To describe and discuss current evidence on the relationship between cognitive performance, bipolar affective disorder (BAD) and metabolic syndrome (MS).
MethodsWe searched for related articles in different bibliographic databases (MEDLINE, EMBASE, Scielo) and performed a narrative review of the literature with the selected articles.
ResultsTo date, evidence has not been conclusive and the effect of MS on BD has not been widely studied, but important correlations have been observed with individual metabolic variables. It is suggested that obesity in patients with BAD is associated with worse performance in verbal memory, psychomotor processing speed, and sustained attention. Hypertriglyceridemia in patients with BAD appears to be associated with a lower score in executive function tasks; hypertension appears to be associated with impairment in overall cognitive function.
ConclusionsDespite the associations between MS and poor cognitive performance in patients suffering from BAD, more studies are required to precisely determine how these variables are related to each other.
Describir y discutir la evidencia actual sobre la relación entre desempeño cognitivo, trastorno afectivo bipolar (TAB) y síndrome metabólico (SM).
MétodosSe buscaron artículos relacionados en distintas bases de datos bibliográficas (MEDLINE, EMBASE, Scielo), y con los artículos seleccionados se realizó una revisión narrativa de la literatura.
ResultadosHasta el momento no se ha estudiado ampliamente el SM en el TAB, pero sí hay datos importantes en la asociación con las variables metabólicas individuales. Se señala que la obesidad de los pacientes con TAB se asocia con peor desempeño en memoria verbal, velocidad de procesamiento psicomotor y atención sostenida. Parecería que la hipertrigliceridemia de los pacientes con TAB está en relación con deterioro en la función ejecutiva, y la hipertensión arterial, con el deterioro en la función cognitiva general.
ConclusionesAunque algunas variables del SM se asocian con peor desempeño cognitivo en pacientes con TAB, faltan estudios para establecer con precisión la naturaleza de esta relación.
Bipolar affective disorder (BPAD) is characterised by sudden, episodic and dysfunctional changes in emotional polarity, which fluctuate with inter-critical periods of euthymia. Its prevalence is 0.6%.1,2 In patients with BPAD there is cognitive impairment; alterations of this are described in relation to emotional polarity, the current episode and the number and type of episodes.3 In a euthymic state, between 40 and 60% of patients present cognitive impairment.4,5 BPAD is associated with high comorbidity, which increases the health costs by 40%.6 The third cause of death in BPAD is cerebrovascular events,7 which are related to metabolic syndrome (MS).8 As with BPAD, MS has also been associated with cognitive abnormalities in patients without the affective disorder. The way in which comorbidity between BPAD and MS influences cognitive performance is currently being studied.9 In this article, a narrative review of the literature on the correlation between MS and cognitive performance in BPAD is carried out.
Cognitive performance in BPADOver the last two decades, research into BPAD has identified that the study of cognitive performance has clinical and functional relevance. There are multi-domain alterations in relation to emotional polarity, the current episode and the number and type of episodes.10 Cognitive impairment is associated with worse quality of life, job performance and functionality, and higher rates of suicide attempts, death and use of health services.11
Some 40–60% of patients in a euthymic state present with cognitive impairment, mainly those with bipolar I disorder and a history of psychosis.4 During a euthymic state, alterations persist in working memory, verbal memory, declarative memory, resolution of problems, attention, executive function and visuospatial function.10,12 A meta-analysis13 of 28 articles found cognitive impairment during a euthymic state with a large effect size (d>0.8) in working memory, executive function, fluidity and verbal memory. It has been observed that general functionality is worse in the subgroup of patients with greater cognitive impairment.14
Another meta-analysis15 of 42 studies, 13 on mania and five on depression, showed moderate to severe cognitive impairment in different domains in subjects who were both euthymic and with affective episodes; the euthymic individuals had impairment in visual processing speed (d=0.69), working memory (d=0.65), verbal learning (d=0.81) and resolution of problems (d=0.61). In a euthymic state in manic episodes, performance was worse in verbal learning and attention; in depression, it was worse in phonemic fluency. The sample was small for some domains and the results were heterogeneous. In many cases, confounding variables such as psychiatric comorbidities were not monitored.
There is cognitive impairment from the first affective episodes in attention and executive function.16 López et al.17 studied the progression of cognitive impairment in bipolar I disorder and its relationship with the number of affective episodes. They found that the number of obsessions is linked to the intensity and type of cognitive alteration. In patients who had presented one obsession, impairment was identified in working memory; in those who had two obsessions, affection was greater in episodic, visuospatial short-term memory, delayed evocation, recognition of logical memory and phonological verbal fluency; and in those who had suffered more than two obsessions, impairment and number of domains affected were greater in executive function and psychomotor processing speed. These findings contradict those of Samamé et al.18 and Bora et al.19 in two meta-analyses in subjects with BPAD in a euthymic state; although they found cognitive impairment, it was not progressive in the follow-up (a mean of 4.6 and 5.5 years, respectively) in the domains studied.
Cognitive domains mediated by frontotemporal circuits are the most affected: executive function, motor functions, attention, verbal fluidity, learning and memory; in particular, the declarative memory is more impaired in patients with a long-term disorder or recurrent affective episodes.11
Three subgroups or cognitive clusters have been described in BPAD; the first (40%) has a similar performance to the general population; the second (30%) has moderate cognitive impairment in psychomotor processing speed, verbal learning, social cognition and attention; and a third group (30%), has severe general impairment comparable to that of schizophrenia.20 Similarly, Solé et al.14 describe three groups of patients with bipolar II disorder in a euthymic state: intact group (n=29 [48.3%]), group with selective impairment (n=24 [40%]) and group with general impairment (n=7 [11.6%]).14
Metabolic syndrome and BPADBPAD increases mortality by 1.5–2.5-fold; the risk of diabetes mellitus five-fold and the risk of MS by 50%.21,22 The prevalence of MS in patients with BPAD varied according to the criteria used; a systematic review5 of 39 articles showed a prevalence of co-existence of two disorders of between 17.6 and 50% according to criteria of the Adult Treatment Panel III, and between 25.7 and 67% according to criteria of the International Diabetes Federation (IDF); other studies show prevalences of between 30 and 49%.23 This comorbidity is more common in Hispanics and women.5 Obesity in BPAD is reported in 20–35%, more than in the general population.23 In BPAD, there are more modifiable risk factors for cardiovascular disease which are related to the onset and perpetuation of MS.
The epidemiology of MS varies according to geographic and cultural factors. The prevalence of MS according to criteria of the Third Report of the National Cholesterol Education Program (NCEP-III) adjusted by age is 23.7%.24 In the United States, between 2009 and 2010 it was 22.9%, higher among Mexican immigrants (34.7%) and white males (22.9%) and lower in black males (18.9%).25 Other studies show prevalences in this country of between 36 and 49%, while in Italy it is 17% and, in Belgium, 27%.26–28
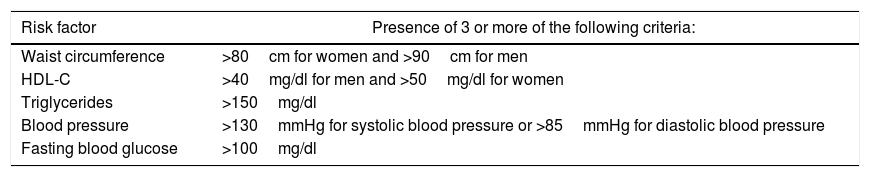
Diagnosis of MSIn MS, risk factors such as hypertension, dyslipidaemia, hyperglycaemia and obesity, which increase the risk of type 2 diabetes mellitus and cardiovascular disease, co-exist.29 In 1988, Reaven called it “syndrome X”; 10 years later, the World Health Organization (WHO) formalised the first definition and since then different diagnostic proposals have emerged. In 2009, the WHO, the IDF, the National Heart, Blood and Lung Institute (NHBLI), the American Heart Association (AHA) and the International Association for the Study of Obesity proposed a “harmonised” definition22 of MS which aims to integrate the different criteria (Table 1). Pajunen et al.22 compared the predictive value of different definitions of MS and concluded that they are all useful for predicting the onset of diabetes mellitus and cardiovascular disease, but the “harmonised” definition was better for predicting cardiovascular disease.
“Harmonised” definition of metabolic syndrome.
| Risk factor | Presence of 3 or more of the following criteria: |
|---|---|
| Waist circumference | >80cm for women and >90cm for men |
| HDL-C | >40mg/dl for men and >50mg/dl for women |
| Triglycerides | >150mg/dl |
| Blood pressure | >130mmHg for systolic blood pressure or >85mmHg for diastolic blood pressure |
| Fasting blood glucose | >100mg/dl |
Additional criteria: those who receive treatment for hyperglycaemia, hypertension, hypertriglyceridaemia or low HDL-C.
The presentation of BPAD varied when combined with MS. McIntyre et al. characterised the relationship of these two disorders in a systematic review2:
- 1.
MS is more common in BPAD.
- 2.
The most common finding of MS in BPAD is increased waist circumference.
- 3.
The presence of MS complicates the course and prognosis of BPAD: more depressive and relapse episodes, worse response to treatment and higher prevalence of suicide attempts; obesity is the greatest metabolic risk factor for suicide (53% versus 36% of those who only have BPAD).
- 4.
Comorbidity between MS and BPAD is associated with the use of psychotropic drugs which increase weight.
Given the relationship between MS and BPAD, Mansur et al.21 state that there is a bidirectional relationship between both, more than a comorbidity, which are a different subtype of disorder that they call “metabolic-affective syndrome”, and they argue its existence with different characteristics common to both:
- •
Phenomenology: patients with BPAD and obesity have greater reactivity to affection, increased appetite, hypersomnia and leaden paralysis. Comorbidity between depression and obesity predicts that less weight is lost and this is maintained.30
- •
Genetics: both disorders have a high genetic load (50–70% for BPAD and 50–90% for obesity).31 The FTO gene32 is related to both obesity and symptoms of depression.
- •
Neurotransmitters and functional tests: in BPAD and MS there are alterations in different areas of brain connectivity. Dopamine, serotonin and endogenous opioids are neurotransmitters involved in the regulation of affection and food intake.33
- •
Environmental factors, such as poverty, poor family support and low education level, and a history of traumatic experiences are involved in the genesis of both disorders.34
There are different factors that explain the metabolic alterations in BPAD27:
- •
Neuroendocrine dysfunction: physiological and psychological stress typical of BPAD causes an increase in the release of corticotropin-releasing hormone, which increases the systemic concentrations of cortisol and may lead to hyperglycaemia and hyperlipaemia.35 Similarly, it has been associated with dysfunction of the hypothalamic–pituitary–adrenal axis and increased circulatory catecholamines which cause hypertension, especially in mania.36 Hypothyroidism has been related to increased cholesterol bound to low-density lipoproteins, endothelial dysfunction, obesity and cardiovascular disease.37 In BPAD, the prevalence of hypothyroidism is greater; different physiopathological mechanisms explain this comorbidity, such as the use of lithium and the inflammatory theory of BPAD.35
- •
Smoking: it has been described as a risk factor of multiple conditions and is clearly related to the increased metabolic risk and mortality from cardiovascular causes.38 Patients with BPAD present greater addiction to nicotine than the general population (35.2 versus 12.8%, respectively).39
- •
Lifestyle: sedentary lifestyle and high-calorie diets are more common in patients with BPAD; some studies indicate that these patients have a lower metabolic rate, which increases the risk of MS.40
- •
Medications: different medications used for BPAD lead to weight gain. Therefore, a study41 with 90 patients reported weight gain in the first year of 13% with lithium and 21% with valproic acid (placebo, 7%). Another study42 showed weight gain of between 1 and 4.2kg with lithium and 1kg with valproic acid and reduction of 2.2kg with lamotrigine. Typical antipsychotics are those that have a smaller scale effect; regarding atypical antipsychotics, olanzapine and clozapine show the greatest weight gain, quetiapine and risperidone have an intermediate effect.43 Another metabolic effect is the diabetogenic effect, specifically with clozapine and olanzapine.23
MS is associated with impaired cognitive performance by producing cerebral small vessel disease and subclinical cerebrovascular events, which is why it has been associated with neurodegenerative diseases.44 A cross-sectional study in a Chinese population found that MS elevated the risk of mild cognitive impairment.45 MS worsens performance of the executive function and verbal fluency (not progressive at six years) and the risk factor with the strongest relationship is increased waist circumference.44
A review of the literature found that 14 of 15 cross-sectional studies demonstrated a link between obesity and cognitive impairment, mainly in executive function; this study did not include patients with mental illness. Some results were contradictory, possibly as confounding variables were not monitored.9
BPAD, MS and cognitive performanceIn a study in adults with BPAD in a euthymic state46 diagnosed according to the DSM-IV-TR, two groups were compared: one overweight or obese group (n=48; body mass index [BMI], 25–40) and another normal weight group (n=18; BMI, 18.5–24.9). In the study, learning and memory (California Verbal Learning Test), attention and psychomotor processing speed (Trail Making Test A and Symbol Digit Modalities Test), executive function (Trail Making Test B and verbal fluency test), general intellectual ability (Shipley Abstraction), recollective and habit memory (Process-Dissociation Procedure) and own perception cognitive failures (Cognitive Failures Questionnaire) were evaluated. Patients with a traumatic brain injury or who had been pregnant in the last year, with diabetes, MS, electroconvulsive therapy in the previous six months and substance abuse or dependence in the previous three months were excluded. The univariate analysis showed that BMI was related significantly and negatively with the score from the Symbol Digit Modalities Test (r=−0.32; p<0.01); the group who were obese and overweight presented a lower mean in the verbal fluency test (59.29±13.87 versus 70.06±19.5) and no statistically significant differences were found in the other cognitive domains. When monitoring by confounding variables such as age and years of education, no effect of obesity on cognitive performance was found.
In 2014, Depp et al.47 published the study with the largest sample size: 314 patients with BPAD and 417 with schizophrenia. Among the objectives, the authors looked for the relationship between BMI, treated hypertension and cognitive performance. The diagnosis of hypertension and diabetes was performed according to the use of medications for both, without considering the clinical criteria used at the time of diagnosis. In the group of patients with BPAD, they found a small and negative correlation between BMI and general cognitive performance (r=−0.185; p=0.001); the effect persisted when adjusted for education level, residential status, negative symptoms and use of atypical antipsychotics (r=−0.144; p=0.009). The effect was greater in verbal memory (F(1.339)=3.3; p=0.036), psychomotor processing speed (F(1.339)=6.3; p=0.001) and sustained attention (F(1.339)=4.6; p=0.011). Patients with BPAD who received antihypertensive treatment had worse cognitive performance than those who did not receive it (Z-score without antihypertensive drugs, mean, −0.32±0.83 versus treatment, −0.60±0.83; F(1.339)=7.5; p=0.006).
Other authors indicate a negative relationship between obesity and cognitive impairment. Lackner et al.48 studied the relationship between cognitive performance, BPAD and obesity in 100 patients with bipolar I and II disorder in a euthymic state and 64 healthy controls with no history of mental illness in first-degree blood relatives. Each group was divided up according to weight into normal (BMI<24.9) and overweight (BMI>25). Different cognitive domains were evaluated: learning and verbal memory (California Verbal Learning Test [CVLT]), psychomotor processing speed and attention (Trail Making Test A, d2 Test of Attention, Stroop Test) and executive function (Stroop interference). BMI, waist circumference, hip circumference, waist-to-hip ratio, waist-to-height ratio, body fat distribution and subcutaneous adipose tissue were measured. The univariate analysis showed that obese individuals from the group of patients with BPAD had worse performance than the BPAD group with normal weight in the Trail Making Test A (mean 39.05±18.08 versus 32.18±11.81). When comparing patients and controls, obesity was linked to worse cognitive performance independently of the diagnosis of BPAD. Furthermore, obese patients with BPAD had worse performance than non-obese patients with BPAD in the short-delay free recall test (mean, 10.43±3.17 versus 12.72±2.60); this difference was not found in the control group. The differences found were no longer observed in the multivariate analysis adjusted for age, gender and education level.
Other variables of MS have been linked to worse cognitive performance in BPAD. The cross-sectional study by Hubenak et al.49 included 40 patients in a euthymic state with a diagnosis of BPAD according to the ICD-10 criteria; they studied the relationship between metabolic variables, treatment with mood stabilisers and cognitive performance. They measured height, blood pressure, BMI, cholesterol, triglycerides, glucose, fasting insulin, neuropsychological profile (verbal learning, memory, attention and executive function). The patients were analysed in two groups, one with studied metabolic disorder and another without it. In the case of MS, the criteria of the NCEP ATP-III were applied. A total of 37.5% of patients had MS and 75% had a BMI>25. They found worse performance in all domains of the cognitive tests, specifically in patients with abdominal obesity (T-score, 43 versus 46.3; p=0.039), hypertension (T-score, 42.6 versus 47.5; p=0.003) and MS (T-score, 41.9 versus 46.1; p=0.011). The post hoc analysis showed an insignificant effect in the impairment of cognitive performance for MS (d=0.74) and abdominal obesity (d=0.55); however, the effect was significant (d=0.87) in the case of hypertension. The other variables studied, including treatment with mood stabilisers, did not have a relationship with cognitive impairment.
Naiberg et al.50 studied the relationship between hypertriglyceridaemia and executive function performance. They included 69 patients aged 13–20, 34 in the group with BPAD and 35 in the group of healthy controls. The neuropsychological evaluation was performed with the Cambridge Neuropsychological Test Automated Battery (FE Intra-Extra Dimensional Set-Shifting Task). They measured weight, height, abdominal circumference, cholesterol, triglycerides and fasting blood glucose level, and MS was diagnosed according to the criteria of the IDF. In both groups, those who presented at least one criterion of MS had worse executive function performance (Z-score, 0.21±0.50 versus 0.45±0.53; p=0.027). In the group of patients with BPAD, the triglyceride and blood pressure figures correlated negatively with the executive function performance (r=−0.396; p=0.020 and r=−0.358; p=0.041, respectively). In the analysis by correlation performance, the concentration of triglycerides was the only thing which was negatively related to the executive function performance (p=0.036); this relationship was not found in the controls. The effect of triglycerides on the executive function remained after monitoring by the affective episodes variable. Patients with symptoms of depression had a worse cognitive performance than asymptomatic patients or those with hypomania and a multivariate analysis was not performed.
Regarding the time of onset of altered cognitive performance, a study by Silveira et al.51 included patients aged 16–35, in a euthymic state and with BPAD after the first episode (mania, mixed, with or without psychosis); they were divided up according to BMI into obese and overweight (BMI>25, n=25) and normal weight (BMI 18.5–24.9, n=40). This population was compared to controls without BPAD (obese and overweight, n=9; normal weight, n=28) and cognitive performance was measured with the MATRICS Consensus Cognitive Battery, an instrument validated for BPAD which includes the attention, verbal and non-verbal memory, working memory and executive function domains. Cognitive performance was compared between groups and between the different subgroups. Although the BPAD group presented with worse performance in all cognitive domains than the control group, no differences were found between the cognitive performance of obese or overweight patients with BPAD and BPAD patients with a normal BMI. However, in the group with BPAD, a negative relationship was found between increased BMI (above normal) and the performance of non-verbal memory (r=−0.246, r2=0.006; p=0.05). The authors suggest that the impact of BMI on cognitive performance in BPAD begins as the disorder progresses, and not from the first episode.
DiscussionPatients with BPAD present a higher rate of MS. Different mechanisms in BPAD explain this link: metabolic effect of antipsychotics, genetic susceptibility to metabolic alterations, non-healthy lifestyles, higher frequency of disorders due to use of substances and neuroendocrine dysfunction.30,52 “Metabolic-affective”21 syndrome has been described, in which BPAD is characterised by a higher rate of suicide, relapses, depression and reactivity of affection and worse response to treatment.
Up to 60% of patients with BPAD present a worse cognitive performance in different domains, such as working, verbal and declarative memory, resolution of problems, attention, executive and visuospatial function.20 Three cognitive clusters have been proposed according to the degree of affection.13 These alterations are related to poor control of the symptoms, more affective episodes, little response to pharmacological treatment and time of progression.11 Metabolic disorders produce cognitive impairment; therefore, obesity is associated with impairment of executive function, and increased waist circumference is the metabolic finding which is most associated with cognitive impairment.9
Currently, there is a lack of evidence to characterise if the cognitive performance of patients with BPAD and MS is different from that of patients who only have BPAD. It is assumed that the combination of risk factors for cognitive impairment (BPAD and MS) known in the same patient may lead to a different cognitive performance, possibly worse than in the patient who only has one of the two disorders.
Literature regarding the cognitive performance of patients with BPAD and MS is limited; in the current review, six articles which study this relationship were found. Two found a link between the body weight of patients with BPAD with worse performance in verbal memory and psychomotor processing speed tests, but the effect size was small and in one of them the link disappeared when adjusted for age, gender and education level.47,48 Another two studies did not find a relationship between cognitive performance and BMI,46,51 and one of them was in patients assessed after a first manic episode.
With respect to hypertension, which is one of the criteria of MS, three studies found a negative relationship with cognitive performance.47,49,50 In two, general cognitive function was evaluated, and the relationship was independent of the presence of confounding variables,47,49 but in one which examined executive function only, the association disappeared when adjusted for multiple tests.50 In this study, it was observed that there was a link between hypertriglyceridaemia and worse performance in executive function.
Only one study49 included criteria of MS according to the IDF and found a significantly worse performance in general cognitive function in the subgroups of MS, obesity and hypertension; the latter was the only subgroup with a significant effect size. In the article, they did not specify what the performance of each cognitive domain was like in the subgroup of patients with MS; likewise, the effect size in this subgroup was small and of little clinical significance.
In general, the findings are of a small effect size and possibly clinically insignificant; however, they could be useful for the study of endophenotypes and psychopathological mechanisms in BPAD. The studies indicate worse cognitive performance of patients with BPAD who have some criteria of MS, such as hypertension, obesity and hypertriglyceridaemia. An indirect link could be made between the cognitive performance of patients with BPAD when they have MS, and it could be considered that they have these alterations, although there is no supporting evidence.
There are different questions on the cognitive performance of patients with BPAD and MS. Well-judged monitoring by confounding variables in future research is important: what relationship is there between treatment with psychotropic drugs and cognitive performance of patients with BPAD and MS? What impact does treatment of MS have on this performance? What is the temporary relationship between these factors? Does the chronicity of MS predispose to greater impairment? What metabolic variables have the greatest effect? Are there differences in the effect of MS on cognitive performance between patients with bipolar I and bipolar II disorder? Studies which take into account variables such as treatment, time of onset of both disorders, progression of cognitive performance impairment, education level and emotional polarity, and which also include patients with a complete diagnosis of MS, are required.
Understanding the relationship between metabolic variables and cognitive deficit in BPAD will make it possible to identify groups of patients in whom cognitive deficit should be evaluated with greater urgency, for those in whom strategies of neuropsychological rehabilitation and functional remediation may be more useful. This line of research may provide advances in the comprehensive care of patients with MS and BPAD: treatment goals, pharmacological treatment, prescription of exercise and the content covered in psychoeducational groups.
ConclusionsDifferent pathophysiological, neuroanatomical and neurocognitive indications explain why the cognitive performance of patients with BPAD and MS is worse than that of those who only have BPAD. There have been contradictory findings on the relationship between hypertriglyceridaemia, hypertension and obesity with a greater impairment of cognitive performance in different domains of patients with BPAD. Current evidence on the cognitive behaviour of patients with BPAD and MS is not conclusive, possibly due to different methodological errors in the few studies available, to a large extent due to not monitoring by confounding variables. To clarify this relationship, studies that include patients with a diagnosis of MS according to international criteria and monitoring by different confounding variables, such as pharmacological treatment, use of psychoactive substances, history of traumatic brain injury and previous cognitive level, among others, are required.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Restrepo Moreno S, García Valencia J, Vargas C, López-Jaramillo C. Desempeño cognitivo de los pacientes con trastorno afectivo bipolar y síndrome metabólico. Rev Colomb Psiquiat. 2019;48:149–155.