Social cognition in schizophrenia is currently one of the major fields of interest in studies of this disorder. It is commonly conceptualised as a set of mental operations underlying social interactions and therefore related to the ability to interpret and predict the behaviour of others in different social contexts. The research community has defined the functional areas that constitute the domain of social cognition, including, at least, the theory of mind, sensory perception, social perception, and attributional bias. Different bodies of evidence have shown that alterations in these functions in patients with schizophrenia are linked to some of their main psychopathological dysfunctions, such as defects in sensory perception, insight and attributional origin, and authorship of human acts. These behavioural alterations have been linked to structural and functional disturbances in the constituents of the so-called social brain. This includes a set of medial parietal, temporal, and pre-frontal areas that have been associated with some anomalies in the theory of mind, the perception of emotions, and the ability to consider the perspective of others, phenomena commonly found in schizophrenia. Future research in the domain of social cognition should be aimed at clarifying its relationship with the social brain and neurocognition.
La cognición social en la esquizofrenia actualmente es uno de los campos de mayor interés en los estudios de este trastorno. Se la conceptualiza comúnmente como el conjunto de operaciones mentales que subyacen a las interacciones sociales y que, por lo tanto, se relacionan con la capacidad para interpretar y predecir la conducta de los otros en los diferentes contextos sociales. La comunidad de investigadores ha definido las áreas funcionales que constituyen el dominio de la cognición social, que incluyen, al menos, la teoría de la mente, la percepción sensorial, la percepción social y la atribución de sesgos. Un variado conjunto de evidencias ha demostrado que las alteraciones de estas funciones en pacientes con esquizofrenia se vinculan con algunas de las manifestaciones clásicas de la psicopatología de la esquizofrenia, como los defectos en la sensopercepción, la conciencia de enfermedad y la atribución del origen y la autoría de actos humanos. Estas alteraciones conductuales se han vinculado a perturbaciones estructurales y funcionales en los constituyentes del llamado cerebro social. Este incluye un conjunto de áreas prefrontales mediales, parietales y temporales que se han asociado a algunas anomalías en la teoría de la mente, la percepción de emociones y la capacidad para considerar la perspectiva de los otros, fenómenos comúnmente encontrados en la esquizofrenia. Las futuras investigaciones en el dominio de la cognición social debieran orientarse a clarificar su vínculo con el cerebro social y la neurocognición.
Schizophrenia is one of the most challenging mental health problems in the world. The statistics published by the international health agencies all indicate that schizophrenia affects approximately 1% of the population, with a slight preponderance of males. Over the last 20 years, accumulating evidence has shown us that schizophrenia has its origins in multiple alterations in brain development involving genes and proteins which support the formation and functioning of extensive neural networks.1 From a clinical point of view, diagnosing schizophrenia continues to be a complex challenge for mental health professionals, and a broad knowledge of clinical psychopathology is required to adequately interpret the signs and symptoms caused by the dysfunction, which mainly affects cognitive, affective, and social aspects of the individual's life. In fact, one of the most pertinent aspects in the current discussion about schizophrenia is the domain known as social cognition (SC). This area has attracted wide interest over the last 10 years, further encouraged by recent evidence that SC may be independent of neurocognition (attention, language, executive functions) and have a mediating role between the neurocognition and social behaviour exhibited by patients with schizophrenia. In general terms, SC refers to the set of mental operations that underlie social interactions2 and it necessarily involves processes related to the interpretation and development of responses to the intentions and behaviours of others. We felt that it would be of interest, through this article, to briefly analyse and discuss both the cognitive aspects and the neurobiological structures and processes on which the SC dynamic is based. We begin with a short discussion of the cognitive aspects that current consensus considers to be components of SC and have been incorporated into the MATRICS3,4 project. We then go on to discuss the neurobiological aspects linked to SC, considering the fundamental components of the so-called social brain. The discussion is based on current knowledge in this particular field of schizophrenia research.
SC and the MATRICS consensusWith SC gaining increasing importance in clinical practice and research in schizophrenia, there was a need to establish a consensus on the criteria and concepts that form its theoretical foundations. This has led to the adoption of recommendations emanating from working meetings held over the last 10 years as part of the Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative, a project sponsored by the United States National Institute of Mental Health (NIMH).3,4 The aim of the meetings was to clarify the main concepts and criteria associated with SC in response to the lack of agreement on the main terms, definitions, and measurement methods which existed in this domain. The resulting consensus was that SC should include several areas of interest.
Theory of mindTheory of mind (ToM) is the ability to attribute mental states, i.e. intentions, desires, and beliefs, to others.5 Although this is a field that opened up in relation to the dysfunctions observed in autism, study of ToM has been extended to schizophrenia research based on evidence that SC alterations may play an important role in the pattern of the clinical symptoms.4 The link between ToM and schizophrenia has been a topic of discussion since the 1990s, based on the Frith studies,6–8 which suggested that schizophrenia triggers a deficit in metarepresentational skills, i.e. in the ability to generate representations of intentional attitudes.9 In Frith's view, this implied that there was a deficit in the mentalisation process prevalent in schizophrenia, determining an inability to think about one's own thoughts and those of others. Furthermore, this dysfunction in the capacity to create metarepresentations (in other words, to attribute emotions, thoughts, and intentions to third parties) would be a fundamental factor in the genesis of the psychotic symptoms exhibited by patients with schizophrenia.10 In effect, both patients with negative symptoms (alogia, abulia, affective flattening, social isolation) and positive symptoms (delusional ideas, auditory hallucinations, disorganised thought, and behaviour) should show poor performance in the ToM tasks, typically in first- and second-order false-belief tests.11,12 While the studies conducted by Frith et al. have produced data that support their hypotheses,8,13 their results have not been repeated in subsequent studies.9 Moreover, from a critical point of view, it seems reductionist to propose a single mechanism (i.e., a deficit in mentalisation) as a causal factor of symptoms as heterogeneous as those observed in schizophrenia. Nonetheless, recent studies have confirmed that deterioration in the mentalisation process plays a fundamental role in the psychopathological process of schizophrenia14–17 and, more importantly, that this deficit would be specific and not attributable to deterioration in the general cognitive functioning that affects intelligence and executive functions.15,18 However, in our opinion, this remains a controversial issue, as the evidence shows that executive functioning and intelligence cannot be considered as unnecessary for the mentalisation process in patients with schizophrenia.9 Interestingly, researchers have explored whether disturbances in the mentalisation process could be a feature of the disorder or whether they are in fact a state dependent on the exacerbation phases. Studies of patients with schizophrenia in remission and even of schizotypal subjects at risk of schizophrenia have revealed that the deficiencies in mentalisation remain in these groups of subjects regardless of the intensity and quality of the psychotic symptoms, which would suggest that the deterioration in mentalisation is a trait and not a state.19,20
There has been special interest in studies linking ToM to the characteristics and disturbances observed in the language of patients with schizophrenia. It has been proposed that deterioration in mentalisation skills could be related to defects in communicative competence observed in these patients. That would mean that deficiencies in mentalisation had a contributory role in the pragmatic disturbances described in people with schizophrenia, particularly in the inability to understand the communicative intentions of the speakers. Several recent studies have shown that people diagnosed with schizophrenia have deficiencies in the interpretation of figurative language. However, the relationship between ToM defects and deficient processing of figurative language has only been shown to be firm in the interpretation of ironies, a use of language particularly demanding in pragmatic skills.16,21 Meanwhile, other studies have confirmed the relationship between ToM and pragmatic processing in schizophrenia. Effectively, studies that evaluated pragmatic skills and ToM in patients with schizophrenia showed that deficiencies in pragmatic tasks correlated with defects in first- and second-order false-belief tasks.22 In fact, researchers assessing the relationship between ToM and pragmatic skills in people with schizophrenia and their relatives found that both groups behaved worse than “normal” people in both false-belief and pragmatic skills tasks in conversational context. These findings open new avenues for research in this field, which should be directed at exploring the genetic basis of disorders of this type.23
Processing of emotionsA second area included in the SC field is the processing of emotions in schizophrenia or, in other words, the adaptive perception and use of emotions.24 In general, studies on schizophrenia have incorporated measurements of both expression and understanding of affect. It is known that patients whose conditions are chronic all tend to show deficiencies in the interpretation of emotions through facial expressions. However, recent studies indicate that patients going through a first episode of schizophrenia also have problems identifying facial expressions, not only interpreting them.25 This certainly has significant theoretical implications, as defects in the perception and identification of facial emotions may be more a marker of susceptibility than one of the sequelae of the chronic nature of the disorder. Moreover, patients with schizophrenia seem to have serious difficulties in regulating the relationship between their emotions and their cognitive functioning. They tend to misinterpret the affective keys of the social context, which leads to alterations in SC and behaviour.26 Once again, this type of limitation in emotional processing is likely to affect patients who are progressing towards their first episode. In fact, recent studies report that patients at this stage show serious defects in processing the emotional component of motor actions that activate the mirror neuron system.26
Social perceptionSocial perception involves a set of social roles and rules that people have to manage in different social contexts. It involves interpreting keys such as nonverbal acts, the intonation of the voice, and the meaning of some verbs in order to make inferences about ambiguous or complex social situations. This includes properly interpreting interpersonal relationships as a type of social act different from that performed individually. It is interesting that insights have been related to factors associated with social interaction and interpersonal relationships. This led to the level of awareness about the condition itself and its consequences being positively related to SC, regardless of neurocognition.27 Associations have also recently been reported between the degree of insights and the ability to establish social28 and interpersonal relationships.29 In fact, insights into their condition are correlated with interpersonal factors such as the perception of support, the frequency of contact with the family, and the alliance with the therapist, regardless of personal factors and cognition.30
Attribution biasThe fourth area of interest in the SC domain is attribution bias, or how people infer the causes of particular positive or negative events. This involves the ability to correctly make attributions about causes originating in other people's behaviour, in situational factors or in ourself.24 Application of these categories to subjects suffering delusions of persecution have shown that they often attribute their problems to others, and not to situations.31 Research in patients with schizophrenia has revealed the tendency of this population to attribute hostile intentions to the actions of others.32 This dynamic would be developed in order to preserve self-esteem, as maintaining a negative perception of others would preserve a positive image of oneself, even with the risk of provoking a growing negative perception on the part of others.33
Neurobiological aspects of SC in schizophreniaSC and the social brainWhen talking about SC and its structural and functional bases in the human brain, it is often necessary to refer to the so-called “social brain”, a concept coined by Brothers a quarter of a century ago. In a landmark study,34 Brothers argued that social knowledge is operationally different from other types of knowledge. Drawing more from studies in primates, he proposed that the social brain is based in three regions: the orbitofrontal cortex, the amygdala, and the temporal cortex. Studies of lesions in monkeys revealed that damage to the amygdala causes social isolation behaviour35; in the orbital frontal cortex, social behaviour disorders, and in the superior temporal sulcus, alterations in the processing of expression and the direction of the gaze.36 Subsequently, the introduction of functional neuroimaging has made it possible to conduct studies in humans, which has contributed to adding a further two brain areas to the social brain: (a) the medial prefrontal cortex and the adjacent region of the anterior paracingulate cortex, involved in tasks that require thinking in mental states; and (b) the mirror neuron system, which allows the sharing of experiences with others.37 Furthermore, Frith36 proposes that, together with the mirror neuron system, which he believes is not linked to any particular brain area, the social brain includes four regions specifically involved in SC: the posterior superior temporal sulcus and the adjacent temporoparietal junction; the amygdala; the temporal poles; the frontal medial region and the adjacent anterior cingulate cortex. The functions performed by each of these regions are not always purely social as they can be applied to other cognitive domains. However, their most important role is in the context of the most complex social interactions. The amygdala assigns emotional value to faces and helps us to interpret expressions of fear and distrust, the posterior superior temporal sulcus facilitates the recognition of the trajectories of agents acting in the world, and the temporoparietal junction plays a critical role in understanding the perspectives of others when faced with complex social situations. The prefrontal medial region is key for thinking about the mental states of others, especially when second-order representations critical in communicative acts are required. Lastly, the mirror neuron system allows us to observe and imitate the actions and emotions of others with whom we are interacting and determines the activation in ourselves of the same brain areas that are stimulated in those we are observing.
SC, schizophrenia, and the social brainSC is represented by a widespread distribution in the human brain, covering several territories in the frontal, parietal, and temporal lobes that correspond to the social brain, understood as a neural system involved in various functions related to social interaction. It has even been suggested, from an evolutionary perspective, that schizophrenia could be a consequence of the evolution of the social brain that allowed the development of extensive frontal–parietal and frontal–temporal networks designated for SC and intellectual life in the context of survival within social groups.38 The evidence, to date, shows that the regions of the social brain are functionally affected in schizophrenia, although it would appear that some of the abnormalities in this disorder affect regions outside the social brain. We should also point out that disturbances in the social brain are not specific to schizophrenia, as they are observed to a greater extent in autism and frontal–temporal dementia.39 Nevertheless, multiple studies support the idea of participation of the social brain as a substrate for SC disorders in schizophrenia. For example, it has been proposed that the anterior cingulate cortex, the adjacent medial frontal cortex (both parts of the social brain), and the dorsolateral prefrontal region may form a network associated with the control of directed efforts, the dysfunction of which in schizophrenia could contribute to explaining symptoms such as abulia, thought disorder, and deficit in the initiation and control of speech.39 Functional neuroimaging studies have made it possible to explore areas and brain circuits involved in the SC abnormalities in schizophrenia. Abnormalities have been reported in the activation of the medial frontal region during the performance of ToM tasks and the processing of emotions.40 Hypoactivations41 and hyperactivations42 have also been found in the amygdala of patients with schizophrenia performing emotional perception tasks, which indicates a functional deregulation in the amygdala in response to social stimuli.40 Alterations in mentalisation tasks involving the frontal medial cortex and in the perception of emotions related to amygdala dysfunctions suggest that patients with schizophrenia have difficulties in attributing both epistemic and affective mental states.40 An interesting finding of various studies was the link between the lower parietal region and SC alterations in people with schizophrenia. Patients studied showed failures in the activation of the right inferior parietal region during tasks in which they were asked to perform voluntary motor acts.43 Alterations in activation of this same region were found when patients with schizophrenia had to perform stimulation source-monitoring tasks.44 This suggests that patients with schizophrenia have difficulties in distinguishing between their own actions and those of others and, consequently, in distinguishing between themselves and others. Another series of studies observed correlations between the brain structure of patients with schizophrenia and the functions attributed to SC. Patients with schizophrenia who show poor performance in ToM tasks usually show decreases in the volume of the grey matter in the ventromedial prefrontal region.45 In the same experimental line, Yamada et al.46 reported that patients who showed difficulties in emotional perception tests also showed considerable decreases in the grey matter of the medial prefrontal area. Studies of connectivity through functional magnetic resonance imaging have shown that patients with poor performance in facial expression recognition tests had decreased connectivity between the amygdala and the insula, associated with hyperactivation of the frontal and medial parietal regions which was interpreted as compensatory.47 Lastly, the involvement of the default mode neural network system underlying SC disorders in first-degree relatives of people with schizophrenia categorised as at high risk of suffering psychosis has recently been described.48 The findings confirmed a reduction in the connectivity of the subsystem of the medial prefrontal dorsal cortex in this population, which also includes the temporoparietal junction, the temporal lateral cortex, and the temporal pole. This structural feature was associated with poor performance in scales of social functioning specifically designed to measure interpersonal relationships, perspective, and empathy. The published results indicate, on the one hand, that the population at risk of psychosis has probably inherited the vulnerability to suffer disturbances of connectivity in the default mode network, and, on the other hand, that the deployment of social behaviour recruits a subsystem of the default mode network, whose centre is the medial prefrontal dorsal cortex.
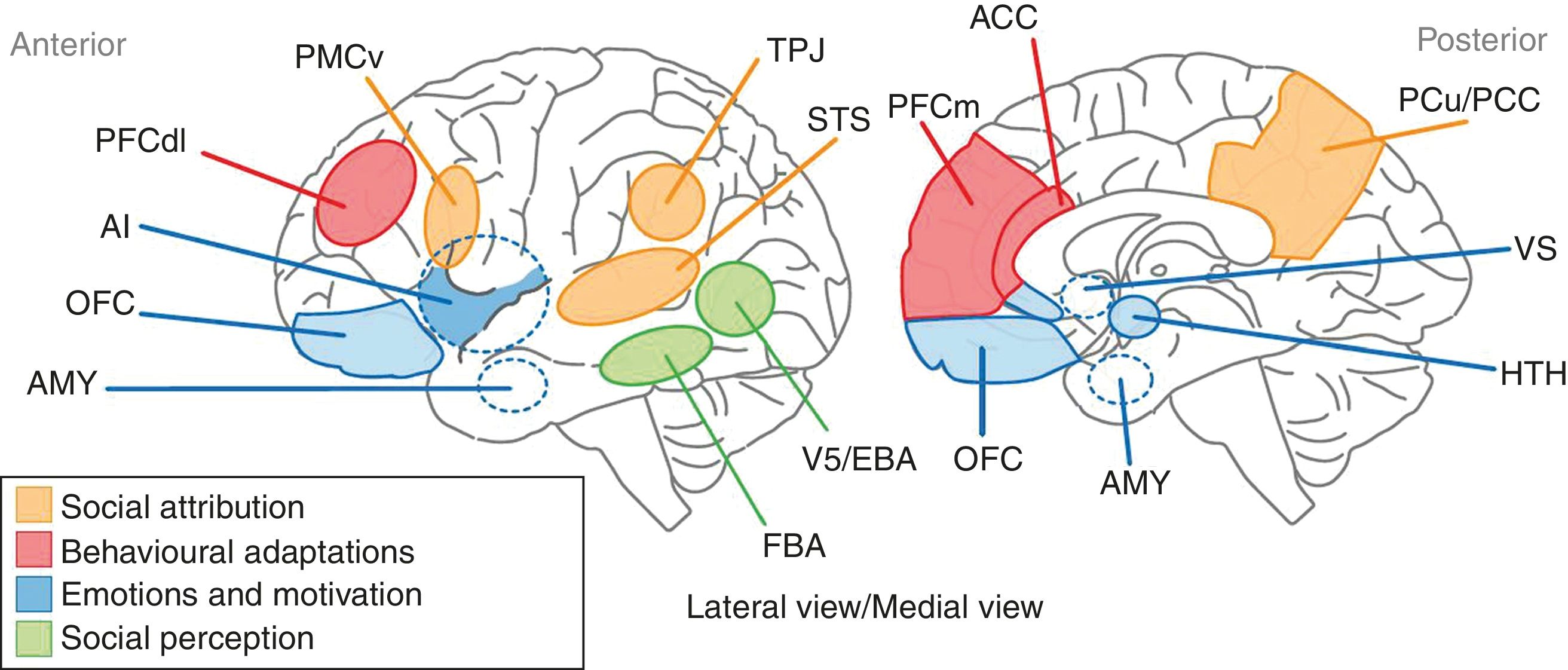
DiscussionSC alterations in schizophrenia cover a vast group of functions that range from the ability to attribute mental states to the ability to recognise the cause of phenomena that arise both from people and from social situations. It is now thought that these varied functions of SC may contribute to explaining some of the components of the psychopathology of schizophrenia, such as certain sensory perception disturbances, the attribution of both epistemic and affective mental states, and the ability to recognise the origin of voluntary human acts. Years ago, it was suggested that the structural support for SC in schizophrenia resided in a set of areas that made up the so-called “social brain”. This incorporates a set of brain areas distributed in the frontal, parietal, and temporal regions that are integrated into extensive neural networks associated with various aspects of cognition. The medial prefrontal cortex, the adjacent anterior cingulate cortex, the temporoparietal junction, the superior posterior temporal cortex, and the temporal poles make up the core of the social brain involved in SC. Billeke and Aboitiz49 recently proposed a neuroanatomical and cognitive model that links a set of brain areas, mostly components of the social brain, with various aspects of SC (Fig. 1): the processing of social stimuli such as biological movement and human faces is represented by areas located in the lower temporal cortex, such as V5 and the fusiform area; emotional and motivational processing includes limbic regions such as the amygdala, the anterior insula and the subgenual and perigenual region, also incorporating the anterior cingulate cortex, the orbitofrontal region and subcortical regions such as the hypothalamus and the ventral striatum; behavioural adaptations are represented by the dorsolateral and medial prefrontal cortices and the anterior cingulate cortex; and lastly, the functions related to the processes of social attribution, with respect to the attribution of mental states, are linked to the ventral premotor region, the superior temporal sulcus, the anterior and posterior cingulate cortices and the precuneus. In turn, the cognitive processes related to ToM are associated with the functioning of areas such as the medial prefrontal cortex and the temporoparietal junction. On the basis of this model, the authors state that schizophrenic patients will have a high degree of deterioration in behaviours such as the processing of emotions and communicative intentions. In addition, the evidence provided by the neuroimaging techniques indicates a significant deterioration in areas such as the amygdala, the anterior cingulate, and the medial prefrontal cortex, which establishes a structural basis for the deterioration observed in schizophrenic patients in emotional processing and ToM, respectively.
Representation of the anatomical areas involved in SC. The anatomical territories linked to the processes of social attribution are shown in yellow. The areas associated with behavioural adaptations are in red. The blue regions are where the processes related to emotions and motivation take place. Participation in sensory perception processes is attributed to the areas shown in green. AI: anterior insula; AMY: amygdala; FBA: fusiform body area; OFC: orbitofrontal cortex; PCC: posterior cingulate cortex; PCu: precuneus; PFCdl: dorsolateral prefrontal cortex; PFCm: medial prefrontal cortex; PMCv: ventral premotor cortex; STS: superior temporal sulcus; TPJ: temporoparietal junction; VS: ventral striatum.
The study of SC in schizophrenia has been an area of great interest over the last 10 years and has included research into social, cognitive, and neurobiological aspects. Recent evidence from behavioural and functional neuroimaging studies has helped to link broad constituent anatomical regions of the social brain with alterations in mentalisation, sensory perception, and goal-directed motor behaviours that are often observed in patients with schizophrenia, including those suffering from a first episode of this disorder. Future research is needed to further clarify the relationships between social brain networks and the psychopathology and neuropathology of schizophrenia. One priority issue in particular is analysis of the relationship between SC and neurocognition, in order to determine whether SC alterations are attributable only to structural and functional defects in the social brain or whether some of the neurocognition components also contribute.50
FundingThis article was written with the support of the Fondecyt 1140733 project assigned by the Chilean Government Comisión Nacional de Ciencia y Tecnología del Gobierno de Chile (Conicyt) [National Commission of Science and Technology].
Conflict of interestsNone.
We would like to thank Mahtías Muñoz Trujillo for his contribution to designing the figure presented in the text.
Please cite this article as: García RR, Aliste F, Soto G. Cognición social en esquizofrenia: aspectos cognitivos y neurobiológicos. Rev. Colomb. Psiquiatr. 2018;47:170–176.