Identify whether rs11179000, rs136494 and rs4570625 polymorphisms of the tryptophan hydroxylase 2 gene, are associated with a major depressive disorder in a sample of the Colombian population.
MethodsCase–control study was conducted in which a comparison was made between subjects diagnosed with major depressive disorder at some point in adulthood or active symptoms at the time of evaluation, and subjects with no psychiatric disease. Subjects were studied in the Department of Psychiatry, Faculty of Medicine and the Institute of Genetics at the National University of Colombia. Polymorphisms were genotyped using Taqman probes in real time PCR. As well as studying the association between major depressive disorder and these single nucleotide polymorphisms (SNPs), the association with other factors previously associated with depression were also analysed.
ResultsNo statistically significant association between genotypic and allelic frequencies of each polymorphism and major depressive disorder was found. Association between sex and complication during pregnancy/childbirth and major depressive disorder was observed. Association between sex and complication during pregnancy/childbirth and major depressive disorder was observed.
ConclusionsThere was no association between any polymorphism and major depressive disorder.
Identificar si los polimorfismos rs11179000, rs136494 y rs4570625 del gen de la triptófano hidroxilasa 2 están asociados a trastorno depresivo mayor en una muestra de población colombiana.
MétodosEstudio de casos y controles en el que se comparó a sujetos con trastorno depresivo mayor diagnosticado en algún momento de la vida adulta o con síntomas activos en el momento de la valoración y sujetos sin enfermedad psiquiátrica. Se estudió a los sujetos en el Departamento de Psiquiatría de la Facultad de Medicina y en el Instituto de Genética de la Universidad Nacional de Colombia. Se genotipificaron los polimorfismos usando reacción en cadena de la polimerasa en tiempo real y sondas Taqman. Además de buscar asociación entre trastorno depresivo mayor y estos polimorfismos de un solo nucleótido, se exploró asociación con otros factores relacionados previamente con depresión.
ResultadosNo se encontró asociación estadísticamente significativa entre las frecuencias genotípicas o alélicas de cada polimorfismo y el trastorno depresivo mayor. Se observó asociación entre sexo y complicaciones durante el embarazo/parto y trastorno depresivo mayor.
ConclusionesNo se halló asociación entre polimorfismo alguno y el trastorno depresivo mayor.
Major depressive disorder (MDD) is associated with high morbidity and mortality rates.1–3 According to the World Health Organisation (WHO), depression is expected to be the second leading cause of disability worldwide by 2020 and the leading cause of disease burden worldwide by 2030.1
MDD is considered to be a genetically complex disease.4 Underlying this hereditary complexity, the expression of multiple genes with minor effect could be modulated by different environmental factors. This would mean that certain risk genotypes confer greater susceptibility to the disease than other genotypes without risk from the same exposure to an environmental risk factor.4,5
The heritability of MDD varies from study to study, but has been estimated at 37–70%,4–6 and the risk of first-degree relatives of patients being affected is 2–3 times greater than that of the general population.1,5,7 A strong association has been found between development of MDD and different genes involved in the production and transport of serotonin.5 One of these genes is tryptophan hydroxylase 2 (TPH2). Tryptophan hydroxylase (TPH) is the enzyme that limits the synthesis of serotonin in rats. TPH1 and TPH2 are isoforms of TPH. Both isoforms are expressed in the brain, but TPH2 is predominantly expressed in the serotonin-producing neurons of the raphe nuclei, whereas TPH1 is also present in peripheral tissues such as the heart, kidneys, lungs, adrenal gland, liver and duodenum.8 TPH2 was discovered after it was found that mice genetically deficient in TPH continued to express serotonin in the brain but not in peripheral tissues, and showed no difference in serotonin-regulated behaviours from animals without TPH deficiency.9
Research studies in mice showed that functional mutations in TPH2 lead to a pronounced reduction in the activity of this enzyme.10–12 In a study by Zhang et al. in humans, a functional polymorphism (Arg441His) was identified which resulted in an 80% reduction in the enzymatic activity of TPH2 when expressed in a cell culture system. There was a greater presence of the mutant allele (1463A) in patients with MDD than in the control subjects,10,13 although this finding was not repeated in later studies.14–16
It has been found that different single nucleotide polymorphisms (SNP) of the TPH2 gene may be risk factors for8,17,18 or protective against19 MDD and its degree of severity.20,21 However, other studies have not found this association.22,23
Psychosocial stressors also influence the onset of MDD, especially if they occur early in the development24; the early loss of a carer is one stressful event strongly associated with depression.25–27 With regard to gene–environment interaction, animal studies show that allele variation in TPH2 function is regulated by stressful events leading to unfavourable outcomes similar to emotional disorders.28,29 Some research studies in humans have found greater reactivity to exposure to stressful life events and higher levels of depressive symptoms in carriers of certain allele variants of the TPH2 gene.24,29
This study was carried out to determine the correlation between the rs11179000,17 rs13864948,20 and rs4570625 SNPs6,21,24 of the TPH2 gene and MDD in a sample of the Colombian population. To the best of our knowledge, this is the first study in Colombia to analyse the association between polymorphisms of TPH2 and MDD.
Materials and methodsThis was a case–control study that included Subjects born in Colombia, aged from 18 to 60 years, who went to the Department of Psychiatry of the Faculty of Medicine at Universidad Nacional de Colombia from February to November 2014, and who were diagnosed with MDD according to the DSM-IV-TR30 criteria. All participants were assessed by two psychiatrists with experience in clinical diagnosis through a psychiatric interview (psychiatry lecturers with more than 10 years of experience). The examination was based on a semi-structured interview model commonly used in the medical record procedures followed by the Department of Psychiatry at Universidad Nacional de Colombia. All the patients were given a survey to collect sociodemographic data and medical history. Severity of depression was assessed in subjects with MDD who had active symptoms at the time of assessment using the 17-item Hamilton Depression Rating Scale (HAMD-17).31 Patients with autoimmune and neurodegenerative diseases, head injuries, cancer and mental disorders other than MDD were excluded. In the case sample, subjects with MDD at some point in adulthood (lax criterion) and subjects with MDD, active symptoms at the time of assessment and score ≥8 on the HAMD-17 (strict criterion) were analysed separately.
As a control group, we included subjects born in Colombia who attended an organised meeting for taking part in the study from February to November 2014. All were assessed in an identical manner with a semi-structured interview by the staff who assessed the subjects forming the case group. Subjects with a history of psychiatric disorder were excluded. Control subjects were aged from 18 to 60 years.
Case and control subjects were included in the study after obtaining their informed consent. This study was approved by the Ethics Committee of the Faculty of Medicine at Universidad Nacional de Colombia.
Laboratory proceduresAll laboratory procedures were blind, by masking the case or control status from the parties involved.
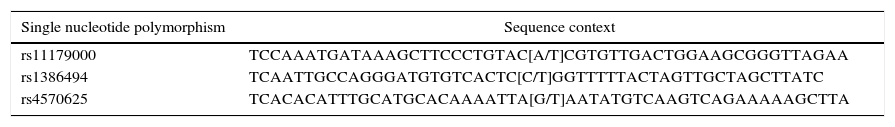
The three genotyped SNPs are listed in the SNP database (dbSNP) of the National Centre for Biotechnology Information (NCBI).32 For rs1386494 the complementary strand was amplified, whereas for the other two SNPs, the template strand was amplified. The context of the genetic sequence is shown in Table 1.
The methodology used for genotyping was real-time polymerase chain reaction (RT-PCR) using TaqMan probes.33 The master mix was prepared for each polymorphism using for each reaction 2.5μl of TaqMan Master Mix 2× (Applied Biosystems) and 0.5μl of TaqMan Assay 10×; the total volume of the master mix depended on the total samples to be processed, including negative and positive control. Then, 3μl of master mix and 2μl of DNA were added to the PCR tube for a total volume of 5μl. Vortex was applied and it was centrifuged. The RT-PCR was performed in a CFX96™ real-time thermocycler (Bio-Rad) using the protocol shown in Table 2.
Statistical analysisThe sample size calculation was performed taking into account a binary logistic regression model, seeking to estimate an odds ratio (OR) of 2.517, given a 1:1 ratio of case and control subjects, assuming a significance value of 5% and a power of 80%. With these assumptions, the calculated sample size was 214 subjects; these calculations were performed with the PASS® software.
For the descriptive statistical analysis we used summary measures according to the characteristics of the variables: median [interquartile range] for the continuous variables, depending on the asymmetry of the distributions, and percentage for the categorical variables. The analyses of association were initially performed using bivariate methods with Fisher's exact test or that of χ2, depending on the characteristics of the contingency tables. Medians were compared between groups of case and control subjects using Wilcoxon rank-sum tests for two samples. The covariates incorporated for analysis and characterisation were: age; gender (female/male); genotype frequencies; allele frequencies; loss of a carer before age 12 (yes/no); complications during pregnancy/childbirth (yes/no); and history of parents with diagnosis of MDD (yes/no). In addition, we used multivariable methods with binary logistic regression in which the dependent variable was being a case subject (lax or strict criterion), for which independent models were used. For all models, the genotype corresponding to each polymorphism was taken as independent variable. For the analysis of covariates in logistic models, those that were significantly associated with p<0.1 when performing bivariate analyses (gender, complications during pregnancy/childbirth, loss of a carer before age 12) were introduced into multivariate models to assess their role as effect modifiers or confounders in the analysis of association. Measures of association were estimated as ORs. For covariate control procedures, interaction terms were assessed within the models (gender, complications during pregnancy/childbirth, loss of a carer before age 12). Except for the initial processes of construction of logistic models, values of p<0.05 were assumed for hypothesis testing purposes. All analyses were performed using the R statistical programme.
The allele frequencies were obtained by direct counting from the observed genotypes and, based on the frequencies found, it was determined whether the population was in Hardy–Weinberg equilibrium.
ResultsCase subjects defined with the lax criterionA total of 102 patients were included in the group of case subjects with a lax criterion and they were compared to 114 subjects in the control group. We found a statistically significant association between gender and being a case or control subject: in the case group, 71% of patients with MDD were female and 29% male, whereas in the control group, 50% were male and 50% female (χ(1)2=9.48; p=0.002; OR=2.4; 95% confidence interval [95% CI], 1.36–4.21). The median age of the case subjects was 26 [14] years and that of the control subjects, 24 [9]; this difference was not statistically significant (rank-sum test, p=0.08).
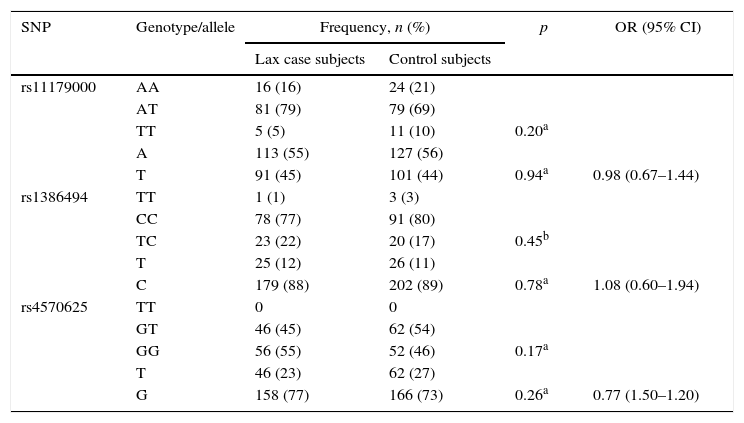
There was no statistically significant association between the genotype and allele frequencies of each of the SNPs studied and MDD. The results are shown in Table 3.
Genotype and allele frequencies of TPH2 polymorphisms in lax case and control subjects.
| SNP | Genotype/allele | Frequency, n (%) | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Lax case subjects | Control subjects | ||||
| rs11179000 | AA | 16 (16) | 24 (21) | ||
| AT | 81 (79) | 79 (69) | |||
| TT | 5 (5) | 11 (10) | 0.20a | ||
| A | 113 (55) | 127 (56) | |||
| T | 91 (45) | 101 (44) | 0.94a | 0.98 (0.67–1.44) | |
| rs1386494 | TT | 1 (1) | 3 (3) | ||
| CC | 78 (77) | 91 (80) | |||
| TC | 23 (22) | 20 (17) | 0.45b | ||
| T | 25 (12) | 26 (11) | |||
| C | 179 (88) | 202 (89) | 0.78a | 1.08 (0.60–1.94) | |
| rs4570625 | TT | 0 | 0 | ||
| GT | 46 (45) | 62 (54) | |||
| GG | 56 (55) | 52 (46) | 0.17a | ||
| T | 46 (23) | 62 (27) | |||
| G | 158 (77) | 166 (73) | 0.26a | 0.77 (1.50–1.20) | |
95% CI: 95% confidence interval; OR: odds ratio; SNP: single nucleotide polymorphism.
For association with the loss of a carer, it was found that 10.8% of subjects with MDD had lost a carer before age 12, compared with 3.5% of subjects in the control group; this difference was statistically significant (χ(1)2=4.40; p=0.03; OR=3.32, 95% CI, 1.02–10.79).
A statistically significant association was found between MDD and history of complications during pregnancy/childbirth – 27% of case subjects versus 13% of control subjects (χ(1)2=6.09; p=0.01, OR=2.37, 95% CI, 1.18–4.77) – as well as the history of threatened abortion, which was more common among case subjects than control subjects (3.9 vs. 0, respectively; Fisher's exact test, p=0.04).
No statistically significant difference was found between the two groups with respect to having a parent with depression: 25% vs. 20%(χ(1)2=1.33; p=0.24; OR=1.6; 95% CI, 0.71–3.56).
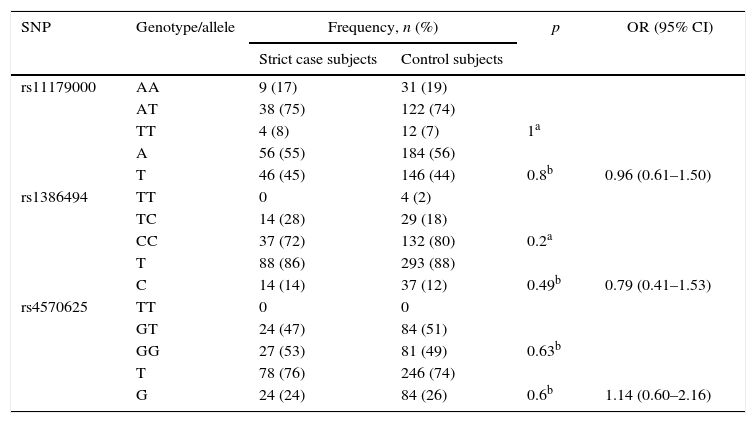
Case subjects defined with the strict criterionIn this category, 51 subjects from the case group and 165 from the control group were assessed. There was no statistically significant association between the genotype and allele frequencies of each SNP and MDD (Table 4). We also found no statistically significant difference with age: case subjects versus control subjects, 26 [20] vs. 25 [12] years. Having depressive disorder was not associated with the loss of a carer before age 12 (9.8% vs. 6.1%; Fisher's exact test, p=0.35; OR=1.7, 95% CI, 0.54–5.17), or with history of depression in a parent (20% vs. 10.9%; χ(1)2=2.79; p=0.09; OR=2.04; 95% CI, 0.87–4.76).
Genotype and allele frequencies of TPH2 polymorphisms in strict case and control subjects.
| SNP | Genotype/allele | Frequency, n (%) | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Strict case subjects | Control subjects | ||||
| rs11179000 | AA | 9 (17) | 31 (19) | ||
| AT | 38 (75) | 122 (74) | |||
| TT | 4 (8) | 12 (7) | 1a | ||
| A | 56 (55) | 184 (56) | |||
| T | 46 (45) | 146 (44) | 0.8b | 0.96 (0.61–1.50) | |
| rs1386494 | TT | 0 | 4 (2) | ||
| TC | 14 (28) | 29 (18) | |||
| CC | 37 (72) | 132 (80) | 0.2a | ||
| T | 88 (86) | 293 (88) | |||
| C | 14 (14) | 37 (12) | 0.49b | 0.79 (0.41–1.53) | |
| rs4570625 | TT | 0 | 0 | ||
| GT | 24 (47) | 84 (51) | |||
| GG | 27 (53) | 81 (49) | 0.63b | ||
| T | 78 (76) | 246 (74) | |||
| G | 24 (24) | 84 (26) | 0.6b | 1.14 (0.60–2.16) | |
95% CI: 95% confidence interval; OR: odds ratio; SNP: single nucleotide polymorphism.
A statistically significant association was found with gender (case subjects: 72.5% of females and 27.5% of males; control subjects: 55.8% of females and 44.2% of males χ(1)2=4.56; p=0.03; OR=2.1; 95% CI, 1.05–4.07), a history of complications during pregnancy/childbirth (37.3% vs. 13.9%; χ(1)2=13.52; p=0.0002; OR 3.7; 95% CI, 1.7–7.5) and history of threatened abortion (8% vs. 0; Fisher's exact test, p=0.002).
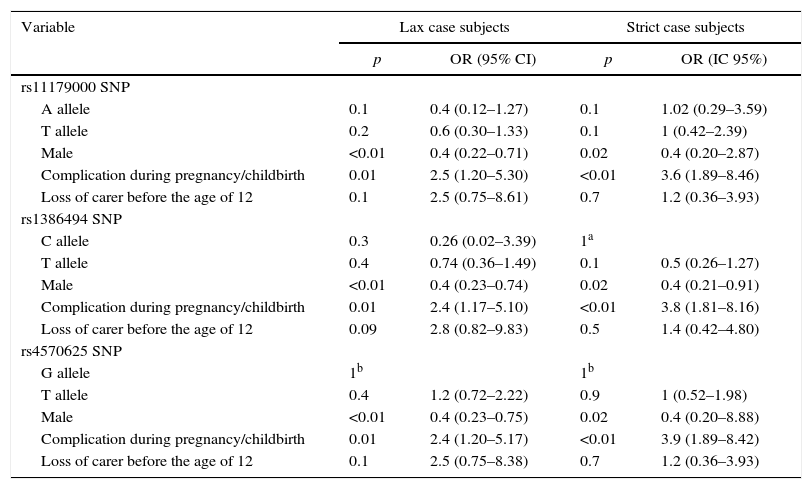
Multivariate analysisAfter performing the multivariate analysis, no statistically significant association was found between MDD and the three SNPs of TPH2. The lack of association was detected by adjusting the possible effect of confounding variables. Within this group, the covariables gender and complications during pregnancy/childbirth introduced into the design showed a statistically significant association with MDD. These results are shown in Table 5.
Multivariate analysis of lax and strict case subjects.
| Variable | Lax case subjects | Strict case subjects | ||
|---|---|---|---|---|
| p | OR (95% CI) | p | OR (IC 95%) | |
| rs11179000 SNP | ||||
| A allele | 0.1 | 0.4 (0.12–1.27) | 0.1 | 1.02 (0.29–3.59) |
| T allele | 0.2 | 0.6 (0.30–1.33) | 0.1 | 1 (0.42–2.39) |
| Male | <0.01 | 0.4 (0.22–0.71) | 0.02 | 0.4 (0.20–2.87) |
| Complication during pregnancy/childbirth | 0.01 | 2.5 (1.20–5.30) | <0.01 | 3.6 (1.89–8.46) |
| Loss of carer before the age of 12 | 0.1 | 2.5 (0.75–8.61) | 0.7 | 1.2 (0.36–3.93) |
| rs1386494 SNP | ||||
| C allele | 0.3 | 0.26 (0.02–3.39) | 1a | |
| T allele | 0.4 | 0.74 (0.36–1.49) | 0.1 | 0.5 (0.26–1.27) |
| Male | <0.01 | 0.4 (0.23–0.74) | 0.02 | 0.4 (0.21–0.91) |
| Complication during pregnancy/childbirth | 0.01 | 2.4 (1.17–5.10) | <0.01 | 3.8 (1.81–8.16) |
| Loss of carer before the age of 12 | 0.09 | 2.8 (0.82–9.83) | 0.5 | 1.4 (0.42–4.80) |
| rs4570625 SNP | ||||
| G allele | 1b | 1b | ||
| T allele | 0.4 | 1.2 (0.72–2.22) | 0.9 | 1 (0.52–1.98) |
| Male | <0.01 | 0.4 (0.23–0.75) | 0.02 | 0.4 (0.20–8.88) |
| Complication during pregnancy/childbirth | 0.01 | 2.4 (1.20–5.17) | <0.01 | 3.9 (1.89–8.42) |
| Loss of carer before the age of 12 | 0.1 | 2.5 (0.75–8.38) | 0.7 | 1.2 (0.36–3.93) |
95% CI: 95% confidence interval; OR: odds ratio; SNP: single nucleotide polymorphism.
Only the rs1386494 SNP was found in Hardy–Weinberg equilibrium, both in the total sample (p=0.80) and in each of the groups: lax case subjects (p=0.93); lax control subjects (p=0.60); strict case subjects (p=0.82); and strict control subjects (p=0.70). The rs11179000 and rs4570625 SNPs were not in equilibrium. Despite that, the results for association between these two SNPs and MDD were marked because the lack of equilibrium was not considered to be due to typing errors, since similar frequencies were obtained in both patients and control subjects. In addition, the TaqMan analysis used for genotyping is a pre-designed analysis in which TaqMan primers and probes have been validated and used previously (Applied Biosystems). It is possible, however, that the lack of equilibrium was due to other factors, such as the size of the sample, or that the effect of the mix in the Colombian population may have an influence.
DiscussionMDD has a major impact on health worldwide. Biological and environmental factors have been investigated in an attempt to clarify the aetiology of MDD, but no one cause has been found to explain the origin of the disease.
Previous studies have associated certain genotypes and alleles of the rs11179000, rs1386494, and rs4570625 TPH2 SNPs with MDD or affective symptoms, but it has not been possible to characterise these SNPs universally for MDD due to the small number of studies and the wide variation in results. This study did not find any association between these three SNPs and MDD in the sample taken from the Colombian population. Some of the research conducted with these SNPs is discussed below.
De Araújo et al.17 found an association between the rs11179000 SNP and late-onset MDD, when they detected a higher frequency of the AA genotype in the group of subjects with MDD, leading them to conclude that being a homozygous carrier of A increases the risk of late-onset depression two-fold (p=0.025).
Zill et al.8 conducted a case–control study to assess the association of the rs1386494 SNP with MDD. They found an increase in the G allele in the case group with respect to the control group (0.86 vs. 0.79; p=0.012). These same authors found a significant association between being a carrier of the G allele of the rs1386494 SNP and suicide (p=0.038).34 Anttilla et al.20 assessed subjects with MDD and resistance to treatment who were treated with electroconvulsive therapy (ECT). Although they found no association between response to ECT and rs1386494, they found that patients with the A/A genotype had a higher score on the Montgomery-Åsberg Depression Rating Scale than patients with the genotypes A/G+G/G (p<0.001). Carriers of the A/A genotype also had more psychotic symptoms. Other studies found no association with this polymorphism.18,23
An association was found between the rs4570625 SNP and conditions characterised by emotional dysregulation. Brown et al.35 assessed the impact of the variant of this polymorphism on the amygdala response to threat, and showed greater activity in the bilateral dorsal area of the amygdala of T-allele carriers than in the homozygous carriers of the G allele (p<0.05). Gutknecht et al.36 found a higher frequency of the T allele of rs4570625 in patients with B and C personality disorders, in whom they found alterations in emotional regulation such as anxiety, depressive symptoms and aggression. Greater comorbidity was also found with affective disorders and anxiety disorders in this study. Serretti et al.21 found that in patients with MDD the rs4570625–rs10748185 G-A haplotype was significantly associated with a higher score on the Montgomery-Åsberg Depression Rating Scale (p=0.006). In addition, Nobile et al.24 analysed the effects of rs4570625 on TPH2 and 5-HTTLPR and the association with an environmental predictor (family structure) on depressive symptoms in a general population of children aged 10–14 years, and found evidence that belonging to a single-parent family and being a carrier of the G variant of the rs4570625 polymorphism (G-703 T) and the short allele of the 5-HTTLPR were also associated, both alone and in an apparent gene–environment interaction, with a higher score on that scale. A meta-analysis published in May 2012 showed that the rs4570625 SNP (G703 T) had a strong epidemiological association with MDD.6 Lastly, Mandelli et al.29 found greater reactivity to exposure to stressful life events and a higher level of depressive symptoms in patients with the TT genotype of TPH2 rs4570625.
It is possible that the lack of concordance of the results between our study and previous ones that show association derives from differences between the genetic structures of the Colombian population and other populations in which this type of study has been conducted. The different clinical manifestations of MDD are caused by the fact that in complex diseases the genetic components among the individuals who suffer from them vary,37 so the lack of homogeneity of the depressive symptoms among the subjects studied may be influenced by the lack of similarity of their genotypes and alleles. Furthermore, the fact that this population was not in Hardy–Weinberg equilibrium for the rs11179000 and rs4570625 SNPs also influences the results. Having said that, not all the subjects who participated in the study came from the same region of Colombia and that could be a factor modifying the results. However, we did not perform a stratified analysis of the population because the majority of the sample was enrolled in the city of Bogota, where the Caucasian/mixed-race population predominates. To carry out a stratified population analysis, we would have to study the Caucasian (pure), Amerindian (pure) and pure black populations and know the composition and rates in each of these groups, which would be almost impossible to do because it is very difficult to find pure groups, mainly Caucasian. There are also very few Amerindian groups in the Colombian Andean region, and the black population is mainly located in regions of the country's Atlantic and Pacific coasts, regions that were not analysed in the sample.
We found a low frequency of the TT genotype in the rs11179000 and rs1386494 SNPs and absence of such genotype in the rs4570625 SNP, which could be related to the fact that being homozygous for the T allele is unfavourable in our population or, simply, that the sample size limits the likelihood of it being found.
Nevertheless, our study did find an association with some sociodemographic and clinical factors. In the case group, the ratio of female to male subjects was 2:1. Being female was therefore considered to be a risk factor for MDD; a statistically significant finding and one in agreement with previous publications which found MDD to be 2–3 times more common in women.1
We found statistically significant differences between complications during pregnancy/childbirth and MDD. Some studies have described an increased risk of depression in adolescents whose mothers suffered from postnatal depression,38 and it has been reported that educational level, low self-esteem and poor social support are related to perinatal depression.39 These are risk factors that are also associated with complications during pregnancy, so it could be hypothesised that mothers of MDD subjects who had complications during pregnancy/childbirth also had some depressive disorder, either associated with the risk factors common to both conditions or as a reactive symptom to the medical complication. Low birth weight can be associated with depression at any age, and in a study by Kaikkonen et al.,40 it was found that in a group of older adults, the short duration of gestation was associated with higher scores in the Beck Depression Inventory and the Centre for Epidemiological Studies-Depression Scale. It is possible that the different complications during pregnancy/childbirth found in this sample (such as threatened abortion, preterm birth, acute foetal distress and pre-eclampsia) were associated with low birth weight or shorter duration of gestation. In any event, the complications during pregnancy/childbirth variable was found to be a statistically significant risk factor for MDD, to the same extent as threatened abortion (among the complications), and could be an aspect to be assessed in future studies.
Although the evidence indicates a greater risk of depression among the immediate relatives of depressed patients, no statistically significant differences were found between subjects with parents or other relatives with MDD and those with no such history. If heritability is considered a contributing factor to this association, it is possible that the above finding is consistent with the lack of association with the genotypes.
Lastly, it has been determined that, in general, there is a lower risk of offspring dysfunction in families in which both the biological mother and father are present.41 Loss of a parent in childhood has been strongly associated with depression.25–27 Our study found an association between loss of a carer before age 12 and MDD in the category of lax case subjects. However, when the cases were assessed with strict criteria and in the multivariate analysis, although this variable continued to be a risk factor for MDD, the statistical significance was lost. Homogenisation of the study population and a larger sample could change the ambiguous results.
ConclusionsNo statistically significant association was found between the rs11179000, rs136494 and rs4570625 SNPs of TPH2 and MDD. Being female and a history of complications during pregnancy/childbirth, threatened abortion in particular, proved to be risk factors associated with MDD. A history of a parent with MDD was not associated with MDD in our study, which may correspond to the lack of association with the genotypes. Lastly, the results were inconclusive regarding the loss of a carer before the age of 12.
For subsequent studies, it would be advisable to homogenise the study population using the following strategies: subdivide the group of subjects with MDD according to the characteristics of the clinical manifestations; and perform the analyses according to the degree of depression and the presence or absence of psychotic or melancholic symptoms. Increasing the sample size could lead to Hardy–Weinberg equilibrium of the SNPs and more statistically significant results.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis project was funded by the Research Unit, Bogota Office, of Universidad Nacional de Colombia (code 23379).
Conflict of interestsThe authors declare that they have no conflicts of interest.
We would like to thank all the subjects who took part in this study.
Please cite this article as: Martínez-Idárraga A, Riveros-Barrera I, Sánchez R, Jaramillo LE, Calvo-Gómez JM, Yunis-Londoño JJ. Caracterización de tres polimorfismos del gen de la triptófano hidroxilasa 2 en una muestra de población colombiana con trastorno depresivo mayor. Rev Colomb Psiquiat. 2017;46:22–30.