| Author/Publication year | Country | Study Design | Population | Sample Size | Diagnostic Criteria | Main Points |
|---|---|---|---|---|---|---|
| Van Beelen et al.24, 2007 | Netherlands | Review | N/A | N/A | N/A | Unique subset of interleukin (IL)-17-producing CD4+ T helper (Th17) cells, distinct from Th1 and Th2 cells discovered and association to inflammatory skin diseases studied. Th17 cells are found to be involved in protection against bacterial pathogens, in addition to being crucial in the pathogenesis of various chronic inflammatory diseases that were formerly categorized as Th1-mediated disorders. |
| Zheng et al.25, 2007 | USA | Review | N/A | N/A | N/A | Results suggest that T(H)17 cells, through the production of both IL-22 and IL-17, might have essential functions in host defence and in the pathogenesis of autoimmune diseases such as psoriasis. IL-22, as an effector cytokine produced by T cells, mediates the crosstalk between the immune system and epithelial cells. |
| Lowes et al.26, 2008 | USA | Longitudinal Study | Healthy volunteers skin (n=4), abdominoplasty normal skin (n=4), moderate to severe psoriasis patients skin(n=4), psoriasis patients (n=11) | 12 skin samples, 11 patients | Unspecified | IL-17 mRNA increased with disease activity, and IL-17 mRNA expression normalized with cyclosporine therapy. IL-22 mRNA expression mirrored IL-17 and both were downregulated in parallel with keratin 16. Th17 cells are a discrete population, separate from Th1 cells (which are also in psoriasis lesions), and Th2 cells. Our findings suggest that psoriasis is a mixed Th1 and Th17 inflammatory environment. Th17 cells may be proximal regulators of psoriatic skin inflammation, and warrant further attention as therapeutic targets. |
| Vissers et al.31, 2004 | USA | Longitudinal Study | patients with chronic plaque psoriasis | 7 psoriasis patients | Unspecified | Calcipotriol proved to have a major effect on the proliferation marker Ki-67 and differentiation marker keratin-10, whereas the effect on T-cell subsets was more selective with major reductions of CD45RO(+) and CD8(+) T cells. In contrast, the effect of betamethasone dipropionate on the epidermis was restricted to a normalization of differentiation with a highly significant increase of keratin-10 positive epidermal surface without a significant effect on Ki-67 positive nuclei, and the effect on T-cell subsets was restricted to a reduction of natural killer T-cell receptors designated by CD94 and CD161 in the epidermis. The combination of the two treatments did not affect the proliferation marker Ki-67 and keratinization marker keratin-10, beyond the effect of calcipotriol monotherapy. However, the combination had a profound effect on, virtually, all T-cell subsets, beyond the effect of the monotherapies. |
| Urpe et al.35, 2005 | USA | Review | N/A | N/A | N/A | There is clinical and experimental evidence that the brain can start, influence, and stop biologic skin events. Studies suggest that the skin, as a relevant part of the “diffuse brain,” can modify the quality of perceptions and feelings. The immune and the endocrine systems seem to represent the protagonists of the modulation of those events and, in this context, psychosocial stressors and interventions can lead to global health changes of great interest for dermatologists. |
| Farber et al.38, 1990 | USA | Cross Sectional Study | Chronic plaque psoriasis patients in whom cutaneous nerve damage resulted in clearance of the disease at that site | 2 patients | Unspecified | Nerve damage resulted in clearance of psoriasis at that site. In both patients reappearance of the psoriasis occurred with recovery of cutaneous sensation. The role of cutaneous sensation in the maintenance of skin disorders and, in particular, the role of neuropeptides in the pathogenesis of psoriasis are discussed. |
| Angiolillo et al.53, 1995 | USA | Longitudinal Study | 6-wk-old female BALB/c nu/nu mice | 4 mice and mice cell cultures | Unspecified | IP-10 is a potent inhibitor of angiogenesis in vivo. IP-10 profoundly inhibited basic fibroblast growth factor-induced neovascularization of Matrigel (prepared by H.K. Kleinman) injected subcutaneously into athymic mice. In addition, IP-10, in a dose-dependent fashion, suppressed endothelial cell differentiation into tubular capillary structures in vitro. IP-10 had no effect on endothelial cell growth, attachment, and migration as assayed in vitro. These results document an important biological property of IP-10 and raise the possibility that IP-10 may participate in the regulation of angiogenesis during inflammation and tumorigenesis. |
| Xia at al.65, 1996 | USA | Case Study | Human cells incubated with either 5 μM clomipramine, 15 μM imipramine or 20 μM citalopram | Unspecified | DSM-IV | In this case, monocytes incubated with TCA for 4 h exhibited only limited inhibition of IL-1 beta and IL-6 release, i.e., 6-25% for all three compounds. The corresponding value for TNF-α release was 20-45% inhibition, with citalopram being the weakest inhibitor. After 10 h of monocytes to LPS exposure, all 3 compounds exerted a strong inhibition of IL-1 beta and TNF-α release, i.e., 60-70% with p-values <.012 for all of them. However the inhibition of IL-6 release was less than 35%. Citalopram was equality as potent as imipramine and clomipramine in inhibiting IL-6 release after long-term exposure of monocytes to LPS. All three TCAs elevated intracellular cAMP concentrations significantly in T lymphocytes and monocytes (P<.001). |
| Galecki et al.57, 2012 | USA | Cross-Sectional Study | Patients with recurrent depressive disorder (rDD) and normal controls | Unspecified | DSM-IV | The mRNA expressions of the genes encoding for COX-2, MPO, iNOS and sPLA2-IIA were significantly increased in the peripheral blood cells of depressed patients versus controls. Results indicate and may confirm the role of peripheral IO&NS pathways in the pathophysiology of depression. The results represent a promising way to investigate biological markers of depression. |
| Kubera et al.74, 2011 | USA | Review | N/A | N/A | N/A | The chronic mild stress (CMS) and learned helplessness (LH) models show that depression-like behaviors are accompanied by peripheral and central inflammation, neuronal cell damage, decreased neurogenesis and apoptosis in the hippocampus. External stress-induced depression-like behaviors are associated with: a) increased interleukin-(IL)1β, tumor necrosis factor-α, IL-6, nuclear factor κB, cyclooxygenase-2, expression of Toll-like receptors, and lipid peroxidation; b) antineurogenic effects and reduced brain-derived neurotrophic factor (BDNF) levels, and c) apoptosis with reduced levels of Bcl-2 and BAG1 (Bcl-2 associated athanogene 1), and increased levels of caspase-3. Stress-induced inflammation, e.g. increased IL-1β, but not reduced neurogenesis, is sufficient to cause depression. Antidepressants: a) reduce peripheral and central inflammatory pathways by decreasing IL-1β, TNF-α and IL-6 levels; b) stimulate neuronal differentiation, synaptic plasticity, axonal growth and regeneration through stimulatory effects on the expression of different neurotrophic factors, e.g. trkB, the receptor for brain-derived neurotrophic factor, and c) attenuate apoptotic pathways by activating Bcl-2 and Bcl-xl proteins, and suppressing caspase-3. |
| Capuron et al.103, 2003 | USA | Longitudinal Study | Patients with malignant melanoma | 26 melanoma patients | DSM-IV | Regardless of antidepressant treatment status, all patients exhibited significant increases in KYN, neopterin, and the KYN/TRP ratio during IFN-α therapy. Among antidepressant-free patients, patients who developed major depression exhibited significantly greater increases in KYN and neopterin concentrations and more prolonged decreases in TRP concentrations than did nondepressed, antidepressant-free patients. Moreover, in antidepressant-free patients, decreases in TRP correlated with depressive, anxious, and cognitive symptoms, but not neurovegetative or somatic symptoms. |
Recent evidence that has been compiled on the topic of major depressive disorder and the skin disorder, psoriasis, suggests that there is indeed a link between the two in regards to their respective underlying mechanisms. In fact, studies have shown that patients suffering from psoriasis, or more specifically the symptoms of psoriasis, often have depressive tendencies and elevated levels of depression, as per DSM standards, may lead to heightened effects of psoriasis. When it comes to issues such as depression, however, DSM standards are not wholly inclusive and as such data regarding depressed psoriatic patients is limited, highlighted in fewer depression observations in comparison studies. Abnormal concentrations of a number of different chemicals have been associated with depression, including melatonin, and this has also been found in psoriatic patients, with incredibly low concentrations present in individuals with both depression and psoriasis. Interestingly, chemical regulation, specifically regarding melatonin, has had positive effects on depressed psoriasis patients in terms of reducing disease morbidity. Although it is known that in many patients with skin disorders there exists an extensive emotional burden, depression and psoriasis have actually been found to be more closely linked through inflammation. Many of the inflammatory molecules and signals that move towards the uppermost layers of skin and create lesions observed in psoriasis, causing the initial formation of said lesions or increasing the multitude of observable lesions, have also been found to be at higher levels in depressed patients. These molecules, increasing in concentration due to psoriasis, can also cross the blood brain barrier and impact mental state, or interfere with proper functioning of the brain circuitry that is related to depressive symptoms and the onset of major depressive disorder. Inflammation related hypothalamic-pituitary-adrenal axis hyperactivity may also lower 5-HT and serotonergic neurotransmitter levels further impacting mental state. Despite all this however, phototherapy has been found to reduce both depressive and psoriatic symptoms. Antidepressant therapy may also reduce psoriasis and anti-inflammation medication used in psoriasis may decrease clinical depression. As such, due to the similarity in the presence of inflammatory molecules and signals and mechanisms by which these molecules function there is significant evidence that a relationship between depression and psoriasis exists. However this relationship warrants further study as specific phenomena or biological substances indicative of disease or present in depression are not necessarily found in skin disorders such as psoriasis with the converse also being true. Additionally, psoriasis is not always present in patients with depression and depressive symptomology is not necessarily present in psoriasis patients indicating the possibility of additional factors being at play.
Conflicts of interestsNone.
The authors are grateful for the help, guidance and support by Nobel Prize nominated professor Dr. Howard I Maibach (UCSF).